ABSTRACT
Objective: Genistein is a recognized isoflavone present in soybeans with antioxidant, anti-inflammatory, antiangiogenic and antitumor activities. This study aimed to test ability of genistein in modulating versican/platelet derived growth factor (PDGF) axis in HCC.
Methods: HCC was experimentally induced in male Sprague-Dawley rats then treated with 25 or 75 mg/kg genistein. Antioxidant activities of genistein was assessed by measuring the gene expression of Nrf2 and the hepatic levels of malondialdehyde (MDA), superoxide dismutase (SOD) and reduced glutathione. Expression of versican, PDGF, protein kinase C (PKC) and ERK-1 protein was assessed by Western blotting and immunostaining.
Results: HCC induced an elevation in oxidative stress, PDGF, versican, PKC and ERK protein expression levels. Genistein significantly reduced an HCC-induced increase in oxidative stress. Moreover, genistein dose-dependently reduced HCC-induced elevation of PDGF, versican, PKC and ERK protein expression levels. Moreover, genistein helped retain a normal hepatocyte structure and reduced fibrous tissue deposition, especially in high dose.
Conclusions: Genistein exerted antitumor and antioxidant effects and therefore suppress HCC development via inhibition of the PDGF/versican bidirectional axis, suppressing both ERK1 and PKC as downstream regulators. Therefore, genistein is a potential novel therapeutic candidate for improving the outcome of patients with HCC.
Highlights
Genistein is an isoflavones present in various soybeans and soy products.
Genistein produced antitumor activity not only due to its antioxidant activity.
Genistein expression of versican, PDGF, PKC and ERK.
Genistein produced hepatoprotective effects.
GRAPHICAL ABSTRACT
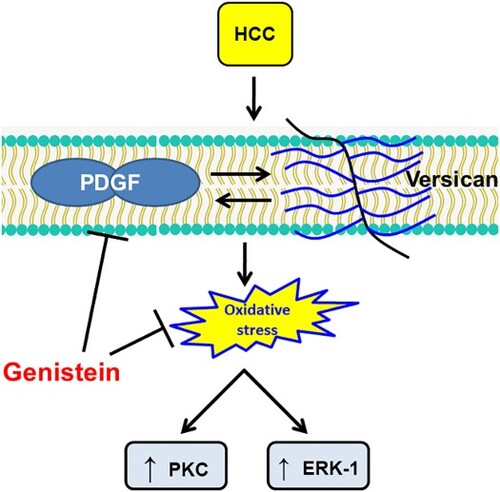
Introduction
Hepatocellular carcinoma (HCC) is the main cause of death in patients with cirrhosis. HCC is the fifth most prevalent liver disease and is characterized by a poor prognosis, being the second most common cause of cancer-related mortality [Citation1]. HCC can result from exposure to numerous factors, including chronic hepatitis C or hepatitis B viral infection, high alcohol consumption and diabetes [Citation2]. HCC is an inflammatory disease, with more than 90% of patients with HCC developing chronic liver damage [Citation3,Citation4]. The prognosis of HCC is poor due to the high rate of metastasis and recurrence of the disease [Citation5]. The molecular pathways that underlie HCC invasion remain elusive, which impedes the discovery of novel therapeutics. Several patients succumb to HCC due to the lack of effective treatments. Therefore, it is crucial to find more effective treatment strategies to ameliorate HCC outcomes.
It has previously been reported that platelet-derived growth factor (PDGF), as a member of the proinflammatory growth factor family, serves an important role in regulating the synthesis of versican. Moreover, PDGF upregulates versican mRNA expression. PDGF expression levels are strongly correlated with HCC grade and invasion, indicating the important role of PDGF in the onset, progression, invasion and metastasis of HCC [Citation6]. Moreover, protein kinase C (PKC) expression levels are strongly correlated with PDGF expression levels; therefore, PKC inhibitors can be used as novel anticancer drugs [Citation7]. Furthermore, the ERK/PDGF signaling pathway contributes to the carcinogenesis of HCC and is considered to be a suitable candidate for the detection of high-risk patients a target for cancer pain treatment [Citation8–10].
Genistein is a soybean isoflavone product that serves as an angiogenesis inhibitor and a phytoestrogen. It exhibits a broad range of important properties, including anti-inflammatory, antioxidant, antiproliferative and antiangiogenic effects, all of which confer genistein chemopreventive potential [Citation11]. Furthermore, genistein has been reported to interfere with estrogen receptors in animals and humans, producing effects similar to estrogen [Citation12], as well as acting on androgenic receptors [Citation13]. Genistein has also been determined to display antiviral activity against rotavirus infection [Citation14].
Genistein is reported to be less harmful to healthy cells than HCC cells by inducing G2/M arrest and apoptosis [Citation15]. However, to the best of our knowledge, there have been no previous studies investigating the effect of versican/PDGF/PKC signaling pathway inhibition by genistein in HCC. Therefore, the aim of the present study was to assess the chemopreventive and hepatoprotective effects of genistein in HCC via investigating its effect on the versican/PDGF/PKC and ERK signaling pathways. For induction of HCC in rats, thioacetamide was used [Citation3,Citation4,Citation16,Citation17].
Materials and methods
Animal experiments. In total, 50 male four weeks old Sprague–Dawley rats (weight, 180-200 g) were used in the present study. The experimental protocols and all procedures were approved by the Faculty of Pharmacy, Mansoura University Research Ethics Committee (Mansoura, Egypt; approval no. 2020-181). Rats were kept in 12 h light / 12 h dark cycle Rats were divided randomly into five groups with 10 animals per group. Animals were kept as 5 rats per each cage at the beginning of the study. The five groups were as follows: (i) Control group, rats were intraperitoneally (i.p.) injected with PBS, 10 mM, pH 7.4; (ii) genistein-treated control group, rats received 75 mg/kg genistein (Sigma-Aldrich; Merck KGaA) daily for 16 weeks by oral gavage; (iii) HCC group, rats received 200 mg/kg thioacetamide (TAA; Tocris Bioscience), i.p. injected twice a week for 16 weeks; (iv) HCC treated with genistein, rats received 25 mg/kg genistein by oral gavage daily for 16 weeks; and (v) HCC treated with genistein, rats received 75 mg/kg genistein by oral gavage daily for 16 weeks. From day one, animals in both HCC groups treated with genistein were injected with 200 mg/kg TAA i.p., twice a week for 16 weeks, along with daily oral genistein treatment. The concentrations of genistein and method of administration used were selected according to those used in previous studies to treat cancer in rats [Citation18,Citation19]. Furthermore, preliminary studies were performed to ensure the effectiveness of the selected doses in cancer treatment.
Sample collection. Blood samples (2 ml) were collected from retro-orbital plexus of each rat anesthetized with thiopental sodium (40 mg/kg, i.p.) via puncture of the retro-orbital plexus. Blood from the rats was centrifuged at 3000 rpm for 5 min and the serum was subsequently stored at −80˚C prior to liver function analysis. Whole rat livers were freshly extracted, rinsed with normal saline and dissected into two parts. One part was fixed in 10% buffered formaldehyde for morphological and histopathological investigation. The second was homogenized in a 10-fold volume of 0.01 M sodium potassium phosphate buffer, pH 7.4 and stored at −80˚C for biochemical analysis.
Morphologic analysis. Formalin fixed liver samples were cut into 5-μm sections and stained with Masson’s trichrome and Periodic acid Schiff (PAS) stains. Sections were anonymously coded and examined in a masked manner using a digital camera-aided computer system (Nikon Corporation).
Immunohistochemistry. Immunohistochemical analyses were performed using 5-μm paraffin sections incubated with monoclonal antibodies for versican, PDGF, PKC and ERK-1 (Abcam) in 1:500 dilution at 4˚C over night. Sections were subsequently treated with secondary antibodies (Abcam) conjugated to HRP. Then, 2% DAB in 50 mM Tris-buffer, pH 7.6, was added as a chromogen. Slides were counterstained with hematoxylin and examined in a masked manner using a digital camera-aided computer system (Nikon Corporation) [Citation20].
Evaluation of hepatoprotective effects. The serum activity of alanine amino transferase (ALT), aspartate amino transferase (AST), alkaline phosphatase, gamma glutamyl transferase (GGT) and albumin (BioDiagnostic Co.) were quantified spectrophotometrically to assess the hepatoprotective effects.
Antioxidant activity. Hepatic tissue levels of malondialdehyde (MDA), hydrogen peroxide, superoxide dismutase (SOD) and reduced glutathione (GSH) were quantified using commercially available kits purchased from BioDiagnostic Co.
ELISA. α-fetoprotein (AFP) levels were analyzed using commercially available ELISA kits (Wuhan USCN Business Co., Ltd.).
Quantitative real-time polymerase chain reaction (RT–PCR). The PCR analysis was performed as described previously by our group [Citation4]. The sequence of Nrf2 the forward primer 5′-GAGACGGCCATGACTGAT-3′ and reverse primer, 5′-GTGAGGGGATCGATGAGTAA-3′ and for GAPDH, the forward primer 5′- CCATCAACGACCCCTTCATT-3′ and reverse primer 5′- CACGACATACTCAGCACCAGC-3′.
Western blotting. The protein expression levels of PDGF, versican, PKC and ERK in liver samples were determined as described previously [Citation21]. Briefly, total protein was quantified using a protein assay (Bio-Rad Laboratories, Inc.). Total protein was separated (20 μg/lane) using SDS-PAGE and subsequently transferred to a nitrocellulose membrane. Primary antibodies (1:500) were purchased from Sigma-Aldrich (Merck KGaA) and were incubated with the membranes at 4˚C overnight. Membranes were reprobed with 1:2000 β-actin at room temperature (Sigma-Aldrich; Merck KGaA) in PBST containing 5% non-fat milk. Following primary incubation, membranes were incubated with HRP-conjugated sheep anti-rabbit secondary antibodies (1:5000). Protein bands were visualized using enhanced chemiluminescence. These data are expressed as the relative optical density.
Statistical analysis. Data are expressed as the mean ± SEM. Normality of sample distribution was examined using the Kolmogorov–Smirnov test. The Kaplan-Meier method was used to assess rat survival. For determining differences among groups, one way ANOVA followed by Bonferroni post hoc test was used. Statistical analysis was performed using SPSS version 20 (IBM Corp.). P < 0.05 was considered to indicate a statistically significant difference.
Results
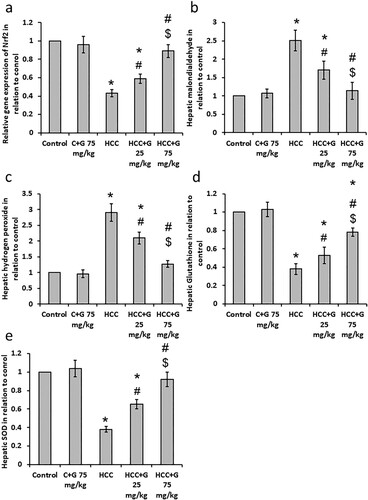
Effect of oral genistein treatment on HCC-induced oxidative stress. HCC rats displayed a 2.51 and 2.92-fold increases in hepatic MDA and hydrogen peroxide levels, respectively. In addition, HCC rats showed 57%, 60% and 63% decrease in hepatic Nrf2, GSH and SOD levels, respectively, compared with the control group. However, treating HCC rats with genistein resulted in a dose-dependent reduction in MDA and hydrogen peroxide levels, as well as a dose-dependent increase in GSH, SOD and Nrf2 levels compared with the HCC group (). These results indicated that genistein may have antioxidant effects in HCC rats.
Figure 1. Effect of genistein at 25 and 75 mg/kg on hepatic oxidative stress and antioxidant markers. (a) gene expression of Nrf2, (b) Malondialdehyde, (c) hydrogen peroxide, (d) superoxide dismutase and (e) reduced glutathione levels compared with the normal control in TAA-induced HCC rats. Data are expressed as the mean ± SEM, *P < 0.05 vs. control; #P < 0.05 vs. HCC group; and $P < 0.05 versus, HCC + 75 mg/kg genistein group. HCC, hepatocellular carcinoma; TAA, thioacetamide; C, control; G, genistein.
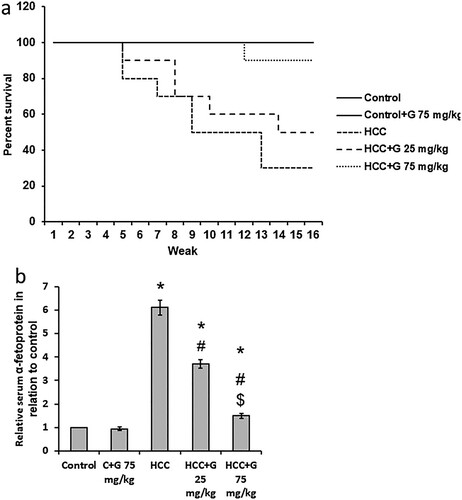
Effect of genistein on HCC-induced mortality and AFP elevation. Liver images from HCC rats showed increased number of nodules as compared with the control group. Treatment of HCC rats with genistein results in a dose dependent reduction in the number of nodules in the liver of rats (). In addition, treating HCC rats with 25 mg/kg of genistein increased the rat survival rate from 30% in the HCC group to 50%. Furthermore, HCC rats treated with 75 mg/kg genistein displayed an increased survival rate of 90%. At the end of the experiment, control rats and control rats treated with genistein 75 mg/kg exhibited 100% survival. Rat survival was also associated with a significant reduction in AFP serum levels compared with the control group (). These results therefore demonstrated that genistein may produce therapeutic effects against HCC by reducing mortality rate and AFP serum levels.
Figure 2. Effect of genistein at 25 and 75 mg/kg on liver images in in (a) the control group, (b) the control group treated with 75 mg/kg genistein, (c) the HCC group, (d) the HCC group treated with 25 mg/kg genistein and (e) the HCC treated with 75 mg/kg genistein.
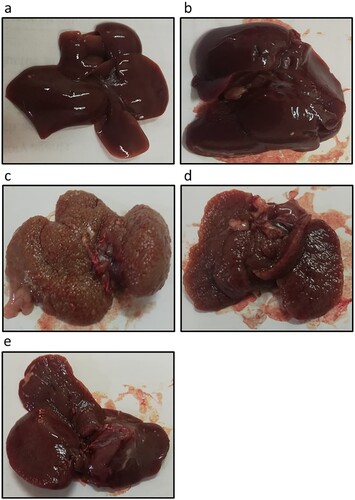
Figure 3. Effect of genistein at 25 and 75 mg/kg on survival rate and AFP serum levels in TAA-induced HCC rats. (a) Rat survival rate. (b) AFP serum levels were determined using ELISA. Data are presented as the mean ± SEM, *P < 0.05 vs. control; #P≤0.05 vs. HCC group; $P < 0.05 vs. HCC + 75 mg/kg genistein group. AFP, α-fetoprotein; TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
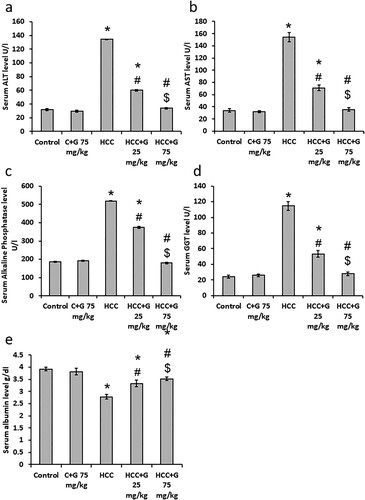
Effect of genistein on liver function tests. As indicated in , compared with the control group, ALT, AST, Alkaline phosphatase and GGT serum levels were significantly elevated in the HCC group, whereas there was a significant reduction in serum albumin levels. Treating HCC rats with genistein resulted in a significant reduction in ALT, AST, Alkaline phosphatase and GGT serum levels and elevated serum albumin levels compared with HCC group, especially in the group treated with 75 mg/kg genistein. These results suggested genistein produced hepatoprotective effects against HCC rats.
Figure 4. Effect of genistein at 25 and 75 mg/kg on serum liver markers levels in TAA-induced HCC rats. (a) ALT, (b) AST, (c) alkaline phosphatase, (d) GGT and (e) albumin levels. Data are expressed as the mean ± SEM, *P < 0.05 vs. control; #P < 0.05 vs. HCC group; and $P < 0.05 vs. HCC + 75 mg/kg genistein group. GPT, glutamine aminotransferase; TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
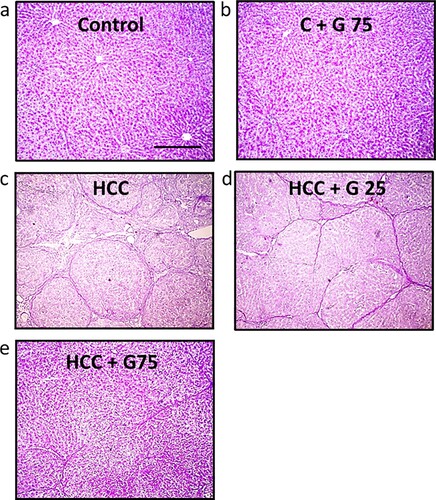
Effect of genistein on HCC-induced morphological changes. Examination of the rat liver samples from the control groups stained with Masson’s trichrome revealed the absence of fibrosis. However, images captured of the liver tissue in the HCC group demonstrated highly stained fibrous septa compared with the control group. Treating HCC rats with genistein decreased fibrous tissue deposition, especially following treatment with 75 mg/kg genistein (). Furthermore, as displayed in , samples stained with PAS displayed a normal appearance in the control groups. The PAS staining was reduced in the HCC group compared with the control group and significantly increased in HCC rats treated with genistein. Therefore, these results suggested that genistein may improve the hepatocyte structure.
Figure 5. Hepatic sections stained with Masson’s trichrome stain. (a) No fibrosis was demonstrated in the control group or the (b) control group treated with 75 mg/kg genistein. (c) HCC displayed green stained broad fibrous septa (arrows). (d) HCC treated with 25 mg/kg genistein demonstrated a mild decrease in fibrous tissue deposition (arrow). (e) HCC treated with 75 mg/kg genistein displayed very mild fibrous tissue deposition (arrow). Scale bars, 100 µm. HCC, hepatocellular carcinoma.
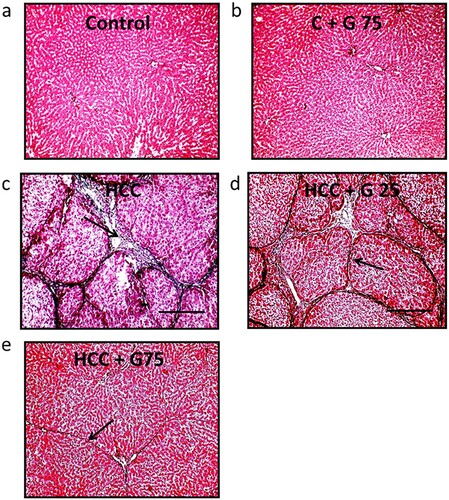
Figure 6. Hepatic sections stained with PAS stain. Livers in (a) the control group and (b) the control group treated with 75 mg/kg genistein displayed a healthy appearance. (c) HCC rats displayed a decreased positivity in staining compared with the control group. (d) HCC rats treated with 25 mg/kg genistein displayed a slight increase in staining positivity compared with the untreated HCC group. (e) HCC treated with 75 mg/kg genistein displayed a markedly increased staining positivity compared with the untreated HCC group. Scale bars, 100 µm. PAS, periodic acid Schiff; HCC, hepatocellular carcinoma.
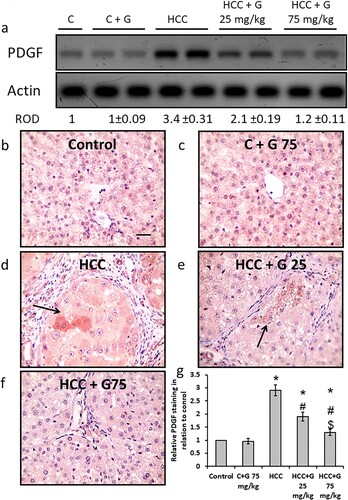
Effect of genistein on HCC-induced PDGF protein expression level elevation in hepatic tissue. HCC rats revealed a significant elevation in PDGF protein expression levels compared with the control groups. However, oral administration of genistein resulted in a significant decrease in PDGF protein expression levels in a dose-dependent manner. Moreover, hepatic sections from the HCC group demonstrated a significant increase in areas stained with anti-PDGF antibodies as compared with the control group. However, treatment of HCC rats with the higher dose of genistein significantly reduced the positively stained area compared with HCC and to be similar levels as those seen in the normal control group (). Therefore, genistein blocked HCC-induced expression of PDGF without affecting the control group.
Figure 7. Effect of genistein at 25 and 75 mg/kg on hepatic PDGF protein expression levels in TAA-induced HCC rats. (a) PDGF protein expression levels were determined via western blotting. Photomicrographs of immunohistochemically stained hepatic sections using anti-PDGF antibodies in the following groups: (b) Control group; (c) control group treated with genistein at 75 mg/kg; (d) HCC group; (e) HCC rats treated with 25 mg/kg genistein; and (f) HCC rats treated with 75 mg/kg genistein. (g) Relative immune-staining score of PDGF showing increase in HCC sections that was reduced by genistein treatment. Scale bars, 50 µm. *P < 0.05 vs. control; #P≤0.05 vs. HCC group; and $P < .05 vs. HCC + 75 mg/kg genistein group. PDGF, platelet derived growth factor; TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
Effect of genistein on HCC-induced versican protein expression level increases. TAA injection resulted in a significant elevation in versican protein expression levels compared with the control groups. However, treating HCC rats with genistein resulted in a decrease in versican protein expression levels in a dose-dependent manner. Furthermore, hepatic sections from the HCC group displayed a significant increase in areas stained with anti-versican antibodies. However, treatment with genistein significantly reduced the positively stained areas to levels similar to those of the normal control group in rats treated with the higher dose of genistein ().
Figure 8. Effect of genistein at 25 and 75 mg/kg on hepatic versican protein expression levels in TAA-induced HCC rats. (a) Versican protein expression levels were determined via western blotting. Photomicrographs of immunohistochemically stained hepatic sections using anti-versican antibodies in the following groups: (b) Control group; (c) control group treated with genistein at 75 mg/kg; (d) HCC group; (e) HCC rats treated with 25 mg/kg genistein; and (f) HCC rats treated with 75 mg/kg genistein. (g) Relative immune-staining score of versican showing increase in HCC sections that was reduced by genistein treatment. Scale bars, 50 µm. *P < 0.05 vs. control group; #P < 0.05 vs. HCC group; and $P < 0.05 vs. HCC + 75 mg/kg genistein group. TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
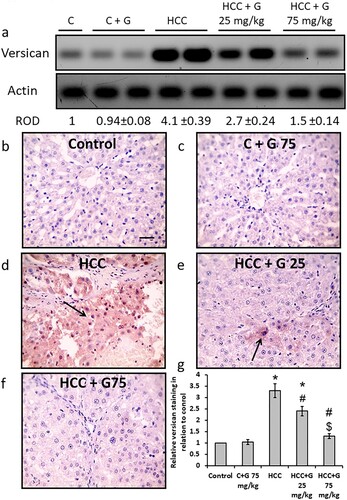
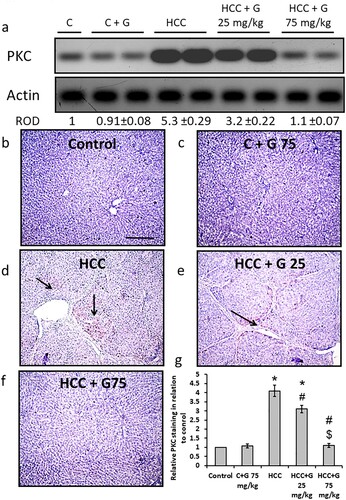
Effect of genistein on HCC-induced PKC protein expression level increases. HCC rats demonstrated a significant increase in PKC protein expression levels compared with the control groups. Hepatic sections stained with anti-PKC antibody revealed an increased area of staining in the HCC group compared with the control groups. However, treating the HCC group with genistein resulted in a PKC protein expression level reduction in a dose-dependent manner. The PKC protein expression levels in the group treated with 75 mg/kg genistein was similar to that of the control group ().
Figure 9. Effect of genistein at 25 and 75 mg/kg on hepatic PKC protein expression levels in TAA-induced HCC rats. (a) PKC protein expression levels were determined via western blotting. Photomicrographs of immunohistochemically stained hepatic sections using anti-PKC antibodies in the following groups: (b) Control group; (c) control group treated with genistein at 75 mg/kg; (d) HCC group; (e) HCC rats treated with 25 mg/kg genistein; and (f) HCC rats treated with 75 mg/kg genistein. (g) Relative immune-staining score of PKC showing increase in HCC sections that was reduced by genistein treatment. Scale bars, 50 µm. *P < 0.05 vs. control group; #P < 0.05 vs. HCC group; $P < 0.05 vs. HCC + 75 mg/kg genistein group. PKC; protein kinase C; TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
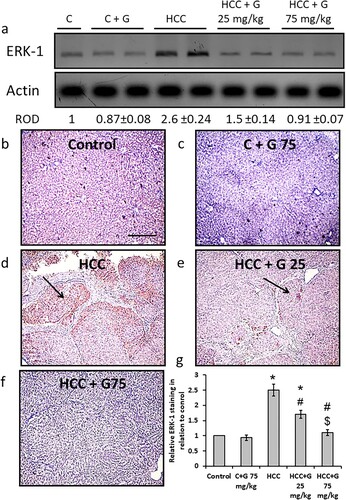
Effect of genistein on HCC-induced ERK-1 protein expression level increases. The HCC group demonstrated a significant elevation in ERK-1 protein expression levels compared with the control groups. Moreover, hepatic sections from the HCC rats displayed an increase in areas stained with anti-ERK-1 antibodies compared with the control groups. However, treating the HCC group with genistein resulted in a reduction in ERK-1 protein expression levels in a dose-dependent manner. The ERK-1 protein expression levels in the group treated with 75 mg/kg genistein were similar to those of the control group ().
Figure 10. Effect of genistein at 25 and 75 mg/kg on hepatic ERK-1 protein expression levels in TAA-induced HCC rats. (a) ERK-1 protein expression levels were determined via western blotting. (b) Photomicrographs of immunohistochemically stained hepatic sections using anti-ERK-1 antibodies in the following groups: (b) Control group; (c) control group treated with genistein at 75 mg/kg; (d) HCC group; (e) HCC rats treated with 25 mg/kg genistein; and (f) HCC rats treated with 75 mg/kg genistein respectively. (g) Relative immune-staining score of ERK-1 showing increase in HCC sections that was reduced by genistein treatment. Scale bars, 100 µm. *P < 0.05 vs. control group; #P < 0.05 vs. HCC group; $P < 0.05 vs. HCC + 75 mg/kg genistein group. TAA, thioacetamide; HCC, hepatocellular carcinoma; C, control; G, genistein.
Discussion
HCC is considered to be the primary hepatic malignancy and is a leading cause of cancer-related mortality worldwide [Citation22]. HCC is highly resistant to radiotherapy and surgical resection ablation therapy [Citation23]. The liver microenvironment consists of several components, including the extracellular matrix (ECM), immune cells, Kupffer cells, endothelial cells, fibroblasts, cytokines and various growth factors, which makes the hepatic carcinogenic tissue microenvironment vulnerable to recurrence as well as the development of de novo HCC tumors [Citation24]. In a carcinogenic tissue microenvironment, proteoglycan expression is altered significantly and, therefore, proteoglycans can be considered as attractive therapeutic targets in HCC [Citation6]. In the present study, HCC significantly increased versican protein expression levels, which could lead to changes in tumor cell survival, angiogenesis and metastasis and facilitate tumor progression [Citation25]. Targeting versican, one of the proteoglycans in the tumor microenvironment, may offer a novel therapeutic approach to cancer. The aim of this study was to investigate the effect of blocking PDGF-modulated versican expression on HCC pathogenicity in vivo.
In the past decade, there has been an increasing interest in potential cancer chemopreventive agents obtained from natural sources [Citation20]. Genistein as a soy isoflavone has drawn attention because of its potential beneficial effects on degenerative diseases, including cancer. A study has reported that genistein can target numerous critical molecular targets. These studies have also demonstrated that genistein also has proapoptotic, cell cycle arrest, antiangiogenic, antimetastatic, antiproliferative and anti-inflammatory properties [Citation11]. However, genistein is considered to be a liver cancer suppressor in vitro [Citation26]. Recently, it has been demonstrated that the chemoprotective effect of genistein may be attributed to its proapoptotic and anti-inflammatory effects via mechanisms involving AMP-activated protein kinase [Citation27].
Therefore, the present study explored a number of new insights concerning the in vivo molecular mechanism of oral genistein as a chemopreventive and hepatoprotective agent on TAA-induced HCC in rats. The results indicated that genistein may possess antiangiogenic, antimetastatic, antiproliferative and antioxidant properties and may therefore suppress hepatocarcinogenesis and HCC progression via inhibition of the PDGF/versican bidirectional axis. This may suppress both ERK1 and PKC as downstream regulators of the interaction between PDGF and its corresponding receptor.
Previous studies have reported that genistein exhibits potent antiangiogenic properties. It is well known that angiogenesis is a fundamental step in tumor growth, as PDGF is a protein essential to pericyte recruitment, which is a critical aspect of blood vessel maturation [Citation28]. Studies have demonstrated that PDGF is a key pathogenic factor in multiple solid tumors [Citation29] and there is strong evidence that the PDGF family serves a fundamental role in liver cirrhosis and, therefore, HCC development [Citation30].
PDGF/PDGF receptor tyrosine kinase activity has been determined to stimulate the transcription, translation and posttranslational processing of versican in arterial smooth muscle. It has also been demonstrated that genistein selectively inhibits versican biosynthesis in monkey arterial smooth muscle cells stimulated by PDGF [Citation31]. Therefore, it can be hypothesized that genistein can act as a blocker of tyrosine kinase activity, which could modulate versican expression levels and hence attenuate HCC development in vivo via numerous signaling pathways.
The present study demonstrated that oral administration of genistein resulted in a dose-dependent downregulation in PDGF protein expression levels in HCC rats. These results are consistent with those reported in other studies, which demonstrated that genistein exerted antiangiogenic activity via downregulating VEGF and PDGF in human bladder cancer cell lines [Citation32]. Furthermore, the genistein-treated groups, especially at a dose of 75 mg/kg, exhibited a significant decrease in versican protein expression levels. Growing evidence has revealed that versican expression levels are upregulated in numerous malignant tumors, which is correlated with a poor prognosis, reduced survival rate and increased mortality rate [Citation33–35]. This data is supported by the results of the present study, whereby in the HCC group significantly higher versican protein expression levels and a decrease in survival rate by 70% were demonstrated, compared with the normal control group. The impact of versican expression in HCC development remains elusive and unclear; however, a recent study by Zhangyuan et al. [Citation35] reported that versican V1 promoted the proliferation and metastasis of HCC via activation of the EGFR/PI3 K/AKT signaling pathway. This study also demonstrated that versican, a component of the ECM, could profoundly affect the tumor microenvironment by affecting cancer-associated fibroblasts (CAFs) [Citation35].
Recent interest has been focused on targeting the cancer microenvironment using PDGF expression as a novel therapeutic target for cancer. It has been reported that CAFs affect the deposition and remodeling of ECM components and release cytokines and growth factors. Among those, PDGF can affect cancer cells in a paracrine manner, which leads to an increase in cancer cell proliferation. Furthermore, CAF-derived PDGF can also indirectly increase tumor angiogenesis via activation of VEGF production, which serves a key role in angiogenesis [Citation36].
The interaction between PDGF and versican could influence cancer cells in a paracrine manner, which may lead to an increase in cancer cell proliferation and tumor angiogenesis [Citation29]. Therefore, genistein reveals a novel and promising mechanism in terms of its chemopreventive effect on HCC in vivo. From the present study it can be hypothesized that genistein attenuates PDGF release, which in turn downregulates versican concentration, which leads to a further reduction in the release of PDGF, representing a positive feedback loop. PDGF and versican also affect PKC isozymes, which act as intermediates in numerous signaling pathways associated with tumorigenesis, proliferation, invasion, migration and metastasis and, hence, they have been the subject of intense research to identify novel drugs for cancer treatment [Citation37].
To the best of our knowledge, the present study is the first to reveal the novel and promising chemopreventive mechanism of genistein in HCC in vivo. The results of the present study suggested that genistein, as a blocker of receptor tyrosine kinase, not only affects tumor cells, but also affects CAFs in the tumor microenvironment. Genistein was demonstrated to attenuate PDGF release, which in turn downregulated versican protein expression levels, which may have resulted in the decreased release of PDGF from CAFs. Therefore, the PDGF/versican positive feedback loop may be considered as a promising target to combat HCC development, invasion and angiogenesis, and overcome tumor therapy resistance.
It has also been reported that the Ras/Raf/MEK/ERK signaling network serves a significant role in HCC progression [Citation38]. To the best of our knowledge, the present study demonstrated for the first time that genistein administration achieved a dose-dependent downregulation of ERK1, as a component of the MAPK signaling pathway, and PKC protein expression levels, as well as a significant decrease in PDGF and versican protein expression levels compared with the HCC group. Previous studies have demonstrated that the overexpression of EGF, PDGF and VEGF as upstream growth factors, combined with RTK, activate the Ras/Raf/MEK/ERK signaling network in HCC. Furthermore, it has been revealed that ERK activation also promotes the expression of EGFR ligands, promoting an autocrine growth loop critical for tumor growth [Citation39]. A previous study also determined that versican has EGF-like motifs [Citation35]. It can therefore be hypothesized that both PDGF and versican could activate ERK1, as a downstream regulator of the MAPK signaling pathway, and PKC. These signaling pathways are implicated in cancer progression, as they induce a number of cellular events, including proliferation, differentiation, cell migration and cell survival.
PKC isozymes sit at the crossroads of multiple signaling pathways associated with proliferation, migration, invasion, tumorigenesis and metastasis and, therefore, they have been the subject of intense research to identify novel therapeutics for cancer [Citation40]. For example, PKC isozymes stimulate the Ras/Raf/MEK/ERK signaling pathway which serves a crucial role in cancer cell survival and proliferation [Citation41].
Signaling via PDGF receptors in tumor cells is tightly regulated and controlled. Certain studies have suggested that there are two main mechanisms that lead to the amplification of PDGF downstream signaling pathways in tumor cells. First, PDGF-stimulated cells produce reactive oxygen species (ROS), which react with cysteine residues at active sites of tyrosine phosphatases leading to their inhibition. Second, MAP-kinase phosphatase 3 degradation as a result of the ubiquitination process, is responsible for the dephosphorylation and inactivation of ERK, which results in increasing tumor proliferation and progression [Citation33]. These results also support the data of the present study, which demonstrated that the HCC group exhibited a significant upregulation in PDGF protein expression levels accompanied by a significant increase in hepatic MDA levels. Furthermore, western blotting data demonstrated a significant upregulation in ERK1 protein expression levels.
Signaling pathways involved in PDGF-mediated increases and PDGF treatments have resulted in increases in versican core protein synthesis. The effects of PDGF on versican mRNA are blocked by the inhibition of either the PKC or the ERK signaling pathways. However, the effect of PDGF can be blocked by PKC inhibition, but not by ERK inhibition [Citation42]. Both PKC and ERK activation are required for versican mRNA core protein expression. Previous studies have indicated that different signaling pathways control different aspects of PDGF-stimulated versican biosynthesis [Citation43].
During oxidative stress, ROS are constantly produced and have deleterious effects on all body cells. It has been reported that oxidative stress serves a key role in the progression of chronic liver disease and hepatocarcinogenesis [Citation44]. The present study demonstrated that oral genistein administration resulted in dose-dependent antioxidant activity, reflected by a significant increase in hepatic Nrf2, GSH and SOD levels and marked suppression of MDA levels. Genistein was reported to produce antioxidant activity in prostate cancer cells in vitro via the induction of antioxidant enzyme expression, such as catalase and superoxide dismutase, which collectively lead to a significant reduction in ROS levels [Citation45].
The present study also demonstrated that genistein, especially at a dose of 75 mg/kg, restored serum GPT, alkaline phosphatase and albumin levels to those seen in the normal control group. Furthermore, liver samples of the genistein-treated groups displayed a decrease in fibrous tissue and collagen deposition compared with the HCC group. Genistein has been demonstrated to exert hepatoprotective effects in non-alcoholic fatty liver disease [Citation46]. Furthermore, in the present study, genistein was demonstrated to markedly decrease AFP levels and, therefore, significantly increased rats survival rate up to 90%.
In conclusion, genistein exhibited antitumor activity, which could not be attributed to its antioxidant activity alone, but also to its inhibition of versican, PDGF, PKC and ERK expression. Genistein could also be a potential therapeutic candidate, improving the outcomes of patients with HCC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Foglia B, Parola M. Of FACT complex and oxidative stress response: a KEAP1/NRF2-dependent novel mechanism sustaining hepatocellular carcinoma progression. Gut. 2020;69(2):195–196.
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2020;73(Suppl. 1):4–13.
- El-Far YM, Khodir AE, Noor AO, et al. Selective cytotoxic activity and protective effects of sodium ascorbate against hepatocellular carcinoma through its effect on oxidative stress and apoptosis in vivo and in vitro. Redox Rep. 2020;25(1):17–25.
- El-Far YM, Khodir AE, Emarah ZA, et al. Fucoidan ameliorates hepatocellular carcinoma induced in rats: effect on miR143 and inflammation. Nutr Cancer. 2020;28:1–13.
- Flynn MJ, Sayed AA, Sharma R, et al. Challenges and opportunities in the clinical development of immune checkpoint inhibitors for hepatocellular carcinoma. Hepatology. 2019;69(5):2258–2270.
- Tanaka Y, Tateishi R, Koike K. Proteoglycans are attractive biomarkers and therapeutic targets in hepatocellular carcinoma. Int J Mol Sci. 2018;19(10):3070.
- Goekjian PG, Jirousek MR. Protein kinase C inhibitors as novel anticancer drugs. Expert Opin Investig Drugs. 2001;10(12):2117–2140.
- Schmitz KJ, Wohlschlaeger J, Lang H, et al. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48(1):83–90. doi:10.1016/j.jhep.2007.08.018.
- Kim ST, Hong JY, Park SH, et al. First-in-human phase I trial of anti-hepatocyte growth factor antibody (YYB101) in refractory solid tumor patients. Ther Adv Med Oncol. 2020;12:1758835920926796. doi:10.1177/1758835920926796.
- Wu XP, Yang YP, She RX, et al. microRNA-329 reduces bone cancer pain through the LPAR1-dependent LPAR1/ERK signal transduction pathway in mice. Ther Adv Med Oncol. 2019;11:1758835919875319.
- Tuli HS, Tuorkey MJ, Thakral F, et al. Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol. 2019;10:1336. doi:10.3389/fphar.2019.01336.
- Lecomte S, Demay F, Ferriere F, et al. Phytochemicals targeting estrogen receptors: beneficial rather than adverse effects? Int J Mol Sci. 2017;18(7):1381.
- Shin SB, Woo SU, Yim H. Cotargeting Plk1 and androgen receptor enhances the therapeutic sensitivity of paclitaxel-resistant prostate cancer. Ther Adv Med Oncol. 2019;11:1758835919846375.
- Donovan SM, Andres A, Mathai RA, et al. Soy formula and isoflavones and the developing intestine. Nutr Rev. 2009;67(Suppl 2):S192–S200.
- Debbabi H, Bonnin P, Ducluzeau PH, et al. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens. 2010;23(5):541–546.
- Huang DQ, Muthiah MD, Zhou L, et al. Predicting HCC response to multikinase inhibitors with in vivo cirrhotic mouse model for personalized therapy. Cell Mol Gastroenterol Hepatol. 2021;11(5):1313–1325. doi:10.1016/j.jcmgh.2020.12.009.
- Hassan HM, Al-Wahaibi LH, Shehatou GS, et al. Adamantane-linked isothiourea derivatives suppress the growth of experimental hepatocellular carcinoma via inhibition of TLR4-MyD88-NF-κB signaling. Am J Cancer Res. 2021;11(2):350–369.
- Min WK, Sung HY, Choi YS. Suppression of colonic aberrant crypt foci by soy isoflavones is dose-independent in dimethylhydrazine-treated rats. J Med Food. 2010;13(3):495–502. doi:10.1089/jmf.2009.1208.
- Rajan RK, Balaji B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 2017;55(1):242–251. doi:10.1080/13880209.2016.1258425.
- Hassan HM, El-Kannishy SMH, Alattar A, et al. Therapeutic effects of blocking β-catenin against hepatocellular carcinoma-induced activation of inflammation, fibrosis and tumor invasion. Biomed Pharmacother. 2021;135:111216.
- Al-Gayyar MMH, Bagalagel A, Noor AO, et al. The therapeutic effects of nicotinamide in hepatocellular carcinoma through blocking IGF-1 and effecting the balance between Nrf2 and PKB. Biomed Pharmacother. 2019;112:108653.
- Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41–53.
- Zhou C, Peng Y, Zhou K, et al. Surgical resection plus radiofrequency ablation for the treatment of multifocal hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2019;8(1):19–28.
- Hernandez-Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma: a review. Gastroenterology. 2013;144:512–527.
- Theocharis AD, Skandalis SS, Tzanakakis GN, et al. Proteoglycans in health and disease: novel roles for proteoglycans in malignancy and their pharmacological targeting. FEBS J. 2010;277(19):3904–3923.
- Chodon D, Ramamurty N, Sakthisekaran D. Preliminary studies on induction of apoptosis by genistein on HepG2 cell line. Toxicol In Vitro. 2007;21(5):887–891.
- Lee SR, Kwon SW, Lee YH, et al. Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer. 2019;19(1):6. doi:10.1186/s12885-018-5222-8.
- Gavalas NG, Liontos M, Trachana S-P, et al. Angiogenesis-Related pathways in the pathogenesis of ovarian cancer. Int J Mol Sci. 2013;14(8):15885–15909. doi:10.3390/ijms140815885.
- Heldin C-H. Targeting the PDGF signaling pathway in tumor treatment: a review. Cell Commun Signal. 2013;11:97.
- Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128(6):1259–1268.
- Schönherr E, Kinsella MG, Wight TN. Genistein selectively inhibits platelet-derived growth factor-stimulated versican biosynthesis in monkey arterial smooth muscle cells. Arch Biochem Biophys. 1997;339(2):353–361. doi:10.1006/abbi.1996.9854.
- Tuli HS, Tuorkey MJ, Thakral F, et al. Molecular mechanisms of action of genistein in cancer: recent advances: a review. Front Pharmacol. 2019;10:1336.
- Nikitovic D, Zafiropoulos A, Katonis P, et al. Transforming growth factor-beta as a key molecule triggering the expression of versican isoforms v0 and v1, hyaluronan synthase-2 and synthesis of hyaluronan in malignant osteosarcoma cells. IUBMB Life. 2006;58(1):47–53. doi:10.1080/15216540500531713.
- Suwiwat S, Ricciardelli C, Tammi R, et al. Expression of extracellular matrix components versican, chondroitin sulfate, tenascin, and hyaluronan, and their association with disease outcome in node-negative breast cancer. Clin Cancer Res. 2004;10(7):2491–2498.
- Zhangyuan G, Wang F, Zhang H, et al. Versicanv1 promotes proliferation and metastasis of hepatocellular carcinoma through the activation of EGFR-PI3K-AKT pathway. Oncogene. 2020;39(6):1213–1230. doi:10.1038/s41388-019-1052-7.
- Tao L, Huang G, Song H, et al. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncol Lett. 2017;14(3):2611–2620.
- Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128(6):1259–1268. doi:10.1002/ijc.25469.
- Li G-DZ, Shi Z, Qi L-L, et al. The Ras/Raf/MEK/ERK signaling pathway and its role in the occurrence and development of HCC. Oncol Lett. 2016;12(5):3045–3050.
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer: a review. Oncogene. 2007;26(22):3291–3310.
- Cooke M, Magimaidas A, Casado-Medrano V, et al. Protein kinase C in cancer: the top five unanswered questions: a review. Mol Carcinog. 2017;56(6):1531–1542.
- Kang J-H. Protein kinase C (PKC) isozymes and cancer: a review. New J Sci. 2014. doi:10.1155/2014/231418.
- Osman N, Getachew R, Thach L, et al. Platelet-derived growth factor-stimulated versican synthesis but not glycosaminoglycan elongation in vascular smooth muscle is mediated via Akt phosphorylation. Cell Signal. 2014;26(5):912–916. doi:10.1016/j.cellsig.2014.01.019.
- Cardoso LEM, Little PJ, Ballinger ML, et al. Platelet-derived growth factor differentially regulates the expression and post-translational modification of versican by arterial smooth muscle cells through distinct protein kinase C and extracellular signal-regulated kinase pathways. J Biol Chem. 2010;285(10):6987–6995. doi:10.1074/jbc.M109.088674.
- Takaki A, Yamamoto K. Control of oxidative stress in hepatocellular carcinoma: helpful or harmful? World J Hepatol. 2015;7(7):968–979.
- Park CE, Yun H, Lee EB, et al. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J Med Food. 2010;13(4):815–820. doi:10.1089/jmf.2009.1359.
- Xin X, Chen C, Hu YY, et al. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed Pharmacother. 2019;117:109047.
