Abstract
Background
Some patients with metastatic breast cancer (MBC) stay on endocrine therapy (ET) for years and others progress quickly. Serum thymidine kinase activity (TKa), an indicator of cell-proliferation, is a potential biomarker for monitoring ET and predicting MBC outcome. We have previously reported TKa as being prognostic in MBC in SWOG S0226. Here, new data on progression within 30/60 days post sampling, with a new, FDA approved version of DiviTum®TKa highlighting differences vs. a Research Use Only version is reported.
Methods
1,546 serum samples from 454 patients were assessed, collected at baseline and at 4 subsequent timepoints during treatment. A new predefined cut-off tested the ability to predict disease progression. A new measuring unit, DuA (DiviTum® unit of Activity) is adopted.
Results
A DiviTum®TKa score <250 DuA provides a much lower risk of progression within 30/60 days after blood draw, the negative predictive value (NPV) was 96.7% and 93.5%, respectively. Patients <250 DuA experienced significantly longer progression-free survival and overall survival, demonstrated at baseline and for all time intervals.
Conclusions
DiviTum®TKa provides clinically meaningful information for patients with HR+ MBC. Low TKa levels provide such a high NPV for rapid progression that such patients might forego additional therapy added to single agent ET.
Trial registration: NCT00075764
Background
The use of endocrine therapy (ET) in patients with hormone receptor (HR) positive, metastatic breast cancer (MBC) provides both palliation and improved survival (Burstein Citation2020). Several different ET modalities are available, including selective oestrogen receptor modulators, aromatase inhibitors and selective oestrogen receptor degraders (Burstein Citation2020). Over the last decade, different targeted therapies, including inhibitors of cyclin-dependent kinase 4 and 6 (CDK4/6), mTOR and mutated PI3K, have been shown to improve outcomes when combined with ET in patients with HR positive MBC. However, these agents also add toxicity and cost.
Tumour biomarker tests (TBT) may provide critical information regarding prognosis, prediction and monitoring for patients with malignant diseases (Hayes Citation2021). Currently, there is no biomarker available for patients with HR positive, HER2 negative MBC that helps clinicians distinguish between those patients who are likely to benefit from early use of one of the ancillary treatments versus those who might safely postpone their use. TBT can be measured in tissue or in circulation. Circulating TBT measured in blood, designated ‘liquid biopsies’, have significant advantages over tissue-based markers (Merker et al. Citation2018); they are non-invasive, can be performed easier and more frequently, and may better represent the integrated biology and tumour burden for a patient. Measuring a circulating enzyme is not a liquid biopsy per se (detecting tumour-specific cells or DNA) but uses the same source (blood) for analysis.
Thymidine kinase (TK) is a metabolic enzyme fundamentally involved in DNA synthesis that plays a critical role in cell proliferation (Welin et al. Citation2004). In resting G0 cells, TK activity (TKa) is absent. In actively dividing cells, TKa increases in the G1/S transition, peaks in the S phase and then disappears during mitosis. Uncontrolled and increased proliferation is a hallmark of cancer cells. Elevated TKa in breast cancer tissues is associated with worse prognosis (Robertson et al. Citation1990). In addition to tissue expression, TKa can be measured in circulation and is frequently elevated in patients with cancer compared to normal, healthy individuals. Healthy individuals typically have very low levels of TKa. By contrast, patients with cancer frequently have elevated serum TKa levels indicating increased cell proliferation. Elevated serum TKa levels serve as a marker of increased tumour growth in patients with malignancies and may precede imaging RECIST (Response Evaluation Criteria In Solid Tumors)-determined disease progression by several months (Krishnamurthy et al. Citation2022).
Several studies support that circulating TKa can predict outcome in breast cancer (Bagegni et al. Citation2017, Bonechi et al. Citation2018, Larsson et al. Citation2020, Cabel et al. Citation2020, Krishnamurthy et al. Citation2022). Because TKa is a marker of cell proliferation and can reflect uncontrolled cell growth, the DiviTum®TKa assay (Biovica, Sweden) was developed for precision measurement of circulating TKa. We previously reported that serum TKa, as measured by the DiviTum®TKa assay predicted progression-free survival (PFS) and overall survival (OS) in MBC (Paoletti et al. Citation2021). The prior publication was conducted using a Research Use Only (RUO) version of the DiviTum®TKa assay in specimens collected from patients with HR positive, HER2 negative MBC who participated in a prospective randomized clinical trial addressing single agent anastrozole vs anastrozole plus fulvestrant. In that report, it was concluded that high serum TKa at baseline and at subsequent timepoints is associated with worse prognosis in patients starting first-line ET, and that patients with low serum TKa at baseline have comparable outcomes on single agent or combination ET (Paoletti et al. Citation2021).
Based on the results from the S0226 trial (Mehta et al. Citation2012, Citation2019), the DiviTum®TKa assay, was approved by the FDA for assessment of TKa in human serum samples for in vitro diagnostic (IVD) use, with the intended application as an aid in monitoring disease progression during treatment of HR positive, postmenopausal patients with MBC. However, the FDA approval was granted based on data generated from S0226 using a refined cut-off and re-analysis than that used for the original publication (Paoletti et al. Citation2021). In this report, differences between the previous RUO assay and the new, FDA approved version of DiviTum®TKa are presented. Additional data with the FDA approved version regarding progression within 30/60 days post sampling and TKa reference values for a healthy female population are also presented. The primary objective of this clinical validation study to achieve FDA clearance was to demonstrate that serum TKa, assessed with DiviTum®TKa at baseline and at subsequent specified time intervals, was prognostic for disease progression within 30/60 days in HR positive postmenopausal women with MBC. Results for PFS and OS will also be presented.
Methods
Patients
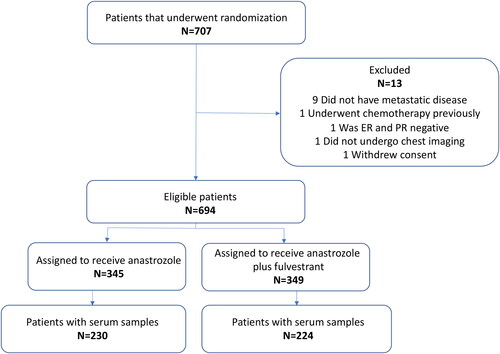
SWOG S0226 was a multicenter phase III trial conducted in the USA and Canada (ClinicalTrials.gov number NCT00075764), conducted in accordance with the Declaration of Helsinki (Mehta et al. Citation2012). Details regarding eligibility and trial design are published elsewhere (Paoletti et al. Citation2021, Mehta et al. Citation2012, Mehta et al. Citation2019). Briefly, HR positive postmenopausal women with MBC were randomly assigned to receive either anastrozole or anastrozole plus fulvestrant as first line therapy, with stratification according to prior adjuvant treatment with tamoxifen. Progression was assessed every 3 months according to RECIST 1.0 (Therasse et al. Citation2000), and OS was assessed every 6 months for the first 2 years and then annually. A total of 707 patients were randomized of whom 694 had data available for analysis and 454 had serum samples collected; 230 received anastrozole and 224 anastrozole plus fulvestrant ().
Clinical validation of the DiviTum®TKa assay
This clinical validation study was designed in accordance with the FDA 510(k) requirements as a blinded, prospective-retrospective study of the DiviTum®TKa assay using serum samples and outcome data from the SWOG S0226 trial. Blood specimen draws at baseline, treatment cycle 2, cycle 3, cycle 4 and cycle 7, processing and archiving are described previously (Paoletti et al. Citation2021). Briefly, 1,726 serum samples from 454 patients were included in the current study. A comparison of patient characteristics and outcomes between the full SWOG S0226 cohort (694 patients) and the clinical validation sub-cohort did not display any significant differences between the two cohorts (Paoletti et al. Citation2021). Aliquots of ∼1 mL were shipped from the SWOG Specimen Repository to the Biovica laboratory in Uppsala, Sweden, for analysis. Serum TKa was assessed by the DiviTum®TKa assay, according to the manufacturer’s instructions for the DiviTum®TKa IVD version (Art. No. 95010, Biovica, Sweden). Serum samples with deidentified patient data from the different timepoints were included (if available). Laboratory staff was blinded to treatment assignment and patient data. All patients included in the study gave consent for use of their serum samples.
Measurement of TK activity
The DiviTum®TKa assay determines enzymatic activity of thymidine kinase. The principles of the DiviTum®TKa assay have previously been presented elsewhere (Bagegni et al. Citation2017). In short, DiviTum®TKa is a multi-step end-point assay involving a cascade of enzymatic reactions and one antibody binding reaction. In accordance with ISO 17511:2003, a new unit, DuA (DiviTum® unit of Activity) with added calibrators to better support quantification of enzyme activity was established for the new, FDA approved IVD version. The DiviTum®TKa assay analytical measuring interval is 100–2000 DuA and the assay has a coefficient of variation (CV) lower than 20%. All specimens in the clinical validation study were assayed in duplicates, and the final result was the mean of the two results. If a sample had a CV >20% between the duplicates, the sample was re-analyzed. Samples exceeding the upper limit were diluted and re-analyzed.
The RUO version of the assay (Art. No. 944) quantified serum TKa as ‘DiviTum® Units per Liter’ (Du/L) and in order to translate the measuring units from the previous versions to the new updated IVD version a conversion equation was established. The new DiviTum® unit of Activity (DuA) is related to the former unit (Du/L) as following: DuA = 134 + 0.53× Du/L.
Clinical cut-off
In the prior publication a predetermined cut-off point of 200 Du/L was chosen based on an approximate TKa median from prior studies for a hormone receptor–positive MBC population included in S0226 (Paoletti et al. Citation2021). Secondary analysis in that report explored the median serum TKa level and divided the TKa values into quartiles.
For the current study, a revised clinical cut-off value for the DiviTum®TKa assay was defined in accordance with FDA guidance and 510(k) requirements, setting the cut-off at the 95th percentile based on serum samples collected from a population of apparently healthy US postmenopausal women of different ethnicities. The cut-off in a healthy population was selected using the same threshold, 95th percentile, as a predicate device and FDA approved IVD (FDA 510(k) substantial equivalence determination decision summary of Cyfra 21-1).
Statistical analysis
Statistical analyses were performed by biostatisticians (Statisticon, Sweden), in collaboration with the original biostatistician from SWOG who analyzed S0226 (Paoletti et al. Citation2021). Landmark analysis were performed calculating time-dependent endpoints (progression within 30/60 days, PFS, OS) at baseline (BL) and each subsequent interval.
Actual time of sample collections deviated from the theoretical/planned time of sample collections due to real-world conditions in a clinical setting. To include all samples, the times of visits were divided into the following intervals: 0–20 (BL), 21–70, 71–98, 99–154 and >154 days. Baseline samples were defined as being (i) the first drawn sample from the patient and (ii) drawn before 21 days. Both criteria had to be fulfilled to be considered as baseline.
In some cases, the same patient had more than one assessment within the same time period. Some of the statistical analyses were based on the number of samples and some analyses on the number of patients. Analyses based on number of patients used the first assessment within a time-interval, if the patient had more than one assessment within the same interval. Analyses for the different time-periods were based on patients who had not progressed in any of the previous time periods.
Sensitivity for progression was calculated as the proportion of samples with a TKa ≥ 250 DuA from patients who progressed within 30 or 60 days following the date of the blood draw. Specificity for non-progression was calculated as the proportion of samples < 250 DuA among the patients who did not progress within 30 or 60 days following the date of the blood draw. Positive predictive value (PPV) was calculated as the proportion of patients with positive test results who actually progressed within 30 or 60 days following the date of the blood draw. Similarly, the negative predictive value (NPV) was calculated as the proportion of patients with negative test results who did not progress within 30 or 60 days following the date of the blood draw.
Concordance was calculated as the proportion of ‘correct’ prediction regarding progression or not, within 30 or 60 days following the date of the blood draw. For the survival analyses, PFS was defined as time from randomization to progression or death due to any cause and OS was defined as time from randomization to death from any cause. The survival analyses were done using Kaplan–Meier curves and Cox-regression analysis and the 95% confidence intervals for median PFS and OS were calculated. In these analyses each patient was only included once, even if they had multiple assessments within the same time period.
Results
Patient characteristics
The clinical characteristics of the 454 patients included in the validation study are listed in and distribution of patients and specimens for purposes of this study is provided in and .
Figure 2. REMARK diagram of specimen distribution. The total number of samples available for the statistical analysis was 1,546 from 454 patients.
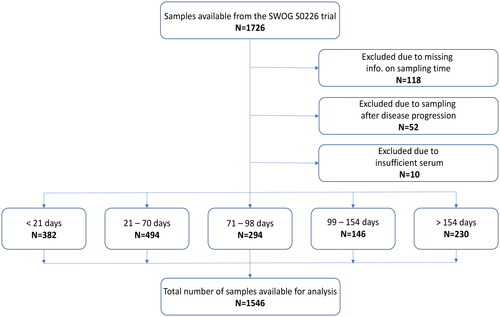
Table 1. Clinical characteristics of the patient cohort with available serum samples for TKa assessment (N = 454).
Patient samples
1,726 serum samples were available from 454 patients (). Number of samples per patient is shown in . Among the 454 patients, 55 had only one sample available. 147 patients (32.4%) had samples available at both baseline and the four subsequent time intervals. Of the 1,726 serum samples available, 180 samples were excluded: 118 due to missing data on sampling time, 52 as they were collected after the patient had progressed and 10 due to insufficient serum available (). In total, 1,546 samples were available for the statistical analysis, an average of 3.4 samples per patient (). The division of time intervals and exclusion of samples deviates from the previous report and impacts the number of samples available. Baseline samples in this analysis were from 382 patients, the previous report contained 432 patients (Paoletti et al. Citation2021).
Table 2. Number of blood specimens per patient.
Cut-off and TKa reference values
The cut-off for the DiviTum®TKa assay was defined in accordance with the FDA 510(k) guidance, setting the cut-off at the 95th percentile based on serum samples collected from a population of apparently healthy US postmenopausal women (n = 123) of different ethnicities (). The distribution of TKa values are shown in . The 95th percentile corresponded to 254 DuA. Hence, the clinical cut-off for low vs high TKa levels was set at <250 DuA. The median TKa value in the healthy population was 125 DuA.
Figure 3. TKa reference interval of 123 apparently healthy post-menopausal females (serum specimens) using Harrel–David non-parametric bootstrap method. The 95th percentile is depicted with corresponding 95% confidence intervals.
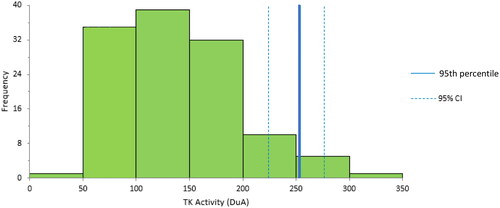
Table 3. TKa values for apparently healthy, US post-menopausal females.
In the previous report, 171 of 432 patients (40%) had TKa values above the cut-off of 200 Du/L at baseline. In this report, the corresponding number is 172 of 382 patients (45%) above the new cut-off of 250 DuA.
TK activity at baseline and subsequent treatment days
As shown in , median serum TKa decreased during treatment with anastrozole or anastrozole plus fulvestrant. At baseline median TKa was 227 DuA: by first follow-up at 21–70 days, it decreased to 160 DuA. During continued treatment the median TKa value decreased further to 142 DuA in the last time period, >154 days (p < 0.0001 for all intervals vs baseline). There were no differences in TKa levels regarding HER2-status irrespective of sample time point. Patients with bone vs. visceral metastatic sites had significantly lower TKa levels at baseline (189 vs. 231 DuA, p = 0.031), a difference that was not seen during treatment.
Table 4. Serum TKa assessment at baseline and during treatment for all patients.
Monitoring of disease progression during treatment
For this study to investigate the clinical validation of a cut-off of ≥ 250 DuA for the DiviTum®TKa assay, the primary objective was to demonstrate that TKa in serum, assessed at specific time intervals, was prognostic for disease progression within 30 and 60 days following blood draw during treatment in HR positive postmenopausal women with MBC. gives the time interval specific estimates for sensitivity, specificity, NPVs, PPVs, concordances and prevalences, as well as the sum of sensitivity and specificity for prediction of disease progression within 30 and 60 days following blood draw. The PPV for all four time points is 13.4% and 23.2%, respectively. A reason for the low PPV is to be found in the way the study evaluated disease progression (volume change) and nature of how TKa reflects disease status. TKa is reflecting the growth status of a tumour and elevated levels can later be picked up by imaging as tumour volume changes. Increased TKa levels predicts subsequent volume change much earlier than when confirmed by imaging/RECIST (Krishnamurthy et al. Citation2022).
Table 5. Time interval specific sensitivity, specificity, PPV, NPV, concordance and prevalence for disease progression during treatment within 30 and 60 days following blood draw.
Corresponding numbers for NPV are 96.7% and 93.5%. At each of the third, fourth and fifth sample time points the NPV for progression within 30 days is >98%. NPVs exceed 90% for all time points analyzed irrespective of progression within 30 or 60 days ().
shows that the DiviTum®TKa assay’s overall clinical sensitivity, specificity and the sum of the sensitivity and specificity for progression within 30 days were 52.4%, 80.7% and 133.0%, respectively. Prognosis of disease progression within 60 days following assessment had a similar outcome (also shown in ) with sensitivity, specificity and the sum of both being 48.7%, 81.9% and 130.6%, respectively. The 30- and 60-day forward looking time intervals are relevant from a clinical perspective as DiviTum®TKa may provide an early first indication of imminent disease progression. With the intended use of the device also comes a need for a high specificity as an indicator of a low rate of cell proliferation and, thereby, no impending disease progression – i.e., the disease seems stable. The clinical validation plan set a target of 125% for the sum of sensitivity + specificity, this represents acceptable clinical performance of the device and the intended use of the device was validated. Furthermore, for samples that had a TKa value above vs. below the 250 DuA reference value, the positive and negative likelihood ratios for disease progression within the next 30 days were 2.71 vs. 0.59 (for progression within 60 days, 2.70 vs. 0.63).
Table 6. Overall clinical sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and concordance with confidence intervals for disease progression within 30 and 60 days following TKa assessment during treatment.
Progression free and overall survival
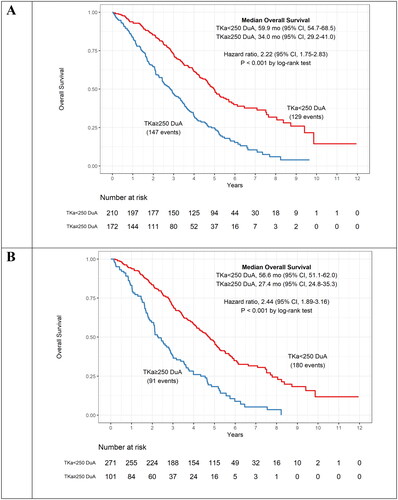
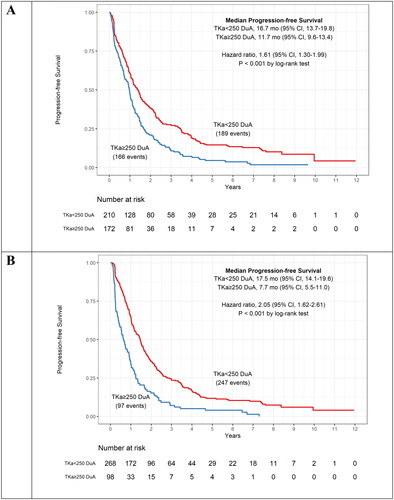
To further evaluate DiviTum®TKa as a prognostic assay, the association between TKa and both PFS and OS was investigated (). Patients with TKa levels below the cut-off experienced significantly longer survival both with respect to PFS and OS, demonstrated for all time intervals. At baseline (<21 days), patients with TKa values above the clinical cut-off (≥250 DuA) had a significantly shorter PFS compared to those with a value below cut-off [median PFS = 11.7 and 16.7 months, respectively; HR: 1.61, 95% CI: 1.30–1.99, p < 0.001] (). At baseline a similar result was observed for OS. Patients with serum TKa values above the clinical cut-off had a significantly shorter OS compared to those with a value below cut-off [Median OS = 34.0 and 59.9 months, respectively; HR: 2.22, 95% CI: 1.75–2.83, p < 0.001] (). The TKa values at later time points demonstrated even more striking differences ( and ). At first follow-up (21–70 days), median PFS for those with TKa levels ≥250 compared to <250 DuA were 7.7 vs. 17.5 months (p < 0.001) () and OS was 27.4 vs. 56.6 months (p < 0.001) (). In the prior publication using a RUO version of the assay, the same results at baseline for PFS and OS was 11.2 vs 17.3 months and 30 vs 58 months, respectively, demonstrating highly consistent and concordant results (Paoletti et al. Citation2021). Results for PFS and OS at subsequent sampling time points are also in agreement.
Figure 4. Kaplan–Meier curves for progression free survival (PFS) for patients below and above the assay cut-off of ≥ 250 DuA. The curves are shown for the serum TKa assessment at baseline (A) and 21–70 days (B) and include median PFS and hazard ratio. TKa, thymidine kinase activity; CI, confidence interval.
Discussion
In this prospective-retrospective, blinded clinical validation study of the DiviTum®TKa assay, a revised cut-off, derived in order to meet requirements for FDA 510(k) clearence, serum TKa was validated as a monitoring biomarker for disease progression in HR positive postmenopausal women with MBC receiving first line ET monotherapy or combination therapy. Submission to the FDA required a new cut-off (250 DuA) for TKa to meet regulatory guidance and a new measuring unit, DuA, to correspond with the ISO 17511:2003 standard. The new cut-off and unit are different but the clinical results and outcome for PFS and OS with the new FDA approved version of the assay is highly consistent with the old RUO version used for analyzing the same samples in S0226 (Paoletti et al. Citation2021).
The results underline the capacity of the DiviTum®TKa assay to predict non-progression of the disease and hence monitor treatment in a patient with HR positive MBC. Following treatment with anastrozole or anastrozole plus fulvestrant a marked median decrease in TKa was observed during the study period for up to 7 months. During cycles 2, 3, 4 and 7, a DiviTum®TKa value <250 DuA is associated with a very low likelihood of disease progression within the next 30 or 60 days. Furthermore, a TKa value above the assay cut-off of ≥ 250 DuA is associated with significantly worse prognosis with respect to both PFS and OS. The results obtained from the current validation study are consistent with ten other studies that have been conducted with the earlier RUO version of the DiviTum® assay in patients with MBC (Bjöhle et al. Citation2013, Bagegni et al. Citation2017, Bonechi et al. Citation2018, McCartney et al. Citation2019, McCartney et al. Citation2020, Larsson et al. Citation2020, Cabel et al. Citation2020, Paoletti et al. Citation2021, Malorni et al. Citation2022, Krishnamurthy et al. Citation2022). These studies have likewise shown that with the assessment of a simple blood sample, DiviTum®TKa can monitor and predict disease progression, PFS and OS in MBC patients receiving ET with or without CDK4/6 inhibitors.
When selecting the cut-off for the DiviTum®TKa assay, emphasis was placed on achieving a high specificity and NPV. A recent study in HR positive postmenopausal women with MBC has shown that the median serum TKa value during treatment with ET was close to the clinical cut-off value of ≥ 250 DuA (Larsson et al. Citation2020). A high clinical specificity, i.e., high assurance that a low TKa value (<250 DuA) is associated with no disease progression, is considered important when monitoring patients with MBC during therapy.
Monitoring disease activity in patients with MBC is an important clinical follow up to detect disease progression early and thereby avoid unnecessary toxicity of ineffective and often costly treatments (Carlson et al. Citation2012). As a corollary, in patients with persistently low circulating TKa values, it offers the opportunity to adjust and limit use of more burdensome monitoring strategies, such as serial imaging, and provides certainty to patients of not experiencing early tumour progression. With a cut-off value of ≥250 DuA, the DiviTum®TKa assay was able to predict disease progression based on the observed change in serum TKa within 30 days following the blood draw with a sensitivity and specificity of 52% and 81%, respectively, providing a PPV for progression of 13.4% and an NPV of 96.7%. Similarly, the PPV and NPV for progression at 60 days were 23.2% and 93.5%. Thus, a patient starting new single agent anastrozole or anastrozole plus fulvestrant with low serum TKa can feel confident that it is unlikely her disease will progress in the next 1–2 months. By contrast, a patient with elevated TKa at baseline has a significantly higher chance of experiencing progression in the successive 1–2 months, and might be better treated with ET plus an ancillary targeted agent, such as a CDK4/6, MTOR or PIK3CA inhibitor, in spite of the additional toxicity.
These data are similar to other studies of TKa in HR positive, HER2 negative MBC. For example, assessment of TKa provided an indication of lack of benefit to palbociclib combined with ET (Bagegni et al. Citation2017, McCartney et al. Citation2020, Cabel et al. Citation2020, Malorni et al. Citation2022, Krishnamurthy et al. Citation2022). Furthermore, rising TKa appears to be a marker of emerging resistance to palbociclib, for which a treatment change might be indicated (Malorni et al. Citation2022, Krishnamurthy et al. Citation2022).
A strength of this clinical validation study is that samples and clinical outcome data originated from a large, well conducted and controlled SWOG trial (Mehta et al. Citation2012, Citation2019). Furthermore, it is shown that the patient cohort used for the clinical validation of the DiviTum®TKa assay is similar with respect to clinical characteristics and outcome as the overall patient population (Paoletti et al. Citation2021). A possible weakness is that the monthly fulvestrant dosing was lower than the currently recommended dose of 500 mg i.m. (Gradishar et al. Citation2017), no samples were available beyond 196 days of treatment, and although ½ of the patients received combination ET, no patients received any ancillary treatments, such as a CDK4/6, mTOR or PIK3CA inhibitors.
In conclusion, serum TKa, which tracks cell proliferation rates, assessed at baseline and serially during treatment, was significantly associated with high NPV and outcomes, including both PFS and OS, in patients with HR positive MBC receiving first line ET. We hypothesize that low serum TKa levels at baseline might identify patients who can do well for a long time with ET monotherapy. Low serum TKa levels provide such a high NPV for early progression that such patients might forego additional therapy added to single agent ET. Combined with a possible reduction of inconvenient and costly serial imaging, such an approach should improve quality of life for these patients, without sacrificing OS benefits from ancillary treatments. Ongoing trials will further document the clinical value of the DiviTum®TKa assay as a tool for early identification of treatment resistance of ET with or without CDK4/6 inhibitors and prediction of long-term benefits for patients with MBC.
Author contributions
Conception and design: M.B., C.P., W.E.B., D.F.H., J.M.R.; Collection and assembly of data: M.B., A.N., A.W., C.P., W.E.B., D.F.H., J.M.R.; Data analysis and interpretation: M.B., A.W., C.P., W.E.B., D.F.H., J.M.R.; Manuscript writing: M.B., A.W., C.P., W.E.B., D.F.H., J.M.R.; Final approval of manuscript: All authors.
Acknowledgment
We thank all patients participating in the study for their contribution.
Disclosure statement
MB, AN and AW are employees at Biovica and holds stock/stock options in the company. Unrelated to this study, CP had received travel reimbursement and research funding from Menarini Silicon Biosystems, Inc.; research funding from AstraZeneca and Pfizer, outside the submitted work. CP is currently working at EISAI, Inc. Nutley, NJ, USA, but this publication is unrelated to her employment. Institution of WB receives research funding from The National Cancer Institute, AstraZeneca and Merck. Unrelated to this study, EFC reports personal income related to consulting or advisory board activities from AstraZeneca, Ayala Pharmaceuticals, Athenex Oncology, bioTheranostics and Immunomedics. Unrelated to this study, RSM reports personal income related to consulting or advisory board activities from Novartis, bioTheranostics and Puma Biotechnology. JRG reports: Roche/Genentech: Steering committee Member, Data Safety Monitoring Committee Member, Astra Zeneca: Advisory Board, Data Safety monitoring committee, Puma: Advisory Board, Novartis: Data Safety Monitoring Committee Member, SeaGen: Consultant, Immunomedics: Data safety monitoring committee member. Unrelated to this work, GH have received compensation for consulting from Novartis and AstraZeneca. PS reports consulting fee and/or honoraria from Pfizer, Merck, Gilead, Seattle Genetics, Novartis, AstraZeneca, GSK; research support to the institution from Novartis, Bristol-Meyers Squibb, Merck, Gilead and royalties from UpToDate. AKG is a co-founder of Sinochips Diagnostics, serves as a scientific advisory board member to Biovica, Clara Biotech, and Sinochips Diagnostics, and receives research funding from Predicine and VITRAC Therapeutics. Unrelated to this study, AMT reports his spouse working for Eli Lilly. Specific to this study, DFH reports personal income related to consulting or advisory board activities from Biovica, the manufacturer of DiviTum®TKa and his institution receives support from AstraZeneca, the manufacturer of anastrozole and fulvestrant, and from Pfizer, the manufacturer of Palbociclib, to support his research. DFH reports support unrelated to this study but provided to his institution in the last 24 months during conduct and analysis of this study from Menarini Silicon Biosystems and Merrimack Pharmaceuticals. Unrelated to this study DFH reports personal income related to consulting or advisory board activities from Cellworks, Cepheid, EPIC Sciences, Freenome, Guardant, L-Nutra, Macrogenics, Oncocyte, Predictus BioSciences, Tempus, Turnstone Biologics, and Xilis. The University of Michigan holds a patent for which DFH is the named investigator and which is licenced to Menarini Silicon Biosystems from whom UM and DFH receive annual royalties. DFH reports personally held stock options from InBiomotion. No conflicts of interest related to this article to report from the other authors.
Data availability statement
Data is available on reasonable request subject to application to SWOG, NIH and Biovica International.
Additional information
Funding
References
- Bagegni, N., et al., 2017. Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast cancer research, 19 (1), 123.
- Bjöhle, J., et al., 2013. Serum thymidine kinase activity compared with CA 15-3 in locally advanced and metastatic breast cancer within a randomized trial. Breast cancer research and treatment, 139 (3), 751–758.
- Bonechi, M., et al., 2018. Plasma thymidine kinase-1 activity predicts outcome in patients with hormone receptor positive and HER2 negative metastatic breast cancer treated with endocrine therapy. Oncotarget, 9 (23), 16389–16399.
- Burstein, H.J., 2020. Systemic therapy for estrogen receptor-positive HER2-negative breast cancer. The new England journal of medicine, 383 (26), 2557–2570.
- Cabel, L., et al., 2020. Plasma thymidine kinase 1 activity and outcome of ER + HER2– metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast cancer research, 22 (1), 98.
- Carlson, R.W., et al., 2012. Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. Journal of the national comprehensive cancer network, 10 (7), 821–829.
- Gradishar, W.J., et al., 2017. NCCN guidelines insights: breast cancer, Version 1.2017. Journal of the national comprehensive cancer network, 15 (4), 433–451.
- Hayes, D.F., 2021. Defining clinical utility of tumor biomarker tests: a clinician’s viewpoint. Journal of clinical oncology, 39 (3), 238–248.
- Krishnamurthy, J., et al., 2022. A phase II trial of an alternative schedule of palbociclib and embedded serum TK1 analysis. NPJ breast cancer, 8 (1), 35.
- Larsson, A.M., et al., 2020. Serial evaluation of serum thymidine kinase activity is prognostic in women with newly diagnosed metastatic breast cancer. Scientific reports, 10 (1), 4484.
- Malorni, L., et al., 2022. Serum thymidine kinase activity in patients with hormone receptor-positive and HER2-negative metastatic breast cancer treated with palbociclib and fulvestrant. European journal of cancer (Oxford, England: 1990), 164, 39–51.
- McCartney, A., et al., 2019. Prognostic role of serum thymidine kinase 1 activity in patients with hormone receptor-positive metastatic breast cancer: analysis of the randomised phase III Evaluation of Faslodex versus Exemestane Clinical Trial (EFECT). European journal of cancer (Oxford, England: 1990), 114, 55–66.
- McCartney, A., et al., 2020. Plasma thymidine kinase activity as a biomarker in patients with luminal metastatic breast cancer treated with palbociclib within the TREnd Trial. Clinical cancer research, 26 (9), 2131–2139.
- Mehta, R.S., et al., 2012. Combination anastrozole and fulvestrant in metastatic breast cancer. The new England journal of medicine, 367 (5), 435–444.
- Mehta, R.S., et al., 2019. Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. The new England journal of medicine, 380 (13), 1226–1234.
- Merker, J.D., et al., 2018. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. Journal of clinical oncology, 36 (16), 1631–1641.
- Paoletti, C., et al., 2021. Evaluating serum thymidine kinase 1 in hormone receptor positive metastatic breast cancer patients receiving first line endocrine therapy in the SWOG S0226 trial. Clinical cancer research, 27 (22), 6115–6123.
- Robertson, J.F., et al., 1990. Thymidine kinase in breast cancer. British journal of cancer, 62 (4), 663–667.
- Therasse, P., et al., 2000. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the national cancer institute, 92 (3), 205–216.
- Welin, M., et al ., 2004. Structures of thymidine kinase 1 of human and mycoplasmic origin. Proceedings of the national academy of sciences, USA, 101: 17970–17975.