Abstract
Background
The conventional markers for hepatocellular carcinoma (HCC), α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), have several limitations; both have low sensitivity in patients with early-stage HCC; low sensitivity for AFP with HCC after eliminating hepatitis C virus (HCV); low specificity for DCP in patients with non-viral HCC, which is increasing worldwide; low specificity for AFP in patients with liver injury; and low specificity for DCP in patients treated with warfarin. To overcome these issues, the identification of novel biomarkers is an unmet need.
Objective
This study aimed to assess the usefulness of serum protein kinase C delta (PKCδ) for detecting these HCCs.
Methods
PKCδ levels were measured using a sandwich enzyme-linked immunosorbent assay in 363 chronic liver disease (CLD) patients with and without HCC.
Results
In both viral and non-viral CLD, PKCδ can detect HCCs with high sensitivity and specificity, particularly in the very early stages. Notably, the value and sensitivity of PKCδ were not modified by HCV elimination status. Liver injury and warfarin administration, which are known to cause false-positive results for conventional markers, did not modify PKCδ levels.
Conclusions
PKCδ is an enhanced biomarker for the diagnosis of HCC that compensates for the drawbacks of conventional markers.
Introduction
Liver cancer and liver failure are the main causes of death among chronic liver disease (CLD) patients. Specifically, hepatocellular carcinoma (HCC) is the sixth most common organ cancer and the fourth leading cause of death worldwide (Bray et al. Citation2018). The mortality rate of HCC is high relative to its morbidity rate because many cases are detected during the advanced stages of the disease (Park et al. Citation2015). HCC usually develops from CLD caused by various aetiologies, such as hepatitis B and C virus (HBV and HCV, respectively) infection and metabolic abnormalities (e.g., excessive hepatic fat accumulation and heavy alcohol consumption). Recently, the incidence of non-viral, especially non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH)-related HCC has been gradually increasing worldwide (Younossi et al. Citation2015, Estes et al. Citation2018, Tateishi et al. Citation2019). The clinical concern for HCC in non-viral CLD patients is highlighted by the fact that one in three people worldwide are obese, and its prevalence will continue to increase (Diehl and Day Citation2017, Singal et al. Citation2020). Meanwhile, with the advent of direct-acting antiviral (DAA; i.e., virus elimination) therapy, sustained virologic response (SVR; i.e., virus elimination) can be achieved in almost all HCV-infected patients, regardless of viral genotype, presence of cirrhosis (including decompensation), and renal dysfunction (Atsukawa et al. Citation2019, Atsukawa et al. Citation2020, Nozaki et al. Citation2020). SVR achievement improves liver function and substantially reduces carcinogenic risk; however, it does not guarantee that the risk of developing HCC is eliminated (Pinero et al. Citation2019). In fact, the occurrence of post-SVR HCC has become a growing concern and has received much attention (Osaki et al. Citation2012, Asahina et al. Citation2013, Toyoda H et al. Citation2022a).
Surveillance strategies for HCC, especially in HBV/HCV-related CLD patients, have been advocated by the American Association for the Study of Liver diseases (AASLD), the European Association for the Study of the Liver (EASL), the Japanese Society of Hepatology, and the Asian Pacific Association for the Study of the Liver. It is recommended that routine radiological examinations using ultrasonography, dynamic computed tomography (CT), and/or gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI) be performed every 3–6 months (Omata et al. Citation2017, EASL Citation2018, Heimbach et al. Citation2018, Kokudo et al. Citation2019). Moreover, not only patients with persistent viral infection but also those with viral elimination or suppression are at high risk for carcinogenesis and need long-term follow-up for HCC surveillance. However, while surveillance strategies for viral HCC have been well addressed in these guidelines, there is no consensus on those for non-viral HCC (e.g., the timing of surveillance and its cost-effectiveness). Therefore, HCC in non-viral CLD patients is often found in the advanced stages.
Early detection of HCC is most important in improving the patient prognosis (Choi et al, Citation2019). Despite the advances in diagnostic imaging, detection of early-stage HCC remains a major challenge because small, well-differentiated HCC usually lacks typical imaging findings (early arterial vascular enhancement and subsequent contrast washout) (Simmons et al. Citation2017). Under such conditions, the presence of early-stage or sporadic cancer can only be suspected by elevated serum/plasma levels of tumour markers, which are conveniently and repeatedly measured. In clinical practice, conventional tumour markers for HCC are α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), which are complementary to each other (Liebman et al. Citation1984, Taketa Citation1990). However, these have several drawbacks: 1) the positivity rate is low (approximately 30%) for early-stage HCC but increases as the disease stage progresses (Toyoda et al. Citation2015, Berhane et al. Citation2016); 2) serum DCP levels are high but less specific to non-viral HCC (Marrero et al. Citation2009, Utsunomiya et al. Citation2015, Hashimoto et al. Citation2017); 3) HCV-related HCC patients who achieve SVR are likely to have lower AFP levels than those with persistent viremia, making it more difficult to detect HCC (Minami et al. Citation2015, Yeh et al. Citation2021, Hsu et al. Citation2022); and 4) serum AFP and DCP levels are modified by liver injury and warfarin treatment, respectively. To overcome these issues, the identification of novel biomarkers is an unmet need.
Protein kinase C delta (PKCδ) is an intracellular serine/threonine kinase expressed in various cancers and is involved in cancer cell survival and invasion (Tsai et al. Citation2000, Wu et al. Citation2007, Yoon et al. Citation2010). PKCδ is extracellularly secreted only in HCC cells (but not in normal hepatocytes or gastrointestinal cancers) via an autophagy mechanism and acts like a growth factor in vitro, contributing to the progression of HCC (Yamada et al. Citation2021, Yamada et al. Citation2022a, Yamada et al. Citation2022b). Furthermore, serum PKCδ has recently become a novel biomarker for the diagnosis of HCC that complements the conventional markers, AFP and DCP, and a useful tool for detecting very early-stage or AFP/DCP double-negative HCC patients (Oikawa et al. Citation2023).This study aimed to investigate the impact of viral or non-viral aetiology on the diagnostic performance of serum PKCδ in detecting HCC and whether serum PKCδ can complement conventional markers, particularly in HCV-infected patients who achieved SVR and in patients who had liver injury or received warfarin treatment.
Patients and methods
Study design
Serum PKCδ, AFP, and DCP levels were simultaneously measured in a total of 363 CLD patients with and without HCC, who included the previous study population (n = 313) (Oikawa et al. Citation2023). These 363 patients were divided into the viral and non-viral groups to investigate the characteristics of PKCδ as an HCC biomarker by comparing with AFP and DCP. The viral group included patients who were positive for hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and/or anti-HCV antibody (HCV-Ab) and had no other liver diseases, such as those described below. Patients with HCV-related HCC were divided into those with SVR (virus elimination) and non-SVR (persistent viremia) to evaluate the influence of viremia on serum PKCδ levels. Meanwhile, the non-viral group included patients without HBsAg, anti-HBc, and HCV-Ab who were diagnosed as alcohol-related liver diseases, metabolic liver diseases (e.g., NAFLD/NASH), autoimmune liver diseases (e.g., autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis), congestive hepatopathy (e.g., Budd-Chiari syndrome), and cryptogenic liver disease. These non-viral CLDs were diagnosed using medical interviews, biochemistry, imaging (ultrasonography, dynamic CT, and/or MRI), and/or histological analyses by biopsy (Heidelbaugh and Sherbondy Citation2006). HCC, including solitary small-sized HCC (≤ 20 mm in diameter), was diagnosed based on contrast-enhanced imaging findings via perflubutane (Sonazoid®, Daiichi Sankyo, Tokyo, Japan) microbubble-enhanced ultrasonography, dynamic iodinated contrast medium-enhanced CT, and/or Gd-EOB-DTPA-enhanced MRI, and/or tumour biopsy according to the AASLD guidelines (Bruix and Sherman al. Citation2011, Marrero et al. Citation2018). HCC stages were determined according to the 8th edition of the TNM classification system released by the American Joint Committee on Cancer/Union for International Cancer Control (UICC) (Brierley et al. Citation2017) and the Barcelona Clinic Liver Cancer (BCLC) staging systems (Reig et al. Citation2022). The diagnosis of very early-stage HCC [isolated small HCC (≤ 20 mm in diameter)] corresponding to BCLC stage 0 was made according to the BCLC staging and treatment strategy in 2022 published by the EASL guidelines. Serum samples of HCC patients were collected before treatment (surgical resection, ablation, transcatheter arterial chemoembolization, and/or systemic chemotherapy). Exclusion criteria were as follows: (1) double cancers (HCC with another extrahepatic cancer); (2) obstructive jaundice and hepatic failure; (3) pregnancy; and (4) treatment with anticoagulants or antibiotics.
Apart from the 363 CLD patients described above, 6 non-HCC patients who had been receiving warfarin were also enrolled in this study to evaluate its influence on serum PKCδ levels.
This study was conducted in accordance with the Declaration of Helsinki and ethical guidelines issued by the administrative department and was approved by the Local Ethics Committee of The Jikei University School of Medicine (approval No. 29-135 [8751]). All participants were over 20 years old and were recruited at The Jikei University School of Medicine. Written informed consent was obtained from all participants.
Measurements of serum PKCδ, AFP, and DCP
Serum PKCδ levels were measured using a sandwich enzyme-linked immunosorbent assay kit, according to the manufacturer’s instructions (MyBioSource, San Diego, USA). Serum AFP and DCP levels were analysed using chemiluminescence enzyme immunoassay (Tosoh bioscience, California, USA). The optimal cut-off value was determined using Youden J statistics for PKCδ and set over 57.7 ng/mL, as described elsewhere (Oikawa et al. Citation2023). The cut-off values for AFP and DCP were over 20.0 ng/mL and 40.0 mAU/mL, respectively, which were considered positive in this study.
Statistical analysis
Fisher’s exact test, chi-squared test, and Mann–Whitney U test were used to compare two groups, as appropriate. Cochran–Armitage trend test was used to evaluate the association between a variable with two categories and a variable with multiple categories. Univariate and multiple logistic regression analyses were used to identify significantly independent factors related to HCC, where in AFP and DCP values were log-transformed [represented as log(AFP) and log(DCP), respectively] with reference to the “GALAD” model (Johnson et al. Citation2014). The diagnostic performance of serum PKCδ for HCC was evaluated in terms of sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), and the area under the receiver operating characteristic curve (AUC). All P values were two-tailed, and P < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 23.0 (IBM Japan, Tokyo, Japan) and R version 4.3.1 (The R Foundation for Statistical Computing, http://www.R-project.org).
Results
Patient characteristics
The 363 CLD patients were divided into two groups (). The viral group (n = 145) included 93 HCC and 52 non-HCC patients, while the non-viral group (n = 218) included 133 HCC and 85 non-HCC patients (P = 0.581). The characteristics of each group are shown in , with no significant differences.
Figure 1. Flow diagram of patients included in this study. CLD, chronic liver disease; HCC, hepatocellular carcinoma.
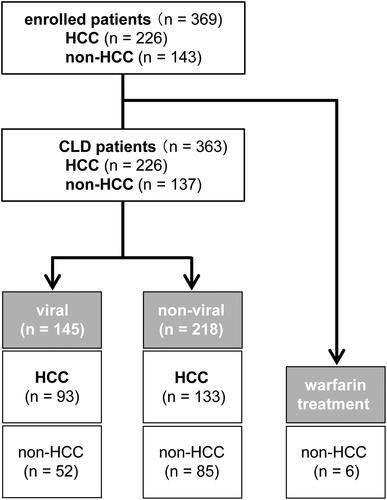
Table 1. Patient characteristics in the viral and non-viral groups.
Three Biomarkers in HCC and non-HCC patients in the viral and non-viral groups
Serum PKCδ levels did not significantly differ between the viral and non-viral groups (). Serum PKCδ levels in HCC patients were significantly higher than those in non-HCC patients in both the viral (median, 50.7 vs. 37.4 ng/mL, respectively; P < 0.001) and non-viral (median, 52.9 vs. 39.9 ng/mL, respectively; P < 0.001) groups (). Meanwhile, serum PKCδ levels in either HCC or non-HCC patients did not significantly differ between the viral and non-viral groups (P = 0.317 and 0.269, respectively; and Supplementary Figure 1).
Figure 2. A. Comparison of serum PKCδ, AFP, and DCP levels in viral CLD (upper) and non-viral CLD (lower) patients with or without HCC. Serum PKCδ was significantly higher in HCC patients in both the viral and the non-viral groups. (HCC vs. non-HCC; P < 0.001 for both). B. Comparison of serum PKCδ, AFP, and DCP levels in HCC patients between the viral and non-viral groups. PKCδ and AFP levels in HCC patients were not significantly different between the viral and non-viral groups, but DCP levels in non-viral HCC were higher than those in viral HCC. The bold line through the middle of each plot represents the median.
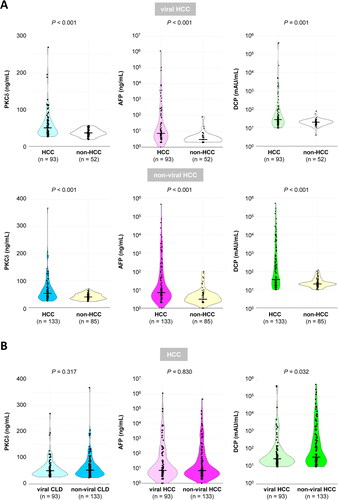
Similarly, serum levels of conventional markers (AFP and DCP) in HCC patients were significantly higher than those in non-HCC patients in both groups (P < 0.001 for AFP and P = 0.001 for DCP in the viral group / P < 0.001 for AFP and DCP in the non-viral group, respectively; ). Serum AFP and DCP levels in non-HCC patients did not significantly differ between the viral and non-viral groups (P = 0.565 and 0.476, respectively; Supplementary Figure 1). However, only serum DCP levels in non-viral HCC patients were significantly higher than those in viral HCC patients (median, 35.0 vs. 28.0 mAU/mL, respectively; P = 0.032; ), whereas serum AFP levels were similar between viral and non-viral HCC patients (P = 0.830; ). Thus, only serum DCP levels showed a significant difference between viral and non-viral HCC patients. This suggests that such an aetiology-dependent marker may be disadvantageous in screening for HCC (i.e., DCP for viral CLD patients).
Serum PKCδ as an independent factor associated with HCC
Age and gender have been identified as independent factors associated with HCC, enhancing the diagnostic performance of AFP and DCP (Johnson et al. Citation2014). On univariate analysis, age, gender (only for non-viral HCC), log(AFP), log(DCP), and PKCδ were significantly associated with HCC. Notably, on multiple logistic regression analysis, PKCδ remained significantly independent, irrespective of viral or non-viral aetiology ().
Table 2. Factors [age, gender, log(AFP), log(DCP), and PKCδ] associated with HCC in multiple logistic regression analyses.
Diagnostic performance of three Biomarkers for HCC in the viral and non-viral groups
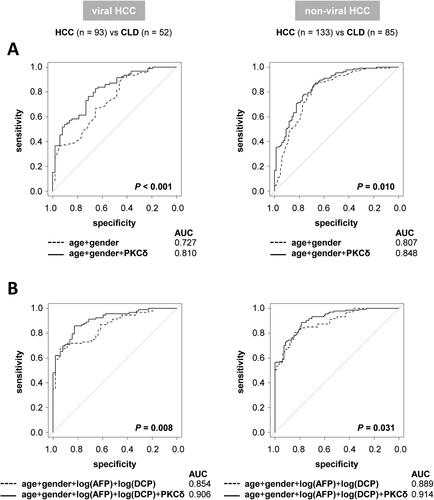
The findings presented in led us to evaluate whether adding PKCδ could enhance the diagnostic performances for HCC on the basis of age, gender, log(AFP), and log(DCP). The addition of PKCδ significantly improved the AUC values of the age/gender- and age/gender/log(AFP)/log(DCP)-based models in both the viral (P < 0.001 and = 0.008, respectively) and non-viral (P = 0.010 and 0.031, respectively) groups ().
Figure 3. Diagnostic performances for HCC. ROC curves for comparisons with the addition of serum PKCδ based on (A) age and gender or (B) age, gender, log(AFP), and log(DCP). The addition of serum PKCδ significantly improved the diagnostic performances for HCC in both the viral and non-viral groups.
shows the number (rate) of PKCδ-, AFP-, and DCP-positive HCC patients in the viral and non-viral groups. Among 93 HCC patients in the viral group, 33 (35.5%), 29 (31.2%), and 34 (36.6%) patients were positive for PKCδ, AFP, and DCP, respectively. Among 133 HCC patients in the non-viral group, 59 (44.4%), 42 (31.6%), and 65 (48.9%) patients were positive for PKCδ, AFP, and DCP, respectively.
Figure 4. Positive patterns of tumour markers in patients with viral HCC (left) and non-viral HCC (right) are shown. The cut-off values for each marker were 57.7 ng/mL for PKCδ, 20.0 ng/mL for AFP, and 40.0 mAU/mL for DCP.
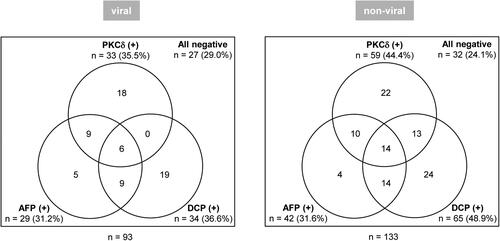
shows the diagnostic performances of PKCδ, AFP, and DCP for detecting HCC with respect to viral or non-viral aetiology. In the viral group, the diagnostic performances were similar among these single markers. However, PKCδ yielded the highest specificity, PPV, NPV, and accuracy, as well as AUC, although not significantly different from other markers. In the non-viral group, PKCδ showed similar results, AFP had the lowest sensitivity (31.6%), and DCP had the lowest specificity (84.7%). Of note, the AUC of PKCδ was highest and significantly higher than that of AFP (P = 0.042). The use of double or triple markers (combination of PKCδ, AFP, and/or DCP) enhanced sensitivity, NPV, and accuracy to h igher or the highest levels in both the viral and non-viral groups. The combination of PKCδ and DCP yielded better diagnostic performance than the combination of AFP and DCP in both the viral and non-viral groups (P = 0.002 and 0.001, respectively).
Table 3. Diagnostic performance of PKCδ, AFP, and DCP for viral or non-viral HCC.
Next, the diagnostic performances for detecting stage 0 HCC were compared between the three markers in the viral and non-viral groups (Supplementary Table 1). The levels of any markers did not significantly differ between the viral and non-viral HCC patients (Supplementary Figure 2). Among single markers, DCP (4.3%) in the viral group and AFP (10.3%) in the non-viral group had extremely low sensitivity, and DCP (84.7%) in the non-viral group had low specificity compared with other markers (Supplementary Figure 3A and Supplementary Table 1). Notably, PKCδ yielded the highest values for all diagnostic parameters in both the viral and non-viral groups. Specifically, PKCδ showed evidently higher sensitivity than conventional markers. Furthermore, the AUC of PKCδ for stage 0 HCC was significantly greater than those of AFP and DCP in both the viral (P = 0.002 and < 0.001, respectively) and non-viral (P < 0.001 for both) groups.
These results suggest that the diagnostic performances of the three biomarkers for HCC are comparable in both groups and that PKCδ is much more useful than conventional markers for detecting very early-stage HCC, irrespective of viral or non-viral aetiology.
Three Biomarkers for detecting HCV-related HCC with viral clearance status
We limited the subjects to 90 HCV-related CLD patients with and without HCC (n = 60 and 30, respectively). All marker levels were significantly higher in HCC patients than in non-HCC patients (). Since all non-HCC patients had achieved SVR, these levels were compared between SVR patients with and without HCC (SVR-HCC, n = 34; and non-HCC, n = 30, upper part, Supplementary Figure 4A). Intriguingly, PKCδ and AFP levels were significantly higher in SVR-HCC patients (P = 0.003 and 0.006, respectively), whereas DCP levels did not significantly differ between groups (P = 0.238). However, all markers were significantly higher in the non-SVR-HCC patients (n = 26) than in the non-HCC (SVR) patients (P < 0.001, < 0.001, and = 0.012 for PKCδ, AFP, and DCP, respectively; lower part, Supplementary Figure 4A). These findings suggested that these marker levels in HCC patients are modified according to the absence and presence of viremia, and especially DCP may be disadvantageous to HCC screening in SVR patients.
Figure 5. A. Comparison of serum PKCδ, AFP, and DCP levels between HCV-related CLD patients with and without HCC. All markers were significantly higher in HCC patients than in non-HCC patients. (P < 0.001, < 0.001, and = 0.040, for PKCδ, AFP, and DCP, respectively). B. Comparison of serum PKCδ, AFP, and DCP levels in HCV-related HCC patients with (SVR) or without (non-SVR) virus elimination. The median levels of PKCδ, AFP, and DCP in SVR-HCC and non-SVR-HCC were 48.9 vs. 58.0 ng/mL (P = 0.256), 6.6 vs. 29.5 ng/mL (P = 0.024), and 24.5 vs. 38.0 mAU/mL (P = 0.213), respectively. Thus, only AFP levels were significantly lower in SVR-HCC than in non-SVR-HCC. The bold line through the middle of each plot represents the median.
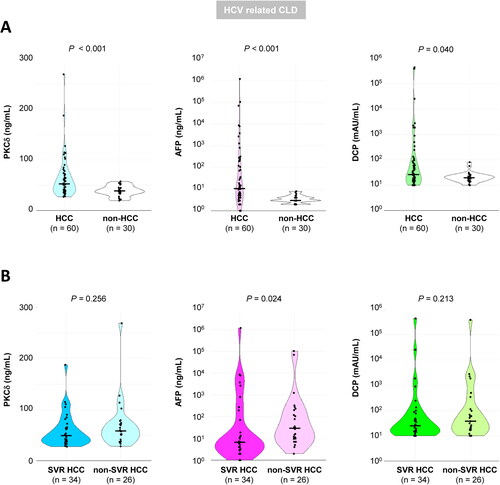
Next, we focused on the 60 HCV-related HCC patients (with SVR, n = 34; and non-SVR, n = 26). The median levels of all markers were lower in SVR-HCC patients than in non-SVR-HCC patients; however, only AFP levels were significantly lower in SVR-HCC patients (P = 0.024, ). Similarly, only the AFP positive rate was significantly lower in SVR-HCC patients than in non-SVR-HCC patients (P = 0.037, Supplementary Figure 4B). Thus, AFP levels or positivity rates were modified by elimination or persistent presence of HCV; therefore, the cut-off value of AFP should be lowered or reset in SVR patients.
Of note, PKCδ yielded the highest sensitivity, specificity, PPV, NPV, and accuracy among the three single markers in both SVR and non-SVR patients (). Moreover, the combination of PKCδ and DCP yielded greater AUC than the combination of AFP and DCP in SVR patients, but not in non-SVR patients (P = 0.048 and 0.173, respectively). The combination of all markers provided the greatest AUC in both SVR and non-SVR patients. These results suggest that PKCδ as a single marker or any combination including PKCδ is useful for detecting HCV-related HCC regardless of viral clearance status.
Table 4. Diagnostic performances of PKCδ, AFP, and DCP for post-SVR and non-SVR HCC.
The association between the tumour marker levels and viremia was not evaluated because > 95% of patients with HBV-associated HCC were under viral control (undetectable HBV) by anti-viral treatment.
Modification by liver injury and warfarin administration
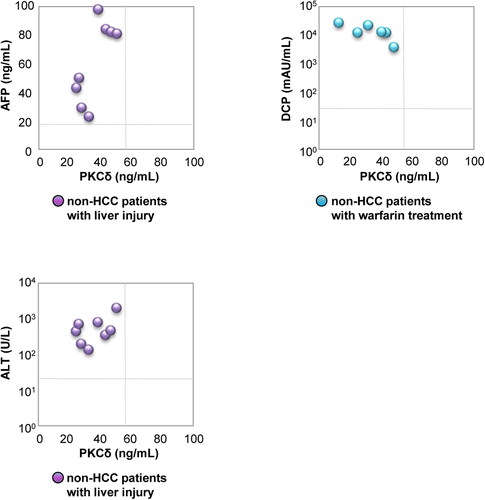
We analysed whether serum PKCδ levels were modified by liver injury or warfarin administration, which are well known to cause false positives for conventional markers, among non-HCC patients. Serum PKCδ were negative (24.5 – 50.6 ng/mL) in all 8 non-HCC patients with liver injury who had elevated serum transaminase (> 100 U/L for AST and ALT; range, 188 – 1603 and 135 – 2002 U/L, respectively) and AFP levels (> 20 ng/mL; range, 29 – 98 ng/mL) (). Meanwhile, all 6 non-HCC patients who had been receiving warfarin treatment had elevated DCP levels (> 40 mAU/mL; range, 3756 – 269788 mAU/mL) but negative PKCδ (12.8 – 48.5 ng/mL). These results suggest that serum PKCδ is useful for screening HCC, to compensate for the disadvantages of conventional markers in patients with liver injury and receiving warfarin treatment.
Discussion
Viral hepatitis is a major cause of HCC worldwide. In recent years, chronic HCV infection has become curable due to innovative therapeutic advances (i.e., emergence of DAAs). Accumulating evidence indicates that hepatocarcinogenesis is inhibited by achieving SVR with interferon- and/or DAA-based regimens (Ikeda et al. Citation1999, Ioannou et al. Citation2017). The prevalence of viral HCC is gradually decreasing, while that of non-viral HCC is increasing and is estimated to increase in the future (Younossi et al. Citation2015, Estes et al. Citation2018, Tateishi et al. Citation2019). Non-viral CLD patients have some carcinogenic factors, including heavy alcohol consumption, obesity, and metabolic syndrome (e.g., type 2 diabetes mellitus, hypertension, and dyslipidemia), and often have HCC found in the advanced stage (Welzel et al. Citation2011, Younossi et al. Citation2016, Diehl and Day Citation2017, Singal et al. Citation2020). Given the complex multifactorial interactions in the NAFLD pathogenesis, the term “NAFLD” has recently been proposed to be replaced with “metabolic dysfunction-associated steatotic liver disease (MASLD)” (Rinella et al. Citation2023). The annual carcinogenic rates in patients with non-cirrhotic NAFLD and NASH-related cirrhosis are < 0.1% and approximately 3% per year, respectively, which are lower than those of viral HCC (Soderberg et al. Citation2010). Nevertheless, the global incidence of steatotic liver disease remains high, and approximately 25% of cases lead to HCC (Younossi et al. Citation2016, Diehl and Day Citation2017). Therefore, the incidence of HCC in MASLD patients is not negligible, and several issues regarding the differences and characteristics in HCC detection according to the underlying aetiology [e.g., HCV-related HCC after viral elimination (i.e., post-SVR HCC) and non-viral HCC, especially MASLD-driven HCC and alcohol-related HCC] need to be addressed.
AFP and DCP have been conventionally used as tumour markers for HCC in clinical practice, and they are complementary to each other (Liebman et al. Citation1984, Taketa Citation1990). PKCδ is a serine/threonine kinase localized to the cytoplasm and is involved in the activation of several signalling pathways through its phosphorylation as well as in cell growth, survival, and apoptosis (Tsai et al. Citation2000, Wu et al. Citation2007, Yoon et al. Citation2010). HCC cells depend on an autophagy mechanism to aberrantly secrete PKCδ from the cytoplasm to the extracellular space. Moreover, the extracellular PKCδ binds to the insulin-like growth factor 1 receptor (IGF-1R) and epidermal growth factor receptor (EGFR) on cell surface and activates mitogen-activated protein kinase 1/2 (ERK1/2) and signal transducer and activator of transcription 3 (STAT3), thereby contributing to tumour growth (Yamada et al. Citation2021, Yamada et al. Citation2022a, Yamada et al. Citation2022b). Furthermore, HCC patients have significantly higher serum PKCδ levels than non-HCC patients and healthy subjects, and serum PKCδ is independent of conventional HCC markers, AFP and DCP. Therefore, PKCδ is a unique biomarker for HCC screening, especially in AFP/DCP double-negative or early-stage HCC individuals (Oikawa et al. Citation2023).
Oncogenic surveillance in viral CLD patients after achieving SVR with anti-HCV treatment is one of the major issues that must be resolved (Osaki et al. Citation2012, Asahina et al. Citation2013, Toyoda H et al. Citation2022a). HCV infection and advanced fibrosis can unexpectedly elevate AFP and DCP (Sterling et al. Citation2012). Post-SVR HCV patients have less AFP production, which makes HCC surveillance difficult due to reduced sensitivity after virus elimination (Minami et al., Citation2015). Consistent with previous reports (Minami et al. Citation2015, Yeh et al. Citation2021, Hsu et al. Citation2022), this study showed that post-SVR HCC patients had significantly lower AFP levels and positive rates than non-SVR HCC patients. The PKCδ levels and positive rates did not significantly differ between HCC patients with SVR and non-SVR. PKCδ provided better diagnostic performance (especially, sensitivity) for HCC than AFP. These findings indicate that serum PKCδ is a reliable biomarker for HCV-related HCC, independent of viral elimination status.
Non-viral HCC is often detected at an advanced stage because 1) conventional markers are often negative in early-stage HCC, and especially DCP has low specificity (Utsunomiya et al. Citation2015, Hashimoto et al. Citation2017), and 2) non-viral CLD patients have few opportunities for imaging examinations due to the lack of an established surveillance system. Several guidelines recommend that imaging examinations should be performed in viral CLD patients at high risk for liver cancer, irrespective of virus elimination or persistent infection (Omata et al. Citation2017, EASL Citation2018, Heimbach et al. Citation2018, Kokudo et al. Citation2019). However, the screening system for non-viral HCC has not yet been established due to the variation in risk factors among non-viral CLDs with different aetiologies (Younossi et al. Citation2015, Estes et al. Citation2018). Viral HCC commonly develops from cirrhosis, whereas non-viral HCC is often found in the non-cirrhotic liver. However, it is not realistic with respect to time consumption or medical economics to perform routine imaging screening on all non-viral CLD patients, even if the HCC incidence continues to increase. Accordingly, a biomarker with high sensitivity and specificity is required to develop a cost-effective screening strategy specific to non-viral HCC. This study revealed the ability of serum PKCδ to detect HCC with high sensitivity and specificity in non-viral CLD patients; the sensitivity of AFP and the specificity of DCP for non-viral HCC were low. Furthermore, the combined use of PKCδ and AFP/DCP improved the diagnostic performance for non-viral HCC. These findings suggest that PKCδ, AFP, and DCP are independent of each other, the diagnostic performance of PKCδ for HCC is comparable (but not superior) to that of conventional tumour markers, and serum PKCδ can be a novel biomarker in non-viral HCC, particularly complementing for the low sensitivity of AFP and the low specificity of DCP. This biomarker may contribute to the establishment of a highly sensitive and specific system for non-viral HCC surveillance.
Early detection of HCC must be seriously addressed to improve poor patients’ prognosis. Diagnosis of early-stage HCC remains challenging due to the lack of typical imaging findings (early arterial vascular enhancement and subsequent contrast washout) and low positive rates of conventional markers (Toyoda et al. Citation2015, Berhane et al. Citation2016, Simmons et al. Citation2017, Choi et al. Citation2019). Aetiology-stratified prognostic analyses have reported that the prognosis of patients with resectable non-viral HCC is better than or compatible with that of resectable viral HCC. Inversely, the prognosis of patients with advanced-stage or palliatively treated non-viral HCC is worse than that of viral HCC patients (Utsunomiya et al. Citation2015, Wakiyama et al. Citation2017, Hsu et al. Citation2020, Toyoda et al. Citation2022b). Thus, accumulating evidence indicates that detection of non-viral HCC at an early stage, when indicated for curative treatment, is essential to improve the prognosis of the disease. This study demonstrated that serum PKCδ had a significantly high sensitivity for very early-stage HCC in both the viral and non-viral groups (47.8% and 51.7%, respectively; Supplementary Table 1 and Supplementary Figure 3A). Consequently, PKCδ had a significantly higher diagnostic performance for detecting very early-stage HCC than conventional markers, independent of viral or non-viral aetiology. Approximately 40% – 50% of both viral and non-viral all-stage HCC patients were double-negative for AFP/DCP, and nearly 40% of them were positive for PKCδ alone (Supplementary Figure 5A). Among PKCδ-positive HCC patients, 13 (22.0%) of 59 non-viral and none (0%) of 33 viral patients were AFP-negative/DCP-positive suggesting that DCP is disadvantageous in screening for viral HCC (Supplementary Figure 5B). Notably, approximately 80% of viral HCC and 60% of non-viral HCC at the very early-stage were negative for conventional markers, but half of them were positive for PKCδ (Supplementary Figure 3B). These findings suggest that PKCδ is a promising biomarker that can identify very early-stage HCC regardless of aetiology, compensating for the disadvantage of conventional markers with low sensitivity and specificity for early-stage HCC.
PKCδ is mainly localized in the cellular cytoplasm, and its activation (activated form) plays an important role in activating several signalling pathways through phosphorylation and contributes to the survival of various cancer types (Tsai et al. Citation2000, Cao et al. Citation2016). We have previously reported novel findings that cancer-related unconventional extracellular protein secretion of PKCδ is observed only in HCC cells but not in normal hepatocytes and gastrointestinal cancer cells, and that secreted PKCδ acts like a growth factor via interaction with heparan sulphate proteoglycans, thereby contributing to HCC progression. Intriguingly, the extracellular secretion of PKCδ occurs only in the inactivated (but not activated) form (Yamada et al. Citation2021). Therefore, intracellular non-secreted PKCδ and extracellularly secreted PKCδ (detectable as serum PKCδ) may have distinct functions in HCC development and progression. Owing to these unique properties, extracellularly secreted PKCδ may be detected in very early-stage HCC patients. Indeed, the positivity rate of PKCδ was higher than that of AFP and DCP in very early-stage HCC patients (Supplementary Figure 3A). These findings suggest that extracellularly secreted PKCδ contributes to the carcinogenic process and progression, especially in very early-stage HCC. Further analysis of functional differences between intracellular non-secreted and extracellularly secreted PKCδ and their association with clinicopathological HCC features is needed.
AFP is elevated even in patients with acute liver injury, chronic liver damage or its abrupt exacerbation, pregnancy, and other malignant tumours (Toyoda et al. Citation2015, Berhane et al. Citation2016). DCP is elevated in vitamin K deficiency or use of anticoagulants (including warfarin administration) or antibiotics (Bertino et al. Citation2012), which is a drawback that reduces the specificity. In this study, we demonstrated the usefulness of serum PKCδ for diagnosing HCC in patients with acute liver injury, abrupt exacerbation of chronic liver damage, or warfarin treatment, as serum PKCδ levels are not modified by such situations.
There are some limitations in this study. First, the sample size was small due to the preliminary single-center nature of the study to clinically characterize PKCδ as an aetiology-independent biomarker for HCC. Second, the non-viral HCC population was more heterogeneous because only two agents (HCV and HBV) were considered for the viral HCC population, while several liver diseases were included for the non-viral HCC population. Third, factors affecting the measurement of PKCδ are unknown (e.g., metabolism, lifestyle, and tumour characteristics including gene signatures) (Hoshida et al. Citation2012, Oikawa Citation2016). Fourth, serum biomarker levels in non-HCC patients with HCV viremia and CLD patients with HBV viremia were not investigated because all HCV-related CLD patients without HCC had achieved SVR and most HBV-infected patients had undetectable HBV with anti-viral treatment. To address these issues, a large-scale, multicentre study in a real-world clinical setting is required.
In conclusion, PKCδ is a more useful biomarker for detecting very early-stage HCC, regardless of viral or non-viral aetiology, and it can compensate for the drawbacks of conventional markers in the diagnosis of HCC, particularly in patients after HCV elimination and those with liver injury or warfarin treatment.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and ethical guidelines issued by the administrative department, and was approved by the Local Ethics Committee of The Jikei University School of Medicine (approval No. 29-135 [8751]).
Author’s contributions
The project was originally conceived and designed by T.O., K.Y., A.T., and K.Y. Acquisition, analyses and interpretation of data were done by C.N., T.O., K.Y., C.S., K.K., N.T., K.U., H.K. Obtained samples were done by M.N., Y.T., T.T., K.H., and T.I. Statistical analysis was done by C.N. and T.O. The article was drafted and edited by C.N, T.O., and A.T. Study supervision was done by M.S., K.Y., and A.T. All of the authors have read and approved of the final manuscript.
Supplemental Material
Download MS Word (1.1 MB)Acknowledgements
We thank Ms. Y. Numata and other physicians who were involved in data collection.
Disclosure statement
The authors who participated in this study declare that they have nothing to disclose regarding funding or conflicts of interest with respect to this manuscript.
Data availability statement
Data, analytical methods, and research materials are not available for public access; however, these could be requested directly from the corresponding authors.
Additional information
Funding
References
- Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H, et al. 2013. Alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 58(4):1253–1262. doi: 10.1002/hep.26442
- Atsukawa M, Tsubota A, Kondo C, Toyoda H, Nakamuta M, Takaguchi K, Watanabe T, Hiraoka A, Uojima H, Ishikawa T, et al. 2020. Real-world clinical application of 12-Week sofosbuvir/velpatasvir treatment for decompensated cirrhotic patients with genotype 1 and 2: a prospective, multicenter study. Infect Dis Ther. 9(4):851–866. doi: 10.1007/s40121-020-00329-y
- Atsukawa M, Tsubota A, Toyoda H, Takaguchi K, Nakamuta M, Watanabe T, Michitaka K, Ikegami T, Nozaki A, Uojima H, et al. 2019. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment: a prospective, multicenter study. Aliment Pharmacol Ther. 49(9):1230–1241. doi: 10.1111/apt.15218
- Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, et al. 2016. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 14(6):875–886 e876. doi: 10.1016/j.cgh.2015.12.042
- Bertino G, Ardiri A, Malaguarnera M, Malaguarnera G, Bertino N, Calvagno GS. 2012. Hepatocellualar carcinoma serum markers. Semin Oncol. 39(4):410–433. doi: 10.1053/j.seminoncol.2012.05.001
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68(6):394–424. doi: 10.3322/caac.21492
- Brierley DJ, Gospodarowicz KM, Wittekind C. 2017. TNM classification of malignant tumours. 8th ed. Oxford (UK): Wiley-Blackwell in affiliation with the Union for International Cancer Control (UICC); p. 90–93.
- Bruix J, Sherman M. . 2011. Management of hepatocellular carcinoma: an update. Hepatology. 53(3):1020–1022. doi: 10.1002/hep.24199
- Cao M, Gao J, Zhou H, Huang J, You A, Guo Z, Fang F, Zhang W, Song T, Zhang T. 2016. HIF-2alpha regulates CDCP1 to promote PKCdelta-mediated migration in hepatocellular carcinoma. Tumour Biol. 37(2):1651–1662. doi: 10.1007/s13277-015-3527-7
- Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. 2019. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 69(5):1983–1994. doi: 10.1002/hep.30233
- Diehl AM, Day C. 2017. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 377(21):2063–2072. doi: 10.1056/NEJMra1503519
- EASL. 2018. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 69(1):182–236. doi: 10.1016/j.jhep.2018.03.019
- Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, Colombo M, Craxi A, Crespo J, Day CP, et al. 2018. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 69(4):896–904. doi: 10.1016/j.jhep.2018.05.036
- Hashimoto M, Tashiro H, Kobayashi T, Kuroda S, Hamaoka M, Ohdan H. 2017. Clinical characteristics and prognosis of non-B, non-C hepatocellular carcinoma: the impact of patient sex on disease-free survival - A retrospective cohort study. Int J Surg. 39:206–213. doi: 10.1016/j.ijsu.2017.01.110
- Heidelbaugh JJ, Sherbondy M. 2006. Cirrhosis and chronic liver failure: part I. Diagnosis and evaluation. Am Fam Physician. 74(5):767– 776.
- Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. 2018. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 67(1):358–380. doi: 10.1002/hep.29086
- Hoshida Y, Moeini A, Alsinet C, Kojima K, Villanueva A. 2012. Gene signatures in the management of hepatocellular carcinoma. Semin Oncol. 39(4):473–485. doi: 10.1053/j.seminoncol.2012.05.003
- Hsu PY, Hsu CT, Yeh ML, Huang CF, Huang CI, Liang PC, Lin YH, Hsieh MY, Wei YJ, Hsieh MH, Dai CY, et al. 2020. Early fibrosis but late tumor stage and worse outcomes in hepatocellular carcinoma patients without hepatitis B or hepatitis C. Dig Dis Sci. 65(7):2120–2129. doi: 10.1007/s10620-019-05938-3
- Hsu PY, Liang PC, Huang CI, Hsieh MH, Tsai YS, Lin TC, Yeh ML, Huang CF, Wang CW, Jang TY, et al. 2022. Effects of achieving SVR on clinical characteristics and surgical outcomes in patients who developed early-stage hcv-related hepatocellular carcinoma and received curative resection: preoperative versus postoperative SVR. Viruses. 14(11):2412. doi: 10.3390/v14112412
- Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H, et al. 1999. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: a long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 29(4):1124–1130. doi: 10.1002/hep.510290439
- Ioannou GN, Green PK, Berry K. 2017. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 5:S0168-8278(17)32273–0. doi: 10.1016/j.jhep.2017.08.030
- Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C, et al. 2014. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 23(1):144–153. doi: 10.1158/1055-9965.EPI-13-0870
- Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, et al. 2019. Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 49(10):1109–1113. doi: 10.1111/hepr.13411
- Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. 1984. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 310(22):1427–1431. doi: 10.1056/NEJM198405313102204
- Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, et al. 2009. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 137(1):110–118. doi: 10.1053/j.gastro.2009.04.005
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. 2018. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 68(2):723–750. doi: 10.1002/hep.29913
- Minami T, Tateishi R, Kondo M, Nakagomi R, Fujiwara N, Sato M, Uchino K, Enooku K, Nakagawa H, Asaoka Y, et al. 2015. Serum alpha-fetoprotein has high specificity for the early detection of hepatocellular carcinoma after hepatitis C virus eradication in patients. Medicine. 94(23):e901. doi: 10.1097/MD.0000000000000901
- Nozaki A, Atsukawa M, Kondo C, Toyoda H, Chuma M, Nakamuta M, Uojima H, Takaguchi K, Ikeda H, Watanabe T, , et al. 2020. The effectiveness and safety of glecaprevir/pibrentasvir in chronic hepatitis C patients with refractory factors in the real world: a comprehensive analysis of a prospective multicenter study. Hepatol Int. 14(2):225–238. doi: 10.1007/s12072-020-10019-z
- Oikawa T. 2016. Cancer stem cells and their cellular origins in primary liver and biliary tract cancers. Hepatology. 64(2):645–651. doi: 10.1002/hep.28485
- Oikawa T, Yamada K, Tsubota A, Saeki C, Nakagawa C, Ueda K, Kamioka H, Taniai T, Haruki K, Nakano M, et al. 2023. Protein kinase C delta is a novel biomarker for hepatocellular carcinoma. GasroHep Advances. 2(1):83–95. doi: 10.1016/j.gastha.2022.07.020
- Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. 2017. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 11(4):317–370. doi: 10.1007/s12072-017-9799-9
- Osaki Y, Ueda Y, Marusawa H, Nakajima J, Kimura T, Kita R, Nishikawa H, Saito S, Henmi S, Sakamoto A, et al. 2012. Decrease in alpha-fetoprotein levels predicts reduced incidence of hepatocellular carcinoma in patients with hepatitis C virus infection receiving interferon therapy: a single center study. J Gastroenterol. 47(4):444–451. doi: 10.1007/s00535-011-0505-8
- Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, et al. 2015. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 35(9):2155–2166. doi: 10.1111/liv.12818
- Piñero F, Mendizabal M, Ridruejo E, Herz Wolff F, Ameigeiras B, Anders M, Schinoni MI, Reggiardo V, Palazzo A, Videla M, Lalrean, et al. 2019. Treatment with direct-acting antivirals for HCV decreases but does not eliminate the risk of hepatocellular carcinoma. Liver Int. 39(6):1033–1043., doi: 10.1111/liv.14041
- Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. 2022. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 76(3):681–693. doi: 10.1016/j.jhep.2021.11.018
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, , et al. 2023. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 79(6):1542–1556. doi: 10.1016/j.jhep.2023.06.003
- Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. 2017. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 45(1):169–177. doi: 10.1111/apt.13841
- Singal AG, Lampertico P, Nahon P. 2020. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 72(2):250–261. doi: 10.1016/j.jhep.2019.08.025
- Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. 2010. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 51(2):595–602. doi: 10.1002/hep.23314
- Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. 2012. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 107(1):64–74. doi: 10.1038/ajg.2011.312
- Taketa K. 1990. Alpha-fetoprotein: reevaluation in hepatology. Hepatology. 12(6):1420–1432. doi: 10.1002/hep.1840120625
- Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, Yoshiji H, Yatsuhashi H, Shimizu M, Torimura T, et al. 2019. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011-2015 update. J Gastroenterol. 54(4):367–376. doi: 10.1007/s00535-018-1532-5
- Toyoda H, Atsukawa M, Uojima H, Nozaki A, Takaguchi K, Hiraoka A, Itobayashi E, Watanabe T, Matsuura K, Shimada N, et al. 2022a. The impact of cirrhosis and history of hepatocellular carcinoma on all-cause mortality after eradication of hepatitis C virus in patients with chronic hepatitis C. Gastro Hep Advances. 1(4):508–515. doi: 10.1016/j.gastha.2022.0
- Toyoda H, Kariyama K, Hiraoka A, Tsuji K, Ishikawa T, Hatanaka T, Naganuma A, Yasuda S, Nouso K, Kakizaki S, et al. 2022b. Improved survival of viral hepatocellular carcinoma but not non-viral hepatocellular carcinoma from 2000 to 2020: a multi-centre cohort study of 6007 patients from high volume academic centres in Japan. Aliment Pharmacol Ther. 56(4):694–701. doi: 10.1111/apt.17088
- Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. 2015. Tumor markers for hepatocellular carcinoma: simple and significant predictors of outcome in patients with HCC. Liver Cancer. 4(2):126–136. doi: 10.1159/000367735
- Tsai JH, Hsieh YS, Kuo SJ, Chen ST, Yu SY, Huang CY, Chang AC, Wang YW, Tsai MT, Liu JY. 2000. Alteration in the expression of protein kinase C isoforms in human hepatocellular carcinoma. Cancer Lett. 161(2):171–175. doi: 10.1016/s0304-3835(00)00597-8
- Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, Matsuyama Y, Sakamoto M, Nakashima O, Ku Y, et al. 2015. A comparison of the surgical outcomes among patients with HBV-positive, HCV-positive, and non-B non-C hepatocellular carcinoma: a nationwide study of 11,950 patients. Ann Surg. 261(3):513–520. doi: 10.1097/SLA.0000000000000821
- Wakiyama S, Matsumoto M, Haruki K, Gocho T, Sakamoto T, Shiba H, Futagawa Y, Ishida Y, Yanaga K. 2017. Clinical features and outcome of surgical patients with non-B non-C hepatocellular carcinoma. Anticancer Res. 37(6):3207–3213. doi: 10.21873/anticanres.11682
- Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, McGlynn KA. 2011. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 54(2):463–471. doi: 10.1002/hep.24397
- Wu TT, Hsieh YH, Wu CC, Hsieh YS, Huang CY, Liu JY. 2007. Overexpression of protein kinase C alpha mRNA in human hepatocellular carcinoma: a potential marker of disease prognosis. Clin Chim Acta. 382(1-2):54–58. doi: 10.1016/j.cca.2007.03.018
- Yamada K, Kizawa R, Yoshida A, Koizumi R, Motohashi S, Shimoyama Y, Hannya Y, Yoshida S, Oikawa T, Shimoda M, et al. 2022a. Extracellular PKCdelta signals to epidermal growth factor receptor for tumor proliferation in liver cancer cells. Cancer Sci. 113(7):2378–2385. doi: 10.1111/cas.15386
- Yamada K, Motohashi S, Oikawa T, Tago N, Koizumi R, Ono M, Tachibana T, Yoshida A, Yoshida S, Shimoda M, et al. 2022b. Extended-synaptotagmin 1 engages in unconventional protein secretion mediated via SEC22B(+) vesicle pathway in liver cancer. Proc Natl Acad Sci U S A. 119(36):e2202730119. doi: 10.1073/pnas.2202730119
- Yamada K, Oikawa T, Kizawa R, Motohashi S, Yoshida S, Kumamoto T, Saeki C, Nakagawa C, Shimoyama Y, Aoki K, et al. 2021. Unconventional secretion of PKCdelta exerts tumorigenic function via stimulation of ERK1/2 signaling in liver cancer. Cancer Res. 81(2):414–425. doi: 10.1158/0008-5472.CAN-20-2009
- Yeh ML, Liang PC, Tsai PC, Wang SC, Leong J, Ogawa E, Jun DW, Tseng CH, Landis C, Tanaka Y, et al. 2021. Characteristics and survival outcomes of hepatocellular carcinoma developed after HCV SVR. Cancers. 13(14):3455. doi: 10.3390/cancers13143455
- Yoon CH, Kim MJ, Park MJ, Park IC, Hwang SG, An S, Choi YH, Yoon G, Lee SJ. 2010. Claudin-1 acts through c-Abl-protein kinase Cdelta (PKCdelta) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J Biol Chem. 285(1):226–233. doi: 10.1074/jbc.M109.054189
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. 2016. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64(1):73–84. doi: 10.1002/hep.28431
- Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. 2015. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 62(6):1723–1730. doi: 10.1002/hep.28123