ABSTRACT
Colony-stimulating factor 1 receptor (CSF1R)-related leukoencephalopathy is one of the most common causes of adult-onset leukodystrophy and is caused by mutation of the CSF1R gene. Brain magnetic resonance imaging (MRI) findings in asymptomatic patients have not been well recognized. We report on the case of a patient with CSF1R-related leukoencephalopathy who had a novel missense variant of the CSF1R gene with a family history of early onset dementia. This is a representative case of CSF1R-related leukoencephalopathy, which shows the progression of brain MRI and cognitive decline from an asymptomatic state.
Introduction
Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) was a comprehensive pathologic term coined for hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) or pigmented orthochromatic leukodystrophy (POLD) based on the common pathologic findings of diffuse myelin and axon loss, pigmented macrophages, and numerous neuroaxonal spheroids. Since the discovery of the CSF1R gene mutation in families with HDLS (Rademakers et al., Citation2011) and POLD (Nicholson et al., Citation2013), CSF1R-related leukoencephalopathy has been used to describe patients with ALSP (Tipton et al., Citation2021). CSF1R-related leukoencephalopathy is autosomal dominant adult-onset leukodystrophy. Patients typically exhibit early onset cognitive or personality changes and motor dysfunction. CSF1R-related leukoencephalopathy is reportedly one of the most common causes of adult-onset leukodystrophy, and accounts for approximately 10% of the cases (Lynch et al., Citation2016). Brain magnetic resonance imaging (MRI) findings have been characterized in symptomatic patients with CSF1R-related leukoencephalopathy, but there are few reports of brain MRI findings in asymptomatic patients. Herein, we describe the case of a patient with CSF1R-related leukoencephalopathy having a novel mutation in the CSF1R gene using serial brain MRI and neuropsychological tests from the asymptomatic state.
Case presentation
A 31-year-old woman was referred with a tentative diagnosis of multiple sclerosis based on brain MRI abnormalities. She has had intermittent vascular headache since in her twenties, which usually lasted 6–7 hours and resolved spontaneously. She had no symptoms other than intermittent headaches, and no history of perinatal or developmental problems. Her father had slow progressive dementia and gait disturbance from his late 40s, and had died at the age of 58 years without a definite diagnosis.
Neurologic examinations did not reveal any abnormalities, and she exhibited a normal gait pattern. Brain computed tomography (CT) had been performed 4 years prior because of her headache, and it showed multifocal discrete calcifications in both cerebral hemispheres, mainly in the gray-white matter junction ()). There were no abnormalities on examination, and she was referred for a regular checkup due to the calcifications. MRI performed during this checkup showed multifocal high signal intensity lesions in bilateral frontal subcortical and periventricular areas ()) with diffusion restriction in some areas ()), which resembled multiple-sclerosis plaques. The lesions were not enhanced after gadolinium injection ()), and the corpus callosum exhibited a normal appearance ()). She underwent neuropsychological assessments including the Mini-Mental State Examination (MMSE), the Clinical Dementia Rating (CDR) scale, the CDR-sum of boxes, and the standardized Seoul Neuropsychological Screening Battery (Ahn et al., Citation2010). Beck Depression Inventory (BDI) was performed for neuropsychiatric testing. The results of the neuropsychological assessments were categorized according to cognitive domains () and the results presented as the percentile of age and education matched normal group. All neuropsychological assessments and BDI revealed no abnormality at first ().
Table 1. Neuropsychological tests included in each cognitive domain
Table 2. The results of serial neuropsychological tests
Figure 1. Brain computed tomography (a, b) and serial brain magnetic resonance imaging (c-h at asymptomatic state and I-L 3 years later) and genetic analysis (m).
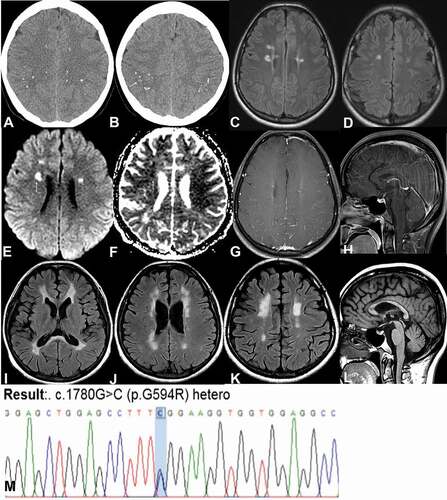
In cerebrospinal fluid (CSF) tests, the white blood cell count was in the normal range (<5), as was the total protein level (16 mg/dL). Oligoclonal band testing was negative, and the IgG index was 0.67. Infectious causes including HIV, syphilis, herpes virus, John Cunningham virus, and tuberculosis were excluded via CSF and serum analyses. Serologic tests for nuclear antibody, ss-A/ss-B antibody, antiphospholipid antibody, and anti-aquaporin four antibodies were negative. Serum very long-chain fatty acids, enzyme activity for Krabbe disease, plasma amino acid profile, and urine organic acid profile were all within the normal ranges. NOTCH3 gene mutation was not detected. Genetic analysis was performed using DNA extraction from the patient’s peripheral blood. All the exons and flanking introns of the CSF1R gene were read using Sanger sequencing. A novel missense variant was heterozygously found in the CSF1R gene (NM_005211.3: ca. 1780 G > C; p.G594R) though it was classified as a variant of uncertain significance according to the ACMG guidelines ()).
Neuropsychological assessments showed little progression of the cognitive decline at 1 year later, and a marked decline in the frontal executive function and memory domain 3 years later (). The neurologic examination including gait analysis conducted 3 years later showed no abnormality. Brain MRI taken 3 years later showed diffuse deep and periventricular white matter lesions ()) with corpus callosum thinning ()).
Discussion
The differential diagnosis of adult-onset leukodystrophy is extensive, and includes a number of acquired and hereditary causes (Ahmed et al., Citation2014). Potential causes such as inflammatory, autoimmune, neoplastic, infections, drugs, and exposure to toxins should be evaluated as initial investigations. If a genetic disorder is suspected, combined clinical features and brain MRI patterns can yield informative indications, helping in diagnosis (Lynch et al., Citation2019).
In symptomatic patients with CSF1R-related leukoencephalopathy, brain MRI typically depicts confluent, largely symmetric T2 hyperintense/T1 hypointense signal abnormality in the frontoparietal and periventricular white matter that spares the U-fibers but involves the pyramidal tracts and corpus callosum, especially thinning of the corpus callosum (Sundal et al., Citation2012). Punctated areas of restricted diffusion are another main feature (Lynch et al., Citation2016). Bilateral calcifications are often observed in the periventricular frontal white matter and subcortical regions of the parietal lobe. The so-called “stepping stone appearance” due to calcification is a characteristic feature of CSF1R-related leukoencephalopathy (Konno et al., Citation2014). In addition to an autosomal dominant inheritance pattern, focal restricted diffusion with calcification in the present case was key to differentiating CSF1R-related leukoencephalopathy from other forms of hereditary leukodystrophy.
Brain MRI findings in asymptomatic patients with CSF1R-related leukoencephalopathy have not been well characterized. Due to focal diffusion restriction, it could be misdiagnosed as central nervous system vasculitis (Lynch et al., Citation2016) or ischemia, or multiple sclerosis, as it was in the current case. One study reported serial brain MRI findings which showed scattered asymmetric T2 hyperintense foci in bifrontal periventricular white matter at presymptomatic state and progressive, confluent, frontal predominant leukodystrophy at 6 and 7 years later, respectively (Van Gerpen et al., Citation2008). However, they did not perform the neuropsychological tests longitudinally.
The present patient was ultimately diagnosed with CSF1R-related leukoencephalopathy based on the clinical features and detection of a novel CSF1R mutation (ca. 1780 G > C; p.G594R). It was within the mutation hotspot, and between the sites of known pathogenic mutations p.G589E and p.L630R. It is predicted to be pathogenic by Mutation Taster (Schwarz et al., Citation2014) because the site is highly conserved in a wide variety of species. It is classified as a variant of undetermined significance according to the ACMG guidelines. (Richards et al., Citation2015)
The average onset age of CSF1R-related leukoencephalopathy is 43 ± 11 years (18–78 years) in the literature, and female patients are younger (40 years) than males (47 years) (Konno et al., Citation2017). The frequent initial symptoms are cognitive decline (59%), psychiatric symptoms (44%), movement symptoms (38%), and speech symptoms (19%) (Konno et al., Citation2017). Cognitive impairments in CSF1R-related leukoencephalopathy are characterized by frontal lobe dysfunctions including executive dysfunction and attention deficit. Psychiatric symptoms include anxiety, depression, apathy, abulia, indifference, irritability, disinhibition, distraction, and other behavioral and personality changes (Ikeuchi et al., Citation2018). Our patient started showing cognitive decline in her early 30s, however, she denied having any difficulty in activities of daily living.
CSF1R protein is mainly expressed in the microglia, resulting in the disease designation of microgliopathy. Moreover, several studies reported microglial therapy in CSF1R-related leukoencephalopathy (Han et al., Citation2020; Tipton et al., Citation2021).
This case showed representatively the clinical and structural progression of CSF1R-related leukoencephalopathy with sequential brain MRI and neuropsychological tests. Asymptomatic CSF1R-related leukoencephalopathy can be confused with several other diseases due to periventricular focal lesions with diffusion restriction in brain MRI, and a thorough investigation with a careful clinical history is crucial for accurate diagnosis and treatment.
Patient consent
Written consent has been obtained from the patient.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ahmed, R. M., Murphy, E., Davagnanam, I., Parton, M., Schott, J. M., Mummery, C. J., … Chataway, J. (2014). A practical approach to diagnosing adult onset leukodystrophies. Journal of Neurology, Neurosurgery & Psychiatry, 85, 770–781. https://doi.org/https://doi.org/10.1136/jnnp-2013-305888
- Ahn, H. J., Chin, J., Park, A., Lee, B. H., Suh, M. K., Seo, S. W., & Na, D. L. (2010). Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. Journal of Korean Medical Science, 25, 1071–1076. https://doi.org/https://doi.org/10.3346/jkms.2010.25.7.1071
- Han, J., Sarlus, H., Wszolek, Z. K., Karrenbauer, V. D., & Harris, R. A. (2020). Microglial replacement therapy: A potential therapeutic strategy for incurable CSF1R-related leukoencephalopathy. Acta Neuropathologica Communications, 8, 217. https://doi.org/https://doi.org/10.1186/s40478-020-01093-3
- Ikeuchi, T., Mezaki, N., & Miura, T. (2018). Cognitive dysfunction and symptoms of movement disorders in adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. Parkinsonism & Related Disorders, 46, S39–s41. https://doi.org/https://doi.org/10.1016/j.parkreldis.2017.08.018
- Konno, T., Tada, M., Tada, M., Koyama, A., Nozaki, H., Harigaya, Y., … Ikeuchi, T. (2014). Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology, 82, 139–148. https://doi.org/https://doi.org/10.1212/wnl.0000000000000046
- Konno, T., Yoshida, K., Mizuno, T., Kawarai, T., Tada, M., Nozaki, H., … Ikeuchi, T. (2017). Clinical and genetic characterization of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia associated with CSF1R mutation. European Journal of Neurology, 24, 37–45. https://doi.org/https://doi.org/10.1111/ene.13125
- Lynch, D. S., Jaunmuktane, Z., Sheerin, U. M., Phadke, R., Brandner, S., Milonas, I., … Houlden, H. (2016). Hereditary leukoencephalopathy with axonal spheroids: A spectrum of phenotypes from CNS vasculitis to parkinsonism in an adult onset leukodystrophy series. Journal of Neurology, Neurosurgery & Psychiatry, 87, 512–519. https://doi.org/https://doi.org/10.1136/jnnp-2015-310788
- Lynch, D. S., Wade, C., Paiva, A. R. B., John, N., Kinsella, J. A., Merwick, A., … Chataway, J. (2019). Practical approach to the diagnosis of adult-onset leukodystrophies: An updated guide in the genomic era. Journal of Neurology, Neurosurgery & Psychiatry, 90, 543–554. https://doi.org/https://doi.org/10.1136/jnnp-2018-319481
- Nicholson, A. M., Baker, M. C., Finch, N. A., Rutherford, N. J., Wider, C., Graff-Radford, N. R., … Rademakers, R. (2013). CSF1R mutations link POLD and HDLS as a single disease entity. Neurology, 80, 1033–1040. https://doi.org/https://doi.org/10.1212/WNL.0b013e31828726a7
- Rademakers, R., Baker, M., Nicholson, A. M., Rutherford, N. J., Finch, N., Soto-Ortolaza, A., … Wszolek, Z. K. (2011). Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nature Genetics, 44, 200–205. https://doi.org/https://doi.org/10.1038/ng.1027
- Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., … Rehm, H. L. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. 17, 405–424. https://doi.org/https://doi.org/10.1038/gim.2015.30
- Schwarz, J. M., Cooper, D. N., Schuelke, M., & Seelow, D. (2014). MutationTaster2: Mutation prediction for the deep-sequencing age. Nature Methods, 11, 361–362. https://doi.org/https://doi.org/10.1038/nmeth.2890
- Sundal, C., Van Gerpen, J. A., Nicholson, A. M., Wider, C., Shuster, E. A., Aasly, J., … Wszolek, Z. K. (2012). MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology, 79, 566–574. https://doi.org/https://doi.org/10.1212/WNL.0b013e318263575a
- Tipton, P. W., Stanley, E. R., Chitu, V., & Wszolek, Z. K. (2021). Is pre-symptomatic immunosuppression protective in csf1r-related leukoencephalopathy? Mov Disord, 36, 852–856. https://doi.org/https://doi.org/10.1002/mds.28515
- Van Gerpen, J. A., Wider, C., Broderick, D. F., Dickson, D. W., Brown, L. A., & Wszolek, Z. K. (2008). Insights into the dynamics of hereditary diffuse leukoencephalopathy with axonal spheroids. Neurology, 71, 925–929. https://doi.org/https://doi.org/10.1212/01.wnl.0000325916.30701.21
