ABSTRACT
Body ownership has mainly been linked to the right hemisphere and larger interhemispheric connectivity has been shown to be associated with greater right hemispheric activation. Mixed-handed participants tend to have more interhemispheric connectivity compared to extreme handed participants. The aim of this study was to examine whether feelings of ownership as assessed with the rubber hand illusion (RHI) are differentiated by handedness and differed between the left and right hand. Sinistrals-, dextrals-, and mixed-handed individuals (n = 63) were subjected to the RHI. Stroking was synchronously and asynchronously performed on both the participant’s hand and a rubber hand. Outcome measures were an embodiment questionnaire and proprioceptive drift. In contrast to our hypotheses we show a similar experience of ownership for all groups, which may indicate no hemispheric specialization for the illusion. In addition, plasticity of ownership and body ownership are similar for the left hand and right hand in all participants, which suggests similar representations for both hands in the brain. This might be useful to maintain a coherent sense of the body in space.
Introduction
When someone points at your hand and asks if that hand belongs to you, you will immediately confirm that it is your hand. But how do we know that our hand actually belongs to our body? This may not be as self-evident as it appears. Indeed, body ownership can be experimentally manipulated and therefore examined with for example the well-known rubber hand illusion (RHI). In this illusion, participants see a rubber hand being stroked, while simultaneously the occluded real hand is being stroked. As a result, the participants refer their tactile sensations to the rubber hand (Botvinick & Cohen, Citation1998). In other words, participants have the illusionary experience that they can feel the touch they visually perceive on the rubber hand. The tactile, proprioceptive, and visual information are integrated into a single experience. And when this multisensory input is congruent to internal models of the body, participants have the impression that the rubber hand becomes part of their own body (Valenzuela Moguillansky, O’Regan, & Petitmengin, Citation2013).
Previous studies suggest a strong link between the right hemisphere and awareness of the subjective experience of body ownership (e.g., Frasinetti, Maini, Romualdi, Galante, & Avanzi, Citation2008; Karnath & Baier, Citation2010; Tsakiris, Hesse, Boy, Haggard, & Fink, Citation2007; Vallar & Ronchi, Citation2009). More specifically, brain areas involved in body ownership include the right temporoparietal junction (Tsakiris, Constantini, & Haggard, Citation2008), the secondary somatosensory cortex (Press, Heyes, Haggard, & Eimer, Citation2008), the posterior parietal and ventral premotor cortices (Ehrsson, Spence, & Passingham, Citation2004; Zeller, Gross, Bartsch, Johansen-Berg, & Classen, Citation2011), and the right posterior insula (Karnath & Baier, Citation2010). This is further supported by the right lateralization of brain lesions that typically lead to somatoparaphrenia (misidentification and confabulation of limbs) (Vallar & Ronchi, Citation2009).
Research with split-brain patients has shown that feelings of ownership depend on interhemispheric communication, specifically in the posterior corpus callosum (Uddin, Citation2011). Interestingly, interhemispheric connectivity appears to vary with handedness. Studies have revealed that the corpus callosum of sinistrals tends to be larger than the corpus callosum of dextrals, which suggests greater interhemispheric connectivity in sinistrals (Gutwinski et al., Citation2011). This is particularly relevant, since one’s percept of one’s own body may entail cross-talk between the two hemispheres. The left hemisphere has been suggested (Ramachandran & Blakeslee, Citation1998; Ramachandran, Rogers-Ramachandran, & Cobb, Citation1995) to serve as a belief maintenance system in an ever-changing world, while the right hemisphere updates and evaluates these beliefs, that is, detects anomalies. This may also have implications for body representations. Research by Hach and Schütz-Bosbach (Citation2010) concerning representation of one’s own body space suggested that implicit body representation differences (e.g., indicate stimuli on one’s left and right body midline with eyes closed) are linked to a stronger lateralization or greater activation imbalance in dextrals. In contrast, sinistrals have greater access to right hemispheric functions, such as an “up to date” body representation following synchronized visuotactile input. As a consequence they might experience ownership over a fake hand to a greater extent (Hach & Schütz-Bosbach, Citation2010).
However, other studies show that body ownership may not depend on the direction (whether one is left or right handed), but on the degree of handedness. Niebauer, Aselage, and Schutte (Citation2002) found that mixed-handed participants are more receptive to ownership of a rubber hand than extreme handed participants. Their results indicated that, when strength of handedness increased, scores on the illusion scale decreased in the left hand RHI. In their second experiment, participants had to say “now” when, during stroking, participants experienced touch coming from the rubber hand or when participants felt the fake hand becoming part of their body. Time to experience these feelings of ownership increased when (absolute) laterality quotients increased (Niebauer et al., Citation2002). Christman, Henning, Geers, Propper, and Niebauer (Citation2008) and Prichard, Propper, and Christman (Citation2013), support this line of reasoning, and suggest that mixed-handed individuals have greater interhemispheric connectivity than extreme-handed individuals. Moreover, research by Luders et al. (Citation2010) showed a negative association between callosal size and handedness lateralization in which extreme handedness was associated with smaller corpus callosum size. Interestingly, when extreme handed participants were compared with mixed-handed participants, a decrease in right hemisphere activation was found (Propper, Pierce, Geisler, Christman, & Bellorado, Citation2012). As body ownership appears to be right lateralized, this may suggest that mixed-handed participants have a better-developed sense of body ownership. In the current study we tested this hypothesis by applying the RHI to participants with varying degrees of left or right handedness. Since handedness constitutes a robust proxy for laterality, we aim to test the effect of handedness on the subjective and objective experience of the RHI.
So far, most studies, that investigated the RHI, tested dextrals with a left rubber hand (e.g., Botvinick & Cohen, Citation1998; Haggard & Jundi, Citation2009; Tsakiris & Haggard, Citation2005) or a right rubber hand (e.g., Armel & Ramachandran, Citation2003; Constantini & Haggard, Citation2007; Kammers, de Vignemont, Verhagen, & Dijkerman, Citation2009; Lloyd, Citation2007; Shimada, Fukuda, & Hiraki, Citation2009). A few studies used both hands but did not statistically compare the hands (e.g., Pavani, Spence, & Driver, Citation2000; Walton & Spence, Citation2004). Mussap and Salton (Citation2006) did compare the hands, but did not find a subjective (using an embodiment questionnaire) difference between the left and right hand. Interestingly, Michael et al. (Citation2012) found that particularly dextrals are more receptive to spontaneous sensations (e.g., beat/pulse, tickle) for their left hand as opposed to their right hand.
With respect to handedness, so far, only a few studies, incorporated a few sinistrals (e.g., Constantini & Haggard, Citation2007; Haans, IJsselsteijn, & de Kort, Citation2008; IJsselsteijn, de Kort, & Haans, Citation2006; Rohde, Di Luca, & Ernst, Citation2011) and only Haans et al. (Citation2008) tested whether handedness influenced experience of the RHI. They reported no difference in experience between sinistrals and dextrals. However, this might be due to a lack of statistical power, since 18 of the 23 participants were right handed and thus the number of sinistrals was very small. To date, only Ocklenburg, Rüther, Peterburs, Pinnow, and Güntürkün (Citation2011) systematically explored whether sinistrals and dextrals differed in experience of the RHI. Experience of the illusion was measured with skin conductance responses (SCR) and an embodiment questionnaire. The SCR was stronger for the left hand, while there was no difference between sinistrals and dextrals when experience of the illusion was objectively measured. Taken together, the right hemisphere, linked to the somatosensory and visual signals from the left side of the body, as opposed to the left hemisphere, seems to update at a faster rate. This is consistent with the enhanced spontaneous sensations for the left hand (Michael et al., Citation2012) and susceptibility of the RHI for the left hand (Ocklenburg et al., Citation2011).
In order to decipher inconsistencies regarding laterality (extreme versus mixed) and the hand used (left versus right) in the sense of ownership, we systematically examine differences in sense of ownership between sinistrals, dextrals (extreme-handed), and mixed-handed individuals, by using relatively large groups. In addition to the classic subjective (embodiment) questionnaires we also use an established objective measure of the RHI, that is, proprioceptive drift instead of using skin conductance, the latter being used in Ocklenburg et al., Citation2011). We also use stringent handedness quotients; participants were considered sinistral if the laterality quotient, according to the Edinburgh Handedness Inventory: The EHI (Oldfield, Citation1971), was below −80, mixed handed if the laterality quotient was between −80 and 80 and dextral if the laterality quotient was above 80. It is important to note that Ocklenburg et al. (Citation2011) differentiated sinistral and dextral as smaller or larger than zero. Niebauer et al. (Citation2002) however found a correlation between ownership over the rubber hand and handedness, but did not specify the average EHI score in their study. Based on previous research we hypothesize a different experience of the RHI for the different handedness groups. We specifically expect differences in experience of the RHI on both the objective and subjective measures for mixed-handed participants as opposed compared to extreme handed (sinistrals and dextrals). In addition, we expected a higher degree of ownership for the left hand than for the right hand in all handedness groups.
Method
Participants
Sixty-three individuals participated. The individuals were screened online prior to participation. Online questionnaires contained questions regarding demographics (age, sex, and education), the EHI (Oldfield, Citation1971), a screening for history of neurological/psychiatric disorders and substance abuse. Participants who reported either a history of neurological/psychiatric disorders or substance abuse were excluded from participation. Participant demographics are shown in . Based on laterality quotients calculated for the EHI (for the equation, see below, materials), 21 participants were assigned to either the group of sinistrals, mixed-handed participants and dextrals. As stated by Hardie and Wright (Citation2014), a majority of laterality studies use a notional median of 80 (e.g., Christman & Butler, Citation2011; Jasper, Barry, & Christman, Citation2008; Lyle & Orsborn, Citation2011; Westfall, Jasper, & Christman, Citation2012). Therefore, participants were considered extreme handed if the laterality quotient was below −80 (sinistrals) or above 80 (dextrals) and mixed handed if the laterality quotient was between −80 and 80. Additionally, in order to make comparisons across former studies possible Ocklenburg et al. (Citation2011) we also calculated a broader, hence more liberal division between left- and right-handed individuals; laterality quotients between −1 and −100 are considered left-handed individuals and laterality quotients ranging from 1 till 100 are considered right-handed individuals.
Table 1. Mean age and SD (standard deviation), gender and mean laterality quotients and SD (as assessed with the EHI (Oldfield, Citation1971)) for handedness groups and total.
Prior to the experiment informed consent was obtained and the nature of the study was clarified. Each participant received course credit or six Euros.
Design
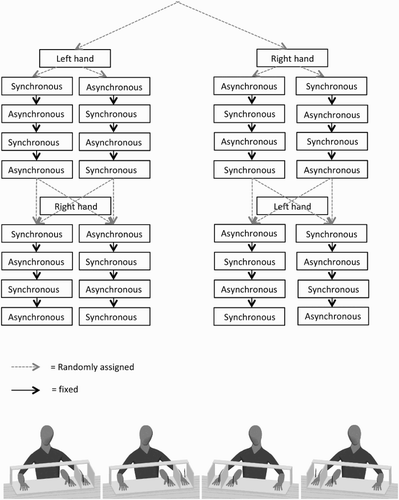
A within-subjects design was used in which all participants completed four stroking conditions twice, resulting in eight trials in total. The four conditions, as shown in , were synchronous left hand stroking, asynchronous left hand stroking, synchronous right hand stroking and asynchronous right hand stroking. Block order was pseudorandomized; four trials for one hand (two synchronous and two asynchronous, see ) were performed before stroking was performed on the other hand. Then, asynchronous stroking in one hand was always followed by synchronous stroking. At random, the experiment started with synchronous or asynchronous stroking of either the left or right hand. The fifth trial, subsequently, started at random with synchronous or asynchronous stroking of the other hand.
Procedure/task/stimuli
Procedure
All measurements were conducted in a quiet lab at Utrecht University, and participants were seated comfortably. Participants received a brief verbal explanation and signed informed consent prior to the experiment. All trials consisted of measuring proprioceptive drift, inducing the RHI, measuring proprioceptive drift, and filling out the embodiment questionnaire, respectively.
Questionnaire
Edinburgh Handedness Inventory: The EHI (Oldfield, Citation1971) consists of 10 items for which the participant is instructed to place a checkmark which hand is preferred. If there is such a strong preference that the other hand would never be used, the participant is instructed to place two checkmarks. Laterality quotients were calculated with the following formula.
LQ stands for laterality quotient, L is the sum of checkmarks for the left hand and R is the sum of checkmarks for the right hand.
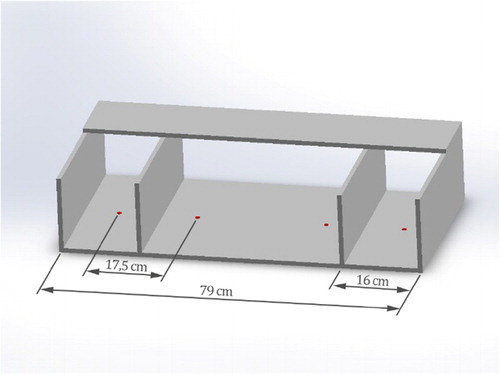
RHI: Participants were seated at a table. The arm of their stimulated hand and the arm of the rubber hand were covered with a black cloth. The cloth prevented the participant to see the proximal end of the rubber hand. As shown in , the rubber hand and the hands of the participant were placed on a fixed (marked) location within a wooden framework (79 cm in width, see for exact dimensions). The space between the stimulated own hand and the RH was approximately 17 cm. In the framework, there is a compartment for the hidden stimulated hand (outer compartments) and a compartment for the rubber hand and the unstimulated hand (inner compartment). The positions of the hands were similar for all stroking conditions and for each hand. The participant’s real hand was placed, depending on which hand was stroked, in the outer compartment (see ) of the box. The center of the wrist was placed on the red marks, and the rubber hand was always on the same position on one of the red dots (depending on which hand was being stroked) in the inner compartment. When measuring proprioception (before and after illusion) a wooden lid was placed on top of the box.
Stroking procedure: During the 90 sec illusion, both the unstimulated hand and the rubber hand were visible for the participant. Two identical brushes were used to stroke the rubber and the real hidden hand. Stroking was performed from knuckle to fingertip. Outcome measures were embodiment questionnaire and proprioceptive drift and will be discussed shortly. In the synchronous conditions, the experimenter stroked the rubber hand and the real hand of the participant synchronously. More specifically, we performed similar but irregular stroke frequencies varying from one stroke per second and one stroke per three seconds. In the asynchronous condition, the rubber hand and the real hand were stroked sequentially in a similar pattern. As such the brush only touched one hand at a time (either the rubber hand or the real hand). In the left hand conditions, the real left hand of the participant and the left rubber hand were stroked and vice versa for the right hand conditions. Positioning of the hands of the participants was similar in all conditions.
Embodiment questionnaire. An embodiment questionnaire was used to measure subjective strength of the illusion (Kammers et al., Citation2009). The questionnaire consisted of 10 statements. Answers were given on a 10-point Likert scale, ranging from 1 (disagree strongly) to 10 (agree strongly). Three of these statements measure the subjective strength of the illusion. These statements are:
It seemed as if I were feeling the touch of the paintbrush in the location where I saw the rubber hand touched, it seemed as though the touch I felt was caused by the paintbrush touching the rubber hand and it felt as if the rubber hand were my hand.
The remaining statements are considered control questions (Kammers et al., Citation2009). Since conditions were induced twice, the average of the conditions was used for analyses. The 10 questions were analysed separately and an average was calculated for the first 3 questions and the remaining questions for each condition.
Proprioceptive drift
Proprioceptive drift was measured in millimetres. To assess proprioceptive drift, a cardboard was placed on top of the box (so the participant could not see to see his/her own hands and the rubber hand). The experimenter moved her own index finger alongside the back of the box (random outwards to inwards or vice versa) and the participant was instructed to say stop when the index finger of the experimenter matched the location of where the participant felt his/her own index finger. Using a small lead weight (hanging on the index finger of the experimenter) and a tape measure, the location was verified. Proprioception of the stimulated hand was measured first and of the unstimulated hand second. Proprioceptive drift was the difference between the estimated location of the index finger after induction of the RHI and the estimated location of the index finger before induction of the RHI. A positive number denoted a drift towards the rubber hand. All conditions were induced twice; therefore the average proprioceptive drift for each condition was used for analyses. The total duration of the experiment was approximately 70 minutes.
Analyses
Most of our data were not normally distributed. We initially used transformation procedures and outlier removal procedures offered by Field (Citation2013). Transforming the data (log transformation, square root transformation, reciprocal transformation) or removing outliers (trimming data by deleting 10% of the highest or lowest scores or excluding data above or below 2.5 SD or 3.5 SD) did not result in normally distributed data. Therefore we used a 2 (Hand-tested; left, right) × 2 (Synchronicity; synchronous, asynchronous) × 2 (Handedness; left-handed, right-handed, × 2 (Handedness strength; extreme, mixed) mixed ANOVA and applied a non-parametric bootstrap over the entire data set. Robust statistics, such as bootstrap has no assumptions about normality. It is also argued that transforming data (e.g., trimming of outliers) is in this type of analyses no longer necessary. In this analysis we used “hand-tested” (left versus right) and “synchronicity” (synchronous versus asynchronous) as within subject factors and “handedness” (left-handers versus right handers) and “handedness strength” (extreme versus mixed) as between subject factors. In our bootstrap we used 1,000 iterations and obtained 95% confidence intervals (CI) of F-values for each statistic and for both outcome measures (i.e., proprioceptive drift and embodiment questionnaire). For readers’ convenience we presented two different divisions of Laterality Quotients (LQ) in each graph; in the left panel we presented data of the extreme handed (LQ’s < −80 (sinistrals) and >80 (dextrals)) and mixed handed (LQ’s between −80 and 80), in the right panel we presented left- (LQ’s between −1 and −100) and right-handed (LQ’s between 1 and 100)Footnote1 data. RStudio (Citation2015) was used for statistical computing and bootstrapping graphics. If not stated otherwise, alpha levels of .05 (two-tailed) were used for the statistical tests.
Results
Hypotheses
We expected the RHI to be stronger for the mixed-handed participants than for the extreme-handed participants. In addition, we examined whether stroking the left hand resulted in a higher degree of ownership over the rubber hand than stroking the right hand in all handedness groups.
Subjective embodiment of the RHI
Questions 1–3 from the embodiment subscale formed the ownership subscale (Kammers et al., Citation2009) and the higher the average score of this subscale, the more subjective strength of the illusion was experienced.
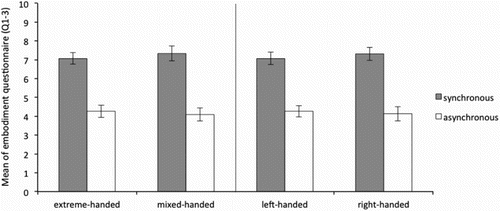
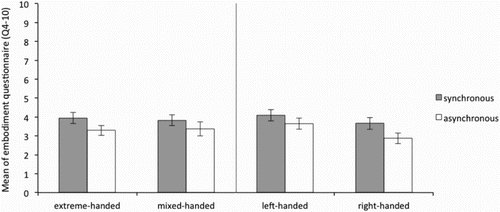
It was therefore expected that questions 1–3 were scored above 5 (neutral) in the synchronous but below 5 in the asynchronous conditions, for both hands in all groups. As can be seen in , and , this was the case for both the left and the right hand respectively. This indicates that in general, for all groups, the illusion was successfully induced.
Figure 3. Average of the “ownership scale” (average Q1–3) of the Embodiment questionnaire for the left hand in the synchronous and asynchronous condition for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
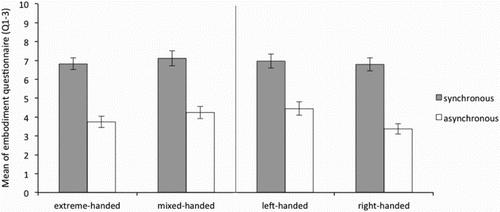
Figure 4. Average of the “ownership scale” (average Q1–3) of the Embodiment questionnaire for the right hand in the synchronous and asynchronous condition for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
The remaining questions served as control questions (Kammers et al., Citation2009). Scores above 5 could indicate that participants were influenced by an “observer-expectancy” bias. These questions were grouped together on the control subscale (average questions 4–10). The control questions are shown in for the left hand and for the right hand stroking conditions. No difference can be observed between synchronous and asynchronous stroking and for both hands the average ratings were below 5, indicating that it is highly unlikely that the subjective data were influenced by experimenter bias.
Figure 5. Average of the “control questions” (average Q4–10) of the Embodiment questionnaire for the left hand in the synchronous and asynchronous condition for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
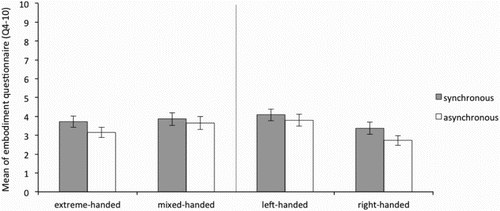
Taken together, visual inspection of the data suggests that the ownership ratings in the synchronous condition were above neutral (average = 5) in all groups, indicating the illusion was successfully induced. No difference can be observed (see and ) between synchronous and asynchronous stroking across groups for the remaining control questions, and for all groups the mean ratings were below 5. Formal statistical analyses were run on the ownership subscale only.
Figure 6. Average of the “control questions” (average Q4–10) of the Embodiment questionnaire for the right hand in the synchronous and asynchronous condition for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
For “synchronicity,” a mixed ANOVA revealed an expected main effect, F(1, 58) = 118.01, p < .001, ηp2 = .67, indicating that the subjective experience of ownership was stronger in the synchronous than the asynchronous condition. Other main-effects (i.e., “hand-tested,” “handedness (left versus right),” “handedness strength” were not significant, summarized: F(1, 58) < 1.46, p > .232.
Against our expectation, there was no two-way interaction between “hand-tested” and “synchronicity,” F(1, 58) = 0.25, p = .618, ηp2 < .01, indicating that the subjective experience of ownership did not differ between the left and right hand. Next, neither the interaction between “handedness strength” and “synchronicity,” F(1, 58) = 0.04, p = .847, ηp2 < .01, nor the interaction between “handedness” and “synchronicity” was significant F(1, 58) = 0.45, p = .506, ηp2 = .01. There was a significant two-way interaction between “handedness” × “hand-tested” F(1, 58) = 4.08, p = .048, ηp2 = .07. However this effect did not interact with “synchronicity.” Other two-way interactions that did not include the factor “synchronicity” and we had no specific hypotheses about (i.e., “handedness” × “handedness strength;” “handedness strength” × “hand-tested”) were not significant, summarized: F(1, 58) < 1.34, p > .252.
Against our expectations, there was no three-way interaction between “hand-tested,” “synchronicity,” “handedness strength,” F(1, 58) = 1.24, p = .270, ηp2 = .02 nor a three-way interaction between “hand-tested,” “synchronicity,” “handedness,” F(1, 58) = 2.60, p = .112, ηp2 = .04. The three-way interaction of “handedness” × “handedness strength” × “hand-tested” was not significant either F(1, 58) = 0.81, p = .373, ηp2 = .01.
Lastly, “synchronicity” did not interact with “hand-tested,” and different “handedness groups” (i.e., left versus right; extreme versus mixed), F(1, 58) = 1.38, p = .244, ηp2 = .02.
All in all, analyses indicate that for the subjective embodiment ownership scale “synchronicity” had no differential impact in either the left or right hand and both handedness groups (i.e., left- and right-handed individuals and, extreme and mixed-handed individuals).
Hereafter we applied a non-parametric bootstrap with 1,000 iterations. We then plotted (see ) the 95% confidence intervals (CI) of these F-values for each statistical test. In we see that the 95% CI of F-values for “synchronicity” (95% CI [127.21–130.52] not displayed in plot) and for “handedness × hand-tested” exceeded criterion F (≈4.01, based on degrees of freedom). The latter effect does not interact with “synchronicity.” The confidence intervals of other effects did not exceed the critical F, hence after resampling the data 1,000 times there is a high level of confidence that the true F for “handedness” × “synchronicity” falls below the critical F.
Proprioceptive drift
Proprioceptive drift was operationalized as the difference between the estimated location of the index finger before induction of the RHI and the estimated location of the index finger after induction of the RHI. A larger value indicates larger shift towards the rubber hand. As expected, synchronous stroking resulted in a larger proprioceptive drift than asynchronous stroking (see and ) for the left and right hand respectively.
Figure 7. Confidence intervals (95%) of F Values (vertical axis) for each statistical effect (horizontal axis: 2 = handedness, 3 = strength handedness, 4 = synchronicity, 5 = hand, 6 = handedness × strength handedness, 7 = handedness × synchronicity, 8 = strength handedness × synchronicity, 9 = handedness × hand, 10 = strength handedness × hand, 11 = synchronicity × hand, 12 = handedness × strength handedness × synchronicity, 13 = handedness × strength handedness × hand, 14 = handedness × synchronicity × hand, 15 = strength handedness × synchronicity × hand, 16 = handedness × strength handedness × synchronicity × hand). Red dashed lines represent criterion F (≈ 4.01) [To view this figure in color, please see the online version of this journal].
![Figure 7. Confidence intervals (95%) of F Values (vertical axis) for each statistical effect (horizontal axis: 2 = handedness, 3 = strength handedness, 4 = synchronicity, 5 = hand, 6 = handedness × strength handedness, 7 = handedness × synchronicity, 8 = strength handedness × synchronicity, 9 = handedness × hand, 10 = strength handedness × hand, 11 = synchronicity × hand, 12 = handedness × strength handedness × synchronicity, 13 = handedness × strength handedness × hand, 14 = handedness × synchronicity × hand, 15 = strength handedness × synchronicity × hand, 16 = handedness × strength handedness × synchronicity × hand). Red dashed lines represent criterion F (≈ 4.01) [To view this figure in color, please see the online version of this journal].](/cms/asset/a2d38d4a-ac4e-4548-b286-4753e0b9233f/plat_a_1273940_f0007_c.jpg)
Figure 8. Average proprioceptive drift in the synchronous and asynchronous condition for the left hand for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
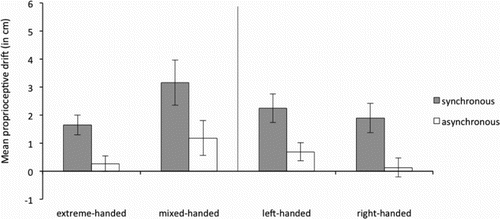
Results for the proprioceptive drift resemble results of the subjective embodiment measurements. For “synchronicity,” a repeated measures ANOVA revealed an expected main effect, F(1, 58) = 37.10, p < .001, ηp2 = .39, indicating that proprioceptive drift was stronger in the synchronous than the asynchronous condition. Other main-effects (i.e., “hand-tested,” “handedness (left versus right),” “handedness strength” were not significant, summarized: F(1,58) < 3.89, p > .053.
Against our expectation, there was no two-way interaction between “hand-tested” and “synchronicity,” F(1, 58) = 0.00, p = .993, ηp2 < .01, indicating that proprioceptive drift did not differ between the left and right hand. Next, neither the interaction between “handedness strength” and “synchronicity,” F(1, 58) = 2.07, p = .155, ηp2 = .03, nor the interaction between “handedness” and “synchronicity” was significant F(1, 58) = 0.26, p = .612, ηp2 < .01. Other two-way interactions that did not include the factor “synchronicity” and we had no specific hypotheses about (i.e., “handedness” × “handedness strength;” “handedness” × “hand-tested;” “handedness strength” × “hand-tested”) were not significant either, summarized: F(1, 58) < 0.36, p > .550.
Against our expectations, there was no three-way interaction between “hand-tested,” “synchronicity,” “handedness strength,” F(1, 58) = 0.01, p = .938, ηp2 < .01, nor a three-way interaction between “hand-tested,” “synchronicity,” “handedness,” F(1, 58) = 0.02, p = .888, ηp2 < .01. The three-way interaction of “handedness” × “handedness strength” × “hand-tested” was not significant either F(1, 58) = 0.43, p = .516, ηp2 = .01.
Lastly, “synchronicity” did not interact with “hand-tested,” and different “handedness groups” (i.e., left versus right; extreme versus mixed), F(1, 58) = 0.48, p = .491, ηp2 = .01.
Taken together, analyses indicate that for the outcome measure proprioceptive drift the type of stroking had no differential impact in either the left or right hand and both handedness groups (i.e., left- and right-handed individuals and, extreme and mixed-handed individuals) ().
Figure 9. Average proprioceptive drift in the synchronous and asynchronous condition for the right hand for the extreme- and mixed-handed division (left panel) and right-handed and left-handed division (right panel). Error bars represent Standard Error (SE) of the mean.
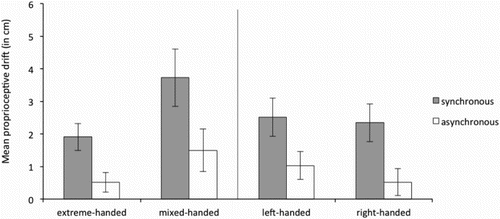
Again, after bootstrapping (1,000 iterations) the entire data set for our outcome measure proprioceptive drift (see ), only the confidence intervals for the effects of “strength handedness” (mixed versus extreme) and “synchronicity” (95% CI [42.96–44.96] not displayed in ) exceeded criterion F (≈4.01, based on degrees of freedom). Interaction-effects including “synchronicity” did not exceed criterion F. This indicates that after numerous replications there is a high level of confidence that type of stroking (synchronicity) had no differential impact in either the left or right hand and both handedness groups.
Figure 10. Confidence intervals (95%) of F Values (vertical axis) for each statistical effect (horizontal axis: 2 = handedness, 3 = strength handedness, 4 = synchronicity, 5 = hand, 6 = handedness × strength handedness, 7 = handedness × synchronicity, 8 = strength handedness × synchronicity, 9 = handedness × hand, 10 = strength handedness × hand, 11 = synchronicity × hand, 12 = handedness × strength handedness × synchronicity, 13 = handedness × strength handedness × hand, 14 = handedness × synchronicity × hand, 15 = strength handedness × synchronicity × hand, 16 = handedness × strength handedness × synchronicity × hand). Red dashed lines represent criterion (≈4.01) [To view this figure in color, please see the online version of this journal].
![Figure 10. Confidence intervals (95%) of F Values (vertical axis) for each statistical effect (horizontal axis: 2 = handedness, 3 = strength handedness, 4 = synchronicity, 5 = hand, 6 = handedness × strength handedness, 7 = handedness × synchronicity, 8 = strength handedness × synchronicity, 9 = handedness × hand, 10 = strength handedness × hand, 11 = synchronicity × hand, 12 = handedness × strength handedness × synchronicity, 13 = handedness × strength handedness × hand, 14 = handedness × synchronicity × hand, 15 = strength handedness × synchronicity × hand, 16 = handedness × strength handedness × synchronicity × hand). Red dashed lines represent criterion (≈4.01) [To view this figure in color, please see the online version of this journal].](/cms/asset/01c38f6b-1214-4b6c-a2cf-aebfa32a6fa0/plat_a_1273940_f0010_c.jpg)
Discussion
In previous studies, body ownership has been demonstrated to be mainly linked to the right hemisphere and greater interhemispheric connectivity has been shown to be associated with greater right hemispheric activation. Mixed-handed participants tend to have more interhemispheric connectivity compared to extreme-handed participants (Christman et al., Citation2008; Prichard et al., Citation2013). The aim of this study was to examine whether individuals with different handedness preferences differentially experienced feelings of ownership as measured with the RHI. In addition, we examined whether the RHI differed for the left and right hand stroking conditions. Handedness was differentiated in both extreme-handed, mixed-handed and left-and right-handed individuals. Based on differences in interhemispheric connectivity, we hypothesized a stronger experience of the RHI for mixed-handed individuals as opposed to extreme-handed individuals. In addition, we expected a higher degree of ownership for the left hand than for the right hand in all handedness groups.
Both subjective (embodiment questionnaire) and objective (proprioceptive drift) outcomes showed that participants experienced the illusion in the synchronous stroking conditions but not the asynchronous stroking conditions, indicating that the RHI was successfully induced. The embodiment questionnaires as well as the proprioceptive drift outcomes indicated that experience of the RHI was similar for sinistrals, mixed-handed participants, and dextrals. Experience of the RHI was also similar in the left and right hand stroking conditions. Bootstrapping the data for both outcome measures confirmed that after random sampling the data multiple times, there is a high level of confidence that the illusion had no differential impact in either the left or right hand and both handedness groups.
Although previous research showed differences in interhemispheric connectivity (Christman et al., Citation2008; Gutwinski et al., Citation2011; Prichard et al., Citation2013), current results suggest that this affects neither the objective nor the subjective experience of a perceptual illusion of visuotactile integration. This may suggest that ownership may not be as lateralized as current literature indicates. However, since corpus callosum size and subsequent interhemispheric connectivity were not measured directly in the current study, the present results are based on a behavioural measure of laterality only and therefore should not be interpreted without caution. Nevertheless, our findings are consistent with Bertamini and O’Sullivan (Citation2014) who suggested that feelings of ownership do not depend on right hemispheric activation only. They state that these feelings of ownership depend more on interhemispheric cross-talk, hence the activation of both hemispheres during the illusion. Indeed, Ehrsson et al. (Citation2004) previously provided evidence of bilateral premotor activation (as measured with fMRI), which correlated with the subjective experience of the RHI. Additionally, Kammers et al. (Citation2009) demonstrated that rTMS over the left inferior posterior lobule attenuated the strength of the illusion in perceptual body judgments (e.g., proprioceptive judgments). Interestingly, other measures, such as subjective self-reports, were unaffected. However, another study did find differences depending on the hand for which the illusion was induced (Ocklenburg et al., Citation2011). The reason why Ocklenburg et al. (Citation2011) found a differential effect on handedness might be due to the usage of a different objective measure (i.e., SCR) under threatening circumstances. A high SCR while watching a syringe approaching the hand reflects an autonomic nervous system arousal response, implying an attentional process. Since the right hemisphere is dominant for attention (Heilman & Van den Abell, Citation1980; Kinsbourne, Citation1970), attentional asymmetries could account for this differential impact. Moreover, recent work (Riemer, Bublatzky, Trojan, & Alpers, Citation2015) have shown physiological changes (i.e., SCR) but not proprioceptive changes in the RHI under threatening circumstances, indicating that measures such as drift and SCR might capture different aspects of RHI, especially in an alarming environment.
One alternative explanation for the current lack of differences in RHI strength between the different handedness groups is that our design was not sensitive enough to detect behavioural changes, that is, we induced the illusion for a fixed time interval (90 seconds). We assumed that the strength of ownership would differentially increase, that is, higher subjective experience and larger proprioceptive drift. However, since we only measured after the 90 seconds interval, a plateau level of ownership might have been reached, which could have masked subtle differences. In this sense, the exact time to experience the illusion might be more sensitive to detect changes. This is in line with results reported by Niebauer and colleagues (Citation2002) found. Here participants had to say “now” as soon as ownership was experienced, and this did result in a differential outcome; time to experience ownership increased when laterality quotients increased (Niebauer et al., Citation2002). Using this manipulation, our results might differ as a function of time instead of strength.
In the present study, we found differences in neither embodiment nor drift between the left and right hand. This is in line with prior research showing no subjective differences for hand being stroked (Mussap & Salton, Citation2006; Niebauer et al., Citation2002). Research published while the current research was conducted also failed to show a difference in proprioceptive drift and embodiment questions 1–3 for the left and right hand in a group of dextrals (Bertamini & O’Sullivan, Citation2014). Interestingly, Bertamini, Berselli, Bode, Lawson, and Wong (Citation2011) reported differences in neither proprioceptive drift nor embodiment when visual information was perceived either directly or through a mirror. The RHI thus appears to be undiminished when the left hand is turned into a right hand (Bertamini et al., Citation2011). Our research is consistent with these results, which is particularly noteworthy concerning literature that linked body ownership to the right hemisphere (Frasinetti et al., Citation2008; Karnath & Baier, Citation2010; Tsakiris et al., Citation2007; Vallar & Ronchi, Citation2009). Our results suggest that neural representations of ownership over the left and right hand might be similar. In the RHI tactile stimulation is first referenced to a mental body representation and the mental body representation is subsequently updated based on visuotactile integration (Serino & Haggard, Citation2010; Tsakiris, Citation2010). Tactile stimulation evokes activity in the contralateral primary somatosensory cortex, the bilateral secondary somatosensory cortex (Ruben et al., Citation2001), the superior and inferior parietal lobule, the supplementary and cingulate motor area and the insula (Pleger & Villringer, Citation2013), indicating that the representation of the left and right hand in the somatosensory cortex are similar. Jung, Baumgärtner, Magerl, and Treede (Citation2011), however found a hemispheric asymmetry of hand representation in the somatosensory cortex, that is, the rostral area was elongated in the left hemisphere as opposed to the right hemisphere due to a larger hand presentation, however this was not statistically linked to handedness. Somatosensory evoked potential studies show differences in neither topography nor response amplitude between the left and right hemisphere (Kakigi & Shibasaki, Citation1992; Zhu, Disbrow, Zumer, McGonigle, & Nagarajan, Citation2007). Taken together, this indicates that the reference frame (i.e., mental body representation) and tactile perception of the left and right hand are similar. Although our study complements existing papers on laterality and ownership using similar sample sizes (Niebauer et al., Citation2002; Ocklenburg et al., Citation2011) we should be careful of making strong claims about a lack of difference, since we are one of the few, if not only, studies that did not find a difference between groups.
In summary, our results show a similar experience of ownership for extreme-handed (i.e. sinistrals, dextrals) and mixed- handed participants. In addition, experience of the RHI is similar for the left hand and right hand in all participants. Ownership therefore appears not to be influenced by handedness. In addition, the modifiability in experiencing ownership over a body part is similar for the left and right hand in a healthy population. These results suggest both hands have a similar representation in the brain, which might be useful to keep a coherent sense of the body in space. After resampling our data we can say with high confidence that our outcomes remained unaltered. However different outcome measures, such as skin conductance may reveal other patterns.
Acknowledgements
We would like to thank A.M. de Haan for providing support for the bootstrap analyses and Dr. M.J. van der Smagt for improving the overall manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes
1 One participant was excluded from this division, as the LQ is exact zero.
References
- Armel, K. C., & Ramachandran, V. S. (2003). Projecting sensations to external objects: Evidence from skin conductance responses. Proceedings of the Royal Society. London. B, 270, 1499–1506. doi: 10.1098/rspb.2003.2364
- Bertamini, M., Berselli, N., Bode, C., Lawson, R., & Wong, L. T. (2011). The rubber hand illusion in a mirror. Consciousness and Cognition, 20, 1108–1119. doi: 10.1016/j.concog.2011.04.006
- Bertamini, M., & O’Sullivan, N. (2014). The use of realistic and mechanical hands in the rubber hand illusion, and the relationship to hemispheric differences. Consciousness and Cognition, 27, 89–99. doi: 10.1016/j.concog.2014.04.010
- Botvinick, M., & Cohen, J. (1998). Rubber hands “feel” touch that eyes see. Nature, 391, 756. doi: 10.1038/35784
- Christman, S. D., & Butler, M. (2011). Mixed-handedness advantages in episodic memory obtained under conditions of intentional learning extend to incidental learning. Brain and Cognition, 77, 17–22. doi: 10.1016/j.bandc.2011.07.003
- Christman, S. D., Henning, B. R., Geers, A. L., Propper, R. E., & Niebauer, C. L. (2008). Mixed-handed persons are more easily persuaded and are more gullible: Interhemispheric interaction and belief updating. Laterality, 13, 403–426. doi: 10.1080/13576500802079646
- Constantini, M., & Haggard, P. (2007). The rubber hand illusion: Sensitivity and reference frame for body ownership. Consciousness and Cognition, 16, 229–240. doi: 10.1016/j.concog.2007.01.001
- Ehrsson, H. H., Spence, C., & Passingham, R. E. (2004). That’s my hand! activity in premotor cortex reflects feeling of ownership of a limb. Science, 305, 875–877. doi: 10.1126/science.1097011
- Field, A. P. (2013). Discovering statistics using IBM SPSS Statistics (4th ed.). London: SAGE.
- Frasinetti, F., Maini, M., Romualdi, S., Galante, E., & Avanzi, S. (2008). Is it mine? Hemispheric assymetries in corporeal self-recognition. Journal of Cognitive Neuroscience, 20, 1507–1516. doi: 10.1162/jocn.2008.20067
- Gutwinski, S., Löscher, A., Mahler, L., Kalbitzer, J., Heinz, A., & Bermpohl, F. (2011). Understanding left-handedness. Deutches Ärzteblatt International, 108, 849–853.
- Haans, A., IJsselsteijn, W. A., & de Kort, Y. A. W. (2008). The effect of similarities in skin texture and hand shape on perceived ownership of a fake limb. Body Image, 5, 389–394. doi: 10.1016/j.bodyim.2008.04.003
- Hach, S., & Schütz-Bosbach, S. (2010). Sinistrals’ upper hand: Evidence for handedness differences in the representation of body space. Brain and Cognition, 72, 408–418. doi: 10.1016/j.bandc.2009.12.001
- Haggard, P., & Jundi, S. (2009). Rubber hand illusions and size—weight illusions: Self presentation modulates representation of external objects. Perception, 38, 1796–1803. doi: 10.1068/p6399
- Hardie, S. M., & Wright, L. (2014). Differences between left- and right-handers in approach/avoidance motivation: Influence of consistency of handedness measures. Frontiers in Psychology, 5, 134. doi: 10.3389/fpsyg.2014.00134
- Heilman, K. M., & Van den Abell, T. (1980). Right hemisphere dominance for attention. The Mechanism underlying hemispheric asymmetries of inattention (neglect). Neurology, 30, 327–330. doi: 10.1212/WNL.30.3.327
- IJsselsteijn, W. A., de Kort, Y. A. W., & Haans, A. (2006). Is this my hand I see before me? The rubber hand illusion in reality, virtual reality, and mixed reality. Presence: Teleoperators and Virtual Environments, 15, 455–464. doi: 10.1162/pres.15.4.455
- Jasper, J. D., Barry, K., & Christman, S. D. (2008). Individual differences in counterfactual production. Personality and Individual Differences, 45, 488–492. doi: 10.1016/j.paid.2008.05.026
- Jung, P., Baumgärtner, U., Magerl, W., & Treede, R. D. (2011). Hemispheric asymmetry of hand representation in human primary somatosensory cortex and handedness. Clinical Neurophysiology, 119, 2579–2586. doi: 10.1016/j.clinph.2008.04.300
- Kakigi, R., & Shibasaki, H. (1992). Effects of age, gender, and stimulus side on the scalp topography of somatosensory evoked potentials following posterior tibial nerve stimulation. Journal of Clinical Neurophysiology, 9, 431–440. doi: 10.1097/00004691-199207010-00011
- Kammers, M. P. M., de Vignemont, F., Verhagen, L., & Dijkerman, H. C. (2009). The rubber hand illusion in action. Neuropsychologia, 47, 204–211. doi: 10.1016/j.neuropsychologia.2008.07.028
- Karnath, H. O., & Baier, B. (2010). Right insula for our sense of limb ownership and self-awareness of actions. Brain Structure and Function, 214, 411–417. doi: 10.1007/s00429-010-0250-4
- Kinsbourne, M. (1970). The cerebral basis of lateral asymmetries in attention. Acta Psychologica, 33, 193–201. doi: 10.1016/0001-6918(70)90132-0
- Lloyd, D. M. (2007). Spatial limits on referred touch to an alien limb may reflect boundaries of visuo-tactile peripersonal space surrounding the hand. Brain and Cognition, 64, 104–109. doi: 10.1016/j.bandc.2006.09.013
- Luders, E., Cherbuin, N., Thompson, P. M., Gutman, B., Anstey, K. J., Sachdev, P., & Toga, A. W. (2010). When more is less: Associations between corpus callosum size and handedness lateralization. NeuroImage, 52, 43–49. doi: 10.1016/j.neuroimage.2010.04.016
- Lyle, K. B., & Orsborn, A. E. (2011). Inconsistent handedness and saccade execution benefit face memory without affecting interhemispheric interaction. Memory, 19, 613–624. doi: 10.1080/09658211.2011.595418
- Michael, G. A., Dupuy, M.-A., Deleuze, A., Humblot, M., Simon, B., & Naveteur, J. (2012). Interacting effects of vision and attention in perceiving spontaneous sensations arising on the hands. Experimental Brain Research, 216(1), 21–34. doi: 10.1007/s00221-011-2901-y
- Mussap, A. J., & Salton, N. (2006). A “rubber-hand” illusion reveals a relationship between perceptual body image and unhealthy body change. Journal of Health Psychology, 11, 627–639. doi: 10.1177/1359105306065022
- Niebauer, C. L., Aselage, J., & Schutte, C. (2002). Hemispheric interaction and consciousness: Degree of handedness predicts the intensity of a sensory illusion. Laterality: Asymmetries of Body, Brain and Cognition, 7, 85–96. doi: 10.1080/13576500143000159
- Ocklenburg, S., Rüther, N., Peterburs, J., Pinnow, M., & Güntürkün, O. (2011). Laterality in the rubber hand illusion. Psychology Press, 16, 174–187.
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
- Pavani, F., Spence, C., & Driver, J. (2000). Visual capture of touch: Out-of-the-body experience with rubber gloves. Psychological Science, 11, 353–359. doi: 10.1111/1467-9280.00270
- Pleger, B., & Villringer, A. (2013). The human somatosensory system: From perception to decision making. Progress in Neurobiology, 103, 76–97. doi: 10.1016/j.pneurobio.2012.10.002
- Press, C., Heyes, C., Haggard, P., Eimer, M. (2008). Visuotactile learning and body representation: An ERP study with rubber hands and rubber objects. Journal of Cognitive Neuroscience, 20, 312–323. doi: 10.1162/jocn.2008.20022
- Prichard, E., Propper, R. E., & Christman, S. D. (2013). Degree of handedness, but not direction, is a systematic predictor of cognitive performance. Frontiers in Psychology, 4, 9. doi: 10.3389/fpsyg.2013.00009
- Propper, R. E., Pierce, J., Geisler, M. W., Christman, S. D., & Bellorado, N. (2012). Asymmetry in resting alpha activity: Effects of handedness. Open Journal of Medical Psychology, 1, 86–90. doi: 10.4236/ojmp.2012.14014
- Ramachandran, V. S., & Blakeslee, S. (1998). Phantoms in the brain. New York, NY: William Morrow & Company.
- Ramachandran, V. S., Rogers-Ramachandran, D., & Cobb, S. (1995). Touching the phantom limb. Nature, 377, 489–490. doi: 10.1038/377489a0
- Riemer, M., Bublatzky, F., Trojan, J., & Alpers, G. W. (2015). Defensive activation during the rubber hand illusion: Ownership versus proprioceptive drift. Biological Psychology, 109, 86–92. doi: 10.1016/j.biopsycho.2015.04.011
- Rohde, M., Di Luca, M., & Ernst, M. O. (2011). The rubber hand illusion: Feeling of ownership and proprioceptive drift do not go hand in hand. PLoS ONE, 6(6), e21659. doi: 10.1371/journal.pone.0021659
- RStudio Team. (2015). RStudio: Integrated development for R. RStudio, Inc. Boston, MA. Retrieved from http://www.rstudio.com/
- Ruben, J., Schwieman, J., Deuchert, M., Meyer, R., Krause, T., Curio, C., … Villringer, A. (2001). Somatopic organization of human secondary somatosensory cortex. Cerebral Cortex, 11, 463–473. doi: 10.1093/cercor/11.5.463
- Serino, A., & Haggard, P. (2010). Touch and the body. Neuroscience & Biobehavioral Reviews, 34, 224–236. doi: 10.1016/j.neubiorev.2009.04.004
- Shimada, S., Fukuda, K., & Hiraki, K. (2009). Rubber hand illusion under delayed visual feedback. PLoS ONE, 4(7), e6185. doi: 10.1371/journal.pone.0006185
- Tsakiris, M. (2010). My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia, 48, 703–712. doi: 10.1016/j.neuropsychologia.2009.09.034
- Tsakiris, M., Constantini, M., & Haggard, P. (2008). The role of the right-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia, 46, 3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004
- Tsakiris, M., & Haggard, P. (2005). The rubber hand illusion revisited: Visuotactile integration and self attribution. Journal of Experimental Psychology, 31, 80–91.
- Tsakiris, M., Hesse, M. D., Boy, C., Haggard, P., & Fink, G. R. (2007). Neural signatures of body ownership: A sensory network for bodily self- consciousness. Oxford Journals, 17, 2235–2244.
- Uddin, L. Q. (2011). Brain connectivity and the self: The case of cerebral disconnection. Consciousness and Cognition, 20, 94–98. doi: 10.1016/j.concog.2010.09.009
- Valenzuela Moguillansky, C., O’Regan, J. K., & Petitmengin, C. (2013). Exploring the subjective experience of the “rubber hand” illusion. Frontiers in Human Neuroscience, 7, 659. doi: 10.3389/fnhum.2013.00659
- Vallar, G., & Ronchi, R. (2009). Somatoparaphrenia: A body delusion. Experimental Brain Research, 192, 533–551. doi: 10.1007/s00221-008-1562-y
- Walton, M., & Spence, C. (2004). Cross-modal congruency and visual capture in a visual elevation discrimination task. Experimental Brain Research, 154, 113–120. doi: 10.1007/s00221-003-1706-z
- Westfall, J., Jasper, J. D., & Christman, S. D. (2012). Inaction inertia, the sunk cost effect, and handedness: Avoiding the losses of past decisions. Brain and Cognition, 80, 192–200. doi: 10.1016/j.bandc.2012.06.003
- Zeller, D., Gross, C., Bartsch, A., Johansen-Berg, H., & Classen, J. (2011). Ventral premotor cortex may be required for dynamic changes in the feeling of limb ownership: A lesion study. The Journal of Neuroscience, 31, 4852–4857. doi: 10.1523/JNEUROSCI.5154-10.2011
- Zhu, Z., Disbrow, E. A., Zumer, J. M., McGonigle, D. J., & Nagarajan, S. S. (2007). Spatiotemporal integration of tactile information in human somatosensory cortex. BMC Neuroscience, 8, 21. doi: 10.1186/1471-2202-8-21