ABSTRACT
Studies have highlighted an association between motor laterality and speech production laterality. It is thought that common demands for sequential processing may underlie this association. However, most studies in this area have relied on relatively small samples and have infrequently explored the reliability of the tools used to assess lateralization. We, therefore, established the validity and reliability of an online battery measuring sequence-based motor laterality and language laterality before exploring the associations between laterality indices on language and motor tasks. The online battery was completed by 621 participants, 52 of whom returned to complete the battery a second time. The three motor tasks included in the battery showed good between-session reliability (r ≥ .78) and were lateralized in concordance with hand preference. The novel measure of speech production laterality was left lateralized at population level as predicted, but reliability was less satisfactory (r = .62). We found no evidence of an association between sequence-based motor laterality and language laterality. Those with a left-hand preference were more strongly lateralized on motor tasks requiring midline crossing; this effect was not observed in right-handers. We conclude that there is little evidence of the co-lateralization of language and sequence-based motor skill on this battery.
Evidence suggests that, at population level, shared left hemisphere networks underpin speech production and fine motor praxis (Flinker et al., Citation2015; Frost et al., Citation1999; Hodgson & Hudson, Citation2018; Ocklenburg & Gunturkun, Citation2017). It is thought that this is because both functions rely on the sequential ordering of information (Grimme et al., Citation2011); for example, sequencing of orofacial movements produces speech, and sequencing of precise finger movements is used to rotate a coin.
Both speech and motor function are lateralized to the left hemisphere at population level, but there are individual differences, with some people showing right bias or no bias for one or both functions. This individual variation allows us to test whether speech and sequential motor skills are necessarily associated (Bruckert et al., Citation2021; Haaland & Harrington, Citation1996). A link between sequence-based motor laterality and language laterality has been highlighted in small non-clinical samples using both functional transcranial Doppler sonography (fTCD; Hodgson et al., Citation2021) and a dual task paradigm (Hodgson et al., Citation2019). Furthermore, Flowers and Hudson (Citation2013) found that motor laterality (assessed using a pegboard task) was a better indicator of language representation (assessed using the Wada test; Wada, Citation1949) than hand preference in a clinical population. Specifically, they showed that those with small between-hand differences on the pegboard task were more likely to display anomalous speech representation (i.e., “speech in the opposite hemisphere to that suggested by their hand preference, and/or ambiguous or bilateral speech representation”; Flowers & Hudson, Citation2013, p. 259); whereas, those with large between-hand differences on the pegboard task all displayed speech lateralized to the hemisphere controlling the better hand (i.e., the hemisphere contralateral to the more skilled hand). Flowers and Hudson (Citation2013) concluded that tasks targeting sequential control in either speech or praxis should show associations between performance across domains. Investigating this association in a large non-clinical sample will help optimize methods for inferring hemispheric dominance for language and extend our understanding of the relationship between motor laterality and language laterality.
Measurement of speech and sequential motor laterality
Motor laterality can be assessed using preference measures (e.g., the Edinburgh Handedness Inventory; Oldfield, Citation1971) or performance measures (e.g., pegboard tasks; Annett, Citation1970). The demands of performance measures can vary widely; with some tasks, such as the grip strength task, not requiring sequencing of intricate finger movements and tasks such as the pegboard task requiring such sequences throughout. Our focus here was on motor laterality assessed using tasks requiring sequence-based motor laterality.
The pegboard task used by Flowers and Hudson (Citation2013), and similar variations (see Richards et al., Citation2021; Roy et al., Citation2003; Schulze et al., Citation2002), are commonly used performance-based measures of motor laterality that require sequences of intricate finger movements for successful execution. Although such tasks are considered valid and reliable measures (Annett et al., Citation1974; Flowers & Hudson, Citation2013), they require the use of specialist equipment and in-person sessions, limiting opportunities for replication and large sample sizes. We developed an online task (the Complex Sequence Tapping Task) requiring a similar pattern of movements to the pegboard task used by Flowers and Hudson (Citation2013) as an alternative method for assessing sequence-based motor laterality.
When examining the relationship between motor laterality and language laterality, it is also vital to consider how we determine language laterality. Several studies have concluded that language is not a unitary construct, and different components of language may lateralize independently (Bradshaw et al., Citation2017; Häberling et al., Citation2016; Hickok & Poeppel, Citation2007; Woodhead et al., Citation2021). Given the involvement of sequencing on speech production tasks and lack thereof on receptive tasks, we might expect weaker associations between receptive language tasks and sequence-based motor laterality, than between language laterality as assessed by expressive language tasks. Indeed, Packheiser et al. (Citation2020) conducted a large-scale analysis on the link between receptive language laterality, measured using a dichotic listening task, and handedness in a sample of 1,554 individuals. It was concluded that only 0.4% of the variance in language lateralization was explained by handedness. Given the evidence showing that language laterality may fractionate, we included a measure of receptive language laterality (the Dichotic Listening Task) and a new measure of speech production laterality (the Verbal Visual Half-Field Task; vVHF). This allowed us to test for an association between sequence-based motor laterality and two components of language laterality (receptive and expressive).
Use of online testing
Parker et al. (Citation2021) demonstrated that online behavioural tasks offer a valid and reliable method for assessing laterality. Specifically, good test-retest reliability was found for a chimeric faces task r = .88, a dichotic listening task, r = .78 and a finger tapping task, r = .76 (N = 392). It was also concluded that each task was valid, insofar as they were all lateralized in the predicted direction. Following these promising findings, we extended the online laterality battery to include new assessments of motor sequencing, as well as a novel speech production task. Here we report data on three novel measures of motor laterality (the Simple Sequence Tapping Task, the Simple Sequence Tapping Midline Task and the Complex Sequence Tapping Task) and a novel measure of speech production laterality (the Verbal Visual Half-Field Task). We also report the relationship of these new measures to hand preference, and to receptive language laterality assessed by the Dichotic Listening Task.
Plan of investigation
Although several studies have focussed on the relationship between speech and motor sequencing, most relied on either a clinical sample (e.g., Flowers & Hudson, Citation2013) or a small non-clinical sample (e.g., Hodgson et al., Citation2019). In the current study, we first examined the validity and reliability of the online battery measuring motor laterality and language laterality. We also assessed the validity of the Complex Sequence Tapping Task by comparing results on this task with laterality assessed using in-person testing on a pegboard task. We then employed the battery to test for an association between motor laterality and language laterality in a large non-clinical sample.
As online testing is less constrained by time, location, and travel, we were able to administer the battery to a large sample, and to test a subset of participants on two occasions to establish between session reliability. Establishing the reliability of measures is particularly important when investigating the link between laterality indices, as the absence of a correlation cannot be interpreted as independence of function unless each task is reliable (Parsons et al., Citation2019). We use a cutoff of r = .65 for satisfactory reliability. This was a threshold used previously by Parker et al. (Citation2021) and represents a lower bound of reliability for a measure to be potentially useful when analysing individual differences.
Additionally, we assessed the validity of each task by testing whether the task was lateralized in the predicted direction based on previous literature with in-person testing. We predicted a right-ear advantage on the Dichotic Listening Task, which would indicate left hemispheric dominance for receptive language. This was based on previous findings highlighting a right-ear advantage among 70% of left-handers and 95% of right-handers (Carey & Johnstone, Citation2014). We predicted a right visual half-field (VHF) advantage on the Verbal Visual Half-Field (vVHF) Task, indicating left hemispheric dominance for speech production. This was based on findings from Van der Haegen et al. (Citation2011) who employed two VHF naming tasks and found that 143 participants showed a consistent right VHF advantage, compared to just 24 showing a consistent left VHF advantage. We preregistered that we would examine population level laterality for all three motor tasks as an indicator of validity. However, as our sample was unrepresentative of the general population in terms of handedness (with excess left-handers), it made more sense to examine laterality separately for those with a left- and right-hand preference. We grouped participants by hand preference based on Edinburgh Handedness Inventory (EHI) scores. We predicted that the left-hand preference group would have a significant left-hand advantage and the right-hand preference group would have a significant right-hand advantage on all three motor tasks (Simple Sequence Tapping Task, Simple Sequence Tapping Midline Task, Complex Sequence Tapping Task). The sample included excess left-handers in an attempt to increase the number of atypically lateralized participants we recruited, to give adequate numbers of participants across the full range of laterality. The tasks used in the current study were administered alongside tasks used in Parker et al. (Citation2022), which investigated inconsistent language lateralization but did not include any performance measures of motor laterality. Four of the 5 tasks used here were not used in Parker et al. (Citation2022), and data from them has not been analysed previously. The Dichotic Listening Task was used in both studies, and the sample used here was a subset of the sample used in Parker et al. (Citation2022).
Our motor laterality tasks also allowed us to investigate an interesting, yet seldom explored, observation within the motor laterality literature. Bryden et al. (Citation1994) found that a pegboard variation and a modified dots task in which participants were forced to reach further across their midline, highlighted a tendency for preferred hand use, even when reaching across the midline. However, exactly how the ability of each hand is impacted by midline crossing has rarely been explored. Our inclusion of a Simple Sequence Tapping Task and a midline variation of this task allowed us to test for a change in motor laterality when operating across one’s midline.
Preregistered hypotheses
The following hypotheses were preregistered on the OSF at https://osf.io/3ungd:
Hypothesis 1A
There will be a significant right-ear advantage at population level on the Dichotic Listening Task, indicating left hemispheric dominance for speech perception.
Hypothesis 1B
There will be a significant right VHF advantage at population level on the vVHF Task, indicating left hemispheric dominance for speech production.
Hypothesis 1C
Those with a left-hand preference will have a significant left-hand advantage on all three motor tasks.
Hypothesis 1D
Those with a right-hand preference will have a significant right-hand advantage on all three motor tasks.
Hypothesis 2
Those who are significantly lateralized on the Complex Sequence Tapping Task will be more likely to be significantly lateralized on the Verbal Visual Half Field (vVHF) Task.
Hypothesis 3
Performance involving midline crossing will result in larger motor laterality indices relative to performance without midline crossing.
Method
Participants
We conducted a priori power analyses for hypotheses 1 and 3 using the pwr.p.test() function from the pwr package (version 1.3-0; Champely, Citation2020) in R (R Core Team, Citation2021). This indicated that, for both hypotheses, 198 participants would be necessary to detect a small effect (d = 0.2) at an alpha-level of 0.05 with at least 80% power. We also conducted a power analysis for hypothesis 2 using the power.cmh.test() function from the samplesizeCMH package (version 0.0.1; Egeler, Citation2017). This indicated that a sample size of 279 would provide sufficient power to test for an association (θk = 3) at an alpha-level of 0.05 with at least 90% power. In practice, our sample size far exceeded that of the a priori power analyses as data for the current study were run alongside tasks forming a separate study investigating inconsistent language lateralization (Parker et al., Citation2022).
In total, 621 participants (265 males, mean age = 27.3 years; SD age = 9.54 years) were recruited through online advertisements across 6 universities (Bangor, Lancaster, Lincoln, Oxford, University College London and University of Western Australia). Participants were aged 16–50 years, had normal or corrected to normal vision, had no history of significant hearing loss, neurological disease, or developmental disorders such as dyslexia or dyspraxia. Participants were also required to have access to a laptop or desktop and were blocked from completing the experiment on a tablet or phone. Participants received either research credits or Amazon vouchers for their participation; at a rate of £10/hour. The study received ethical approval from the University Oxford’s Central University Research Ethics Committee [R72866/RE002]. A subgroup of 32 participants, from the original 621, attended an in-person pegboard session. These participants were selected based on willingness to travel to Lincoln for in-person testing. Fifty-two participants, from the original 621, completed session 2, which was an online session where the behavioural tasks from session 1 were administered again. These participants were selected based on willingness to complete a second online session. The in-person pegboard session took place between session 1 and session 2, but not all participants from the pegboard session completed session 2. Fifteen participants completed both session 2 and the pegboard session.
Materials
The online battery included a demographics questionnaire, a hand preference questionnaire and five experimental assessments of behavioural laterality. The web-based Gorilla Experimental Builder (www.gorilla.sc; Anwyl-Irvine et al., Citation2020) was used to build and administer the tasks. Working versions of the behavioural laterality tasks can be found on Gorilla Open Materials: https://app.gorilla.sc/openmaterials/278292.
Demographics questionnaire
Participants reported their age, gender, and highest level of education. Participants were asked questions to determine hand and foot preference. The question used to determine hand preference was: which is your dominant hand (i.e., which hand do you prefer to use for tasks such as writing, cutting and catching a ball)? The question used to determine foot preference was: which foot do you normally step up on a ladder/step? Participants could answer “left”, “right”, or “no preference / don’t know”. These were to simply characterize the sample and are not reported here for the sake of brevity.
Edinburgh handedness inventory
Participants completed the Edinburgh Handedness Inventory (EHI; Oldfield, Citation1971). This allowed us to place hand preference on a continuum. Participants were required to indicate their preferred hand on a 5-point scale (right hand strongly preferred, right hand preferred, no preference, left hand preferred, left hand strongly preferred) for 10 activities. Standard scoring was used for the EHI, this combined information about direction and exclusiveness of hand preference. Participants were grouped into left-handers (indices less than 0), right-handers (indices greater than 0) and those with no hand preference (indices of 0). Those who scored zero (N = 3) were excluded due to the analyses focusing on left- and right-handers.
LexTALE
The Lexical Test for Advanced Learners of English (LexTALE; Lemhöfer & Broersma, Citation2012) was used to screen for native level English proficiency. Sixty letter strings were presented, and participants had to indicate which words they knew. Forty of the strings were actual words in English and 20 were non-words. Scores were corrected to account for the unequal proportion of words and non-words using the following formula: ((number of words correct/40 × 100) + (number of non-words correct/20 × 100))/2. This resulted in scores ranging from 0 to 100. Based on norms provided by Lemhöfer and Broersma (Citation2012), those who scored below 80 (N = 65) were excluded. This ensured that all participants had native level English proficiency. This resulted in a sample size of 553 after lexTALE and EHI exclusions.
Dichotic listening task
The Dichotic Listening Task has been used previously as part of an online battery testing its reliability (Parker et al., Citation2021). The description of the task provided here uses similar wording to Parker et al. (Citation2021). The task was developed using stimuli from the app “iDichotic”, which has been shown to be a valid and reliable measure of receptive language laterality (Bless et al., Citation2013). Parker et al.’s implementations of this dichotic listening paradigm within Gorilla yielded a test-retest reliability of r = .78.
The task used six consonant vowel (cv) stimuli, formed by pairing a stop-consonant (/b/,/d/,/g/,/p/,/t/,/k/) with the vowel /a/. Each of the six consonant–vowel stimuli (/ba/, /da/, /ga/, /pa/, /ta/, /ka/) were paired and, on each trial, both sounds were played simultaneously, one through each headphone sound channel. This resulted in 36 pairings including homonyms.
Participants were screened to check they were using stereo headphones. This was done by playing a sound into one ear channel on six trials, participants had to correctly identify which channel the sound was played through on four trials to pass this screening.
Participants then completed three blocks of the Dichotic Listening Task. Each block contained 36 trials, resulting in a total of 108 trials. Each trial began with a fixation cross appearing at the centre of the screen and lasting 250 ms, this was followed by the audio stimulus. Following the presentation of each stimulus, participants were asked to report the sound they heard clearest via a mouse click. Responses triggered the start of the next trial. The count of correct answers for stimuli presented to each ear, and reaction times (RT) for each ear were recorded.
Verbal visual half-field (vVHF) task
The vVHF task was designed to engage lateralized speech production processes and was adapted from Brysbaert (Citation1994; see also Van der Haegen et al., Citation2011). On each trial, participants were asked to overtly name an image shown in either the left visual field (LVF) or right visual field (RVF). The task was developed with parameters suggested by Hunter and Brysbaert (Citation2008), who highlighted that speech laterality indices (LI’s) calculated using a vVHF task correlated well with speech LI’s calculated using fMRI, r = .77.
The task used five images, all of which were labelled during familiarization practice trials. The labels were: “boat”, “book”, “house”, “lamp” and “star”. On each trial, a pair of images were presented bilaterally, and an arrow indicated which image participants had to name.
Following familiarization, 160 pairs of stimuli were presented across 4 blocks. This resulted in a total of 40 trials in each block. Each image was displayed the same number of times in each visual field and trial order was randomized.
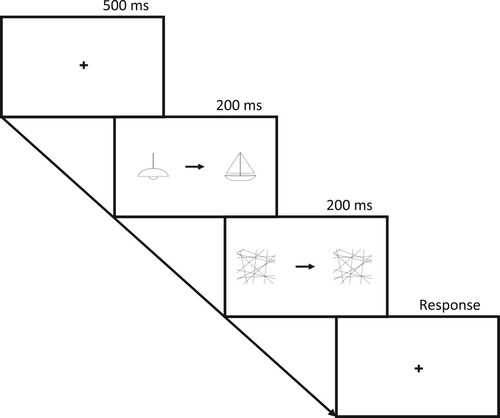
Each trial began with a fixation cross presented centrally for 500 ms. Then two images were presented bilaterally (one in the LVF and one in the RVF), with a centrally positioned arrow pointing towards one of the images, for 200 ms. These were then masked, and the arrow remained on screen for a further 200 ms. Following this, participants had 3000 ms to overtly name the image which the arrow pointed towards. During this report phase a blank screen was shown, followed by a countdown for the final 2000ms. Voice recordings were taken during each trial and stored in the cloud. The recordings were available to download after the participant had finished the study, which allowed response times to be calculated. A schematic illustration of a trial on this task is shown in .
Motor tasks
Three tasks were administered to assess motor laterality, with each task tapping into a range of motor skill components, including: sequencing, precision pressing and finger dexterity. These components were also tested under different conditions, such as: when operating across the midline versus when operating in ipsilateral space. All motor tasks in this study were designed to be completed on a standard “qwerty” keyboard. Prior to the start of each task participants were asked to align their keyboards using the following instructions: “1) The near edge of the keyboard/laptop should be parallel with your torso; 2) The left edge of the “G” key should be perpendicular with your midline.” Participants were also instructed to only tap keys using their index fingers.
Simple sequence tapping task
Participants completed a Simple Sequence Tapping Task, adapted from the finger tapping task reported by Parker et al. (Citation2021). Participants pressed a series of keys in a clockwise fashion with their left and right hand. Participants pressed “W”, “R”, “V” and “X” in sequence with their left hand or “T”, “U”, “M” and “B” in sequence with their right hand as many times as possible in 30 s. These sequences are highlighted in .
Figure 2. Illustration of the sequence of movements for each motor tasks.
Note. For the Complex Tapping Task, the initial sequence of movements on Left Hand trials is shown, moving from left to right, starting with Q. Following this sequence, the task continues moving from right to left, starting with a repeat press of M.
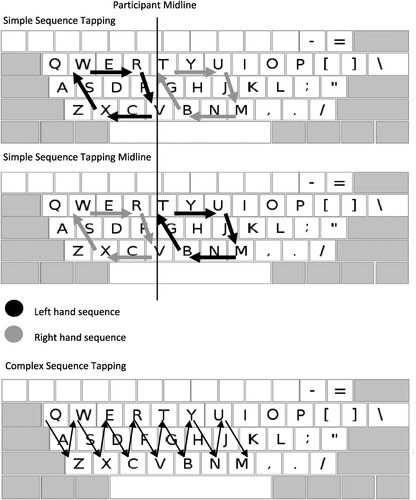
These two sequences made up a block of this task and participants completed two blocks back to back. The order of trials was pseudo-randomized between participants, alternating between sequence 1: right hand, left hand; and sequence 2: left hand, right hand. Each keypress was recorded along with a timestamp relative to the start of the trial. For each trial the number of complete sequences pressed was recorded (e.g., on left hand trials, pressing “W”, “R”, “V” and “X” in order scored 1 point).
Simple sequence tapping midline task
This task was similar to the Simple Sequence Tapping Task; however, participants reached across their midline with each hand. On this task participants pressed “T”, “U”, “M” and “B” with their left hand and “W”, “R”, “V” and “X” with their right hand. The number of blocks, trial order and scoring were consistent with the Simple Sequence Tapping Task. The sequences for this task are highlighted in .
Complex sequence tapping task
This task was designed to be an online version of the pegboard task used by Flowers and Hudson (Citation2013). Many of the pegboard task components are retained, but the grip and release mechanism is not required for successful execution. The task requires a sequence of motor movements, during which participants move between rows of keys, similar to the movements required on the pegboard task.
On left hand trials, participants were required to press the sequence “q,z,w,x,e,c,r,v,t,b,y,n,u,m,m,u,n,y,b,t,v,r,c,e,x,w,z,q”, in order as many times as possible in 30 s. This sequence is highlighted in . On right hand trials, participants were required to press the sequence “o,m,i,n,u,b,y,v,t,c,r,x,e,z,z,e,x,r,c,t,v,y,b,u,n,i,m,o”, in order as many times as possible in 30 s. Each block of this task contained two right hand trials and two left hand trials. Participants completed two blocks of this task back to back. The order of trials was pseudo-randomized between participants, alternating between sequence 1: right hand, right hand, left hand, left hand; and sequence 2: left hand, left hand, right hand, right hand. Each keypress was recorded with a timestamp that corresponded to the start of each trial. Piloting showed that few participants completed many full correct sequences on this task. This meant that a different scoring system was required to reveal fine between-hand performance differences. The task was scored by calculating the inter-keystroke interval (IKI) between correct presses (e.g., the time taken between pressing q and pressing z). Average IKI’s were calculated for each hand.
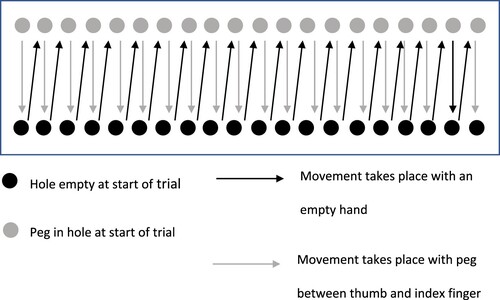
Pegboard task
An additional motor task (the pegboard task) was administered to those who attended the in-person pegboard session. The pegboard task used a wooden board 280-mm X 100-mm X 20-mm in size. The board had two rows of 20 holes (7 mm in diameter) that were drilled 5 mm apart along the length on each side. The rows were spaced 70 mm apart and the board had a Fitts index difficulty of 7.6 (Fitts, Citation1954). This difficulty suggests that it is likely that the task required some “online” control of movements.
The task began with the row of holes on the far side of the board containing 20 pegs, the aim was to move each peg to the opposite hole in sequence from right to left (on right hand trials) or left to right (on left hand trials). Once the first row of 20 pegs were moved across the board, the task continued with the aim of moving the pegs back to the opposite side. This pattern continued for 30 s, and participants were instructed to make as many moves as possible. Participants completed two blocks of this task containing four trials with each hand. Trial order was pseudo-randomized between participants, alternating between sequence 1: right hand, right hand, left hand, left hand; and sequence 2: left hand, left hand, right hand, right hand. The number of pegs moved on each trial was recorded. The sequence of movements required on left hand trials of this task is shown in .
Figure 3. Illustration of the Sequence of Movements for the Pegboard Task on Left Hand Trials.
Note. The figure shows the initial sequence of movements required, moving pegs from top to bottom (starting with the leftmost peg). Following the sequence shown, the task continues with the aim of moving pegs from bottom to top (starting with the rightmost peg).
Procedure
Session 1
Participants completed the full battery of questionnaires and laterality tasks. The session lasted around 2 h and participants accessed the battery through a URL link. Participants completed this session in a location of their choice, using a device with a keyboard, headphones and a microphone. Participants were asked to choose a time and place where they would not be disturbed or distracted.
Session 2
Participants completed all laterality tasks (except the Dichotic Listening Task) but did not repeat the questionnaires or lexTALE. The session lasted just under 2 h. Participants also completed this session in a location of their choice and accessed the battery through a URL link.
Pegboard session
All pegboard sessions took place at the University of Lincoln and the task took around 10 minutes to complete.
Data analyses
Data cleaning
For each motor task, participants were removed if there were more than 20 incorrect key presses across the left- and right-hand trials. This usually occurred due to technical problems, such as keyboard keys continuing to register presses after they had been released.
For both language tasks, we used Hoaglin and Iglewicz’s (Citation1987) procedure to remove outlier response times that were above or below 1.65 times the difference between the first and third quartiles for each participant. This resulted in the exclusion of 4.93% of Dichotic Listening trials from session 1, 4.74% of vVHF trials from session 1, and 5.30% of vVHF trials from session 2.
For the Dichotic Listening Task, participants were removed if they scored lower than 75% on catch trials where the same sound was played to each ear. For the vVHF Task, 10 random trials for each participant were used to check for task compliance. If participants gave an invalid response on 20% or more of these trials, then all trials were checked. Then, if invalid responses were given on 25% or more of all trials, participants were removed. The number of participants who met inclusion criteria for each task are shown in .
Table 1. The number of participants meeting inclusion criteria for each task.
Laterality indices
Lateralization was measured by computing a z-score that represented, for each participant on each task, a comparison between left- and right-sided responses. This is referred to as an LI z-score. This standardized score provided a common metric for reaction time, accuracy, and count measures, and allowed significantly lateralized participants to be identified. Specifically, when using a 2-sided alpha of .05, individuals whose absolute LI z-scores were greater than 1.96 or less than −1.96 (for a specific task) can be categorized as significantly lateralized (on that task).
For the Dichotic Listening Task, LI z-scores were calculated using trials in which participants correctly identified a consonant–vowel stimulus. The number of correct responses corresponding to each ear were used to calculate the LI z-scores. The formula used to calculate the LI z-score was: z = (pR -.5)/sqrt (pR x pL/n), where pR is the proportion of correct right ear responses, pL is the proportion of correct left ear responses and n is the total number of correct responses. LI z-scores on the Dichotic Listening Task were censored at −10 and 10. This was to avoid effects from a handful of participants with unusually extreme scores.
For the vVHF Task, response times were calculated using the peak volume of each sound file. Calculating response times in R using the onset of sound was not suitable due to minor background noise falsely registering as a response. In contrast, the peak volume usually occurred at the onset of, or at a consistent point within, the participants’ response. We cross validated this method with Chronset, a previously validated tool which can be used to mark speech onset (Roux et al., Citation2017). To do this, we took a subsample of random trials (N = 50) and correlated peak reaction times calculated in R with onset reaction times calculated using the online Chronset tool. The reaction times were highly correlated (r = .99) suggesting concordance between the two methods. LI z-scores were derived from a t-test conducted separately on each participant's data, where mean log response times (after outlier exclusion) were the dependent variable and visual field was the independent variable.
For the motor tasks, LI z-scores were derived from t-tests conducted separately on each participant's data. The independent variable for each task was hand used (left or right). For the Simple Sequence Tapping Task and Simple Sequence Tapping Midline Task, the dependent variable was the number of correct sequences tapped. For the Complex Sequence Tapping Task, the dependent variable was the mean IKI between correct presses. For the pegboard task, the dependent variable was total pegs moved.
Reliability
To establish the between-session reliability of each task, we calculated LI z-scores for each task separately for session 1 and session 2. As some LI z-scores were non-normal, Spearman’s correlations were used to examine the correlation of coefficients between session 1 and session 2 LI z-scores.
Complex sequence tapping cross validation
We used a Spearman’s correlation to examine the correlation between Complex Sequence Tapping Task LI z-scores and pegboard task LI z-scores.
Hypothesis 1A,1B,1C and 1D
Population level laterality was examined for each of the behavioural tasks. Shapiro–Wilk tests were used to check the LI z-scores for normality. As some LI z-scores were non-normal, a series of one sample Wilcoxon signed rank tests were conducted to compare mean LI z-scores of the sample to zero. A Bonferroni adjusted alpha of 0.01 (0.05/5) was used.
Hypothesis 2
Participants were grouped based on their LI z-scores on the vVHF Task and Complex Sequence Tapping Task. Participants were categorized as either significantly lateralized or not significantly lateralized on each task. Participants were deemed significantly lateralized if their LI z-score was equal to or greater than 1.96, or, their LI z-score was equal to or less than −1.96. Participants grouped as significantly lateralized on the Complex Sequence Tapping Task had a between-hand difference significantly larger than we would expect to see by chance. Participants grouped as significantly lateralized on the vVHF Task had a between-VHF difference significantly larger than we would expect to see by chance. A Cochran-Mantel-Haenszel test was used to test for an association between the number of people in each laterality group on the vVHF Task and the number of people in each laterality group on the Complex Sequencing Task for each level of hand preference (right, left, no preference), indicated by EHI indices.
Hypothesis 3
It was posited that participants would show stronger lateralization on the Simple Sequence Tapping Midline Task compared to the Simple Sequence Tapping Task, resulting in larger absolute LI z-scores. Firstly, LI z-scores were converted to absolute LI z-scores by multiplying negative z-scores by minus one. Following this, a two-way mixed ANOVA was conducted to investigate the impact of hand preference (left vs right) and task (Simple Sequence Tapping vs Simple Sequence Tapping Midline) on absolute LI z-scores, whilst also testing for an interaction effect (hand preference x task).
Exploratory analyses
Given that the Dichotic Listening Task had good reliability and had weak association with language production laterality in another study (Parker et al., Citation2022), the analysis for hypothesis 2 was repeated using the Dichotic Listening Task in place of the vVHF task. This tested for an association between LI z-scores on the Complex Sequence Tapping Task (motor laterality) and the Dichotic Listening Task (receptive language laterality).
We also examined the correlations between LI z-scores for each of the motor tasks and the Edinburgh Handedness Inventory (EHI) indices. This highlighted which hand skill measures correlated best with an established self-report measure of hand preference.
Results
Reliability
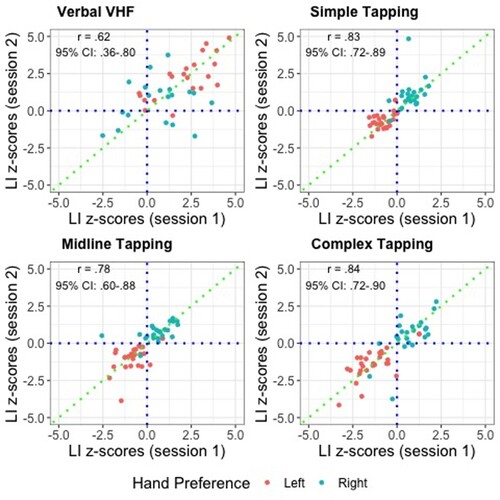
Spearman correlations were used to test the reliability of each laterality task. All tasks showed satisfactory reliability (r ≥ .65) except the vVHF Task. These correlations are displayed as annotations on the scatterplots in along with the 95% confidence intervals.
Complex sequence tapping cross validation
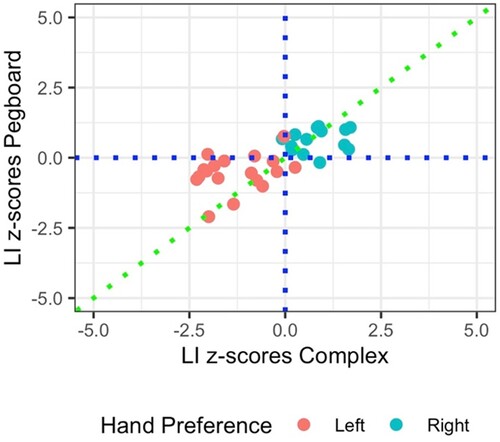
LI z-scores calculated from the Complex Sequence Tapping Task significantly correlated with LI z-scores calculated from the pegboard task, r = .73, 95% CIs = [0.54, 0.84], p < .001, N = 32. This correlation is shown in .
Hypothesis 1
shows the median, minimum and maximum LI z-score for each hand preference group on each laterality task. The table also displays the alpha statistic from Wilcoxon one-sample signed rank tests comparing median LI z-scores to zero.
Table 2. Median, Minimum and Maximum LI z-scores for Each Hand Preference Group on Each Laterality Task. Alpha Statistics from Wilcoxon One-Sample Signed Rank Tests Comparing Median LI z-scores to Zero are also displayed.
One-sample Wilcoxon signed rank tests indicated that median LI z-scores on the Dichotic Listening Task (Mdn = 1.12, N = 526) and vVHF Task (Mdn = 0.92, N = 500) were significantly different from zero, p <. 001, V = 99822 (Dichotic), 96025 (vVHF) indicating a right ear advantage and a right visual field advantage at population level as predicted.
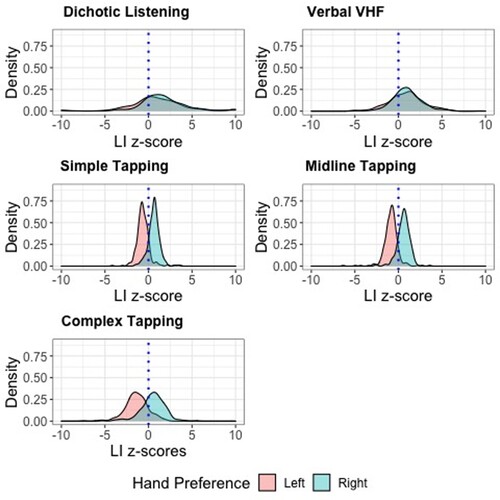
On all three motor tasks, hand preference groups were significantly lateralized in the predicted direction, see . Density plots displaying the distribution of LI z-scores on each task are shown in .
Hypothesis 2
There was no significant association between laterality on the Complex Sequence Tapping Task and laterality on the vVHF Task across hand preference groups, χ2 = 0.74, p = .39. The number of participants who were significantly lateralized on the Complex Sequence Tapping Task and the vVHF Task are displayed in . It is important to consider the observation that the LI z-scores from the vVHF had relatively poor retest reliability meaning that this null relationship is difficult to disentangle from measurement error. In our exploratory analyses we consider the relationship between laterality indices on the Complex Sequence Tapping Task and the Dichotic Listening Task, which typically has stronger retest reliability.
Table 3. Laterality Counts for the vVHF Task and Complex Sequence Tapping Task by Hand Preference Group.
Hypothesis 3
A two-way mixed ANOVA was conducted to investigate the impact of hand preference and task (Simple Sequence Tapping vs Simple Sequence Tapping Midline) on absolute LI z-scores. There was a significant main effect of hand preference, F (1, 546) = 7.81, p = .005, with left-handers having significantly larger absolute LI z-scores (M = .87) than right-handers (M = .76). There was also a significant main effect of task, F (1, 546) = 8.68, p = .003, with larger absolute LI z-scores on the Midline Tapping Task (M = .87) than the Simple Tapping Task (M = .77). Additionally, there was a significant interaction between hand preference and task, F (1,546) = 4.65, p = .031. Violin plots showing raw LI z-scores for each hand preference group on each task are shown in .
Figure 7. Violin Plot Showing LI z-scores for Each Hand Preference Group on the Simple Sequence Tapping Task and the Simple Sequence Tapping Midline Task.
Note. The violin plot displays original LI z-scores before they were transformed into absolute LI z-scores for the ANOVA.
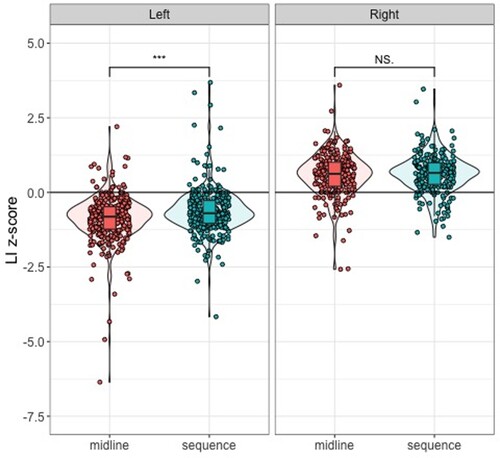
Post-hoc Paired Sample t-tests revealed that while left-handers were more strongly lateralized on the Midline Task (M = .95, SD = .70) compared to the Simple Task (M = .80, SD = .60), t(310) = 3.62, p < .001; right-handers showed no difference in lateralization between the Midline Tapping Task (M = .77, SD = .54) and the Simple Tapping Task (M = .74, SD = .50), t(236) = .58, p = .56.
Post-hoc Independent Sample t-tests revealed that while left-handers were more strongly lateralized on the Midline Task (M = .95, SD = .70) compared to right-handers (M = .77, SD = .54), t(549) = 3.41, p < .001; left-handers absolute LI z-scores on the Simple Task (M = .80, SD = .60) showed no difference to right-handers absolute LI z-scores on the Simple Task (M = .74, SD = .50), t(541) = 1.10, p = .27.
Exploratory analyses
A Cochran-Mantel-Haenszel test was used to test for an association between lateralization on the Complex Sequence Tapping Task and lateralization on the Dichotic Listening Task across hand preference groups. We categorized individuals into groups for each task. Those who were significantly lateralized (with an LI z-score ≥ 1.96 or an LI z-score ≤ −1.96) and those who were not significantly lateralized (with an LI z-score <1.96 and >−1.96). The test revealed no significant association between laterality on the two tasks across hand preference groups, x2 = 0.13, p = .72. The numbers of participants who were significantly lateralized on each task are displayed in .
Table 4. Laterality Counts for the Dichotic Listening Task and Complex Sequence Tapping Task by Hand Preference Group.
Correlations between EHI scores and LI z-scores on each motor task are displayed in .
Table 5. Spearman Correlations Between EHI LI’s and Motor Task LI z-scores.
Discussion
In this study, we used online behavioural testing to examine the relationship between motor laterality and language laterality while removing some of the limitations encountered by previous work. We established good validity and reliability of the behavioural measures and used them to confirm two (1 and 3) of our three preregistered hypotheses.
Reliability and validity of new online measures
Previous work has established good retest reliability for the Dichotic Listening Task when administered in person (r = .78; Bless et al., Citation2013) and online (r = .78; Parker et al., Citation2021). To save time and resources, the Dichotic Listening Task was only administered in session one. Due to similarities in the methods used, and to be consistent with recent work in this area, we examined the retest reliability of the four remaining behavioural tasks based on the cut off for satisfactory reliability set by Parker et al. (Citation2021; r = .65). Based on these standards; the Simple Sequence Tapping Task (r = .83), the Simple Sequence Tapping Midline Task (r = .78) and the Complex Sequence Tapping Task (r = .84) all showed satisfactory reliability. In comparison, McManus et al. (Citation2016) reported retest reliability of r = .83 for The Tapley and Bryden test (Tapley & Bryden, Citation1985) and r = .60 for a pegboard task when administered in person. In summary, the novel motor laterality measures showed excellent reliability and exceeded the reliability of the widely used pegboard task. The reliability of the Verbal Visual Half-Field Task (r = .62) was below the threshold of .65 and therefore cannot be considered satisfactory.
Given previous studies showing that hand skill correlates well with hand preference (e.g., Steenhuis, Citation1999), we assessed the validity of the motor tasks by testing whether participants were lateralized in concordance with their hand preference group, as indicated by EHI scores (left and right). We found that both hand preference groups were significantly lateralized in the predicted direction on all three tasks. Additionally, we found that laterality indices on the Complex Sequence Tapping Task correlated well with laterality indices on the pegboard task on which it was based (r = .73), suggesting that the online task offers a useful alternative to the in-person measure. In summary, all three motor tasks were shown to be valid measures of motor laterality.
Based on findings from many lab studies highlighting a right ear advantage among left- and right-handers (e.g., Bless et al., Citation2015), we examined the validity of the online Dichotic Listening Task by testing whether there was a significant right ear advantage at population level. We found a significant right ear advantage, suggesting left hemispheric dominance for speech perception, at population level and within each hand preference group. Further inspection revealed that the proportion of those with a right ear advantage in each group varied somewhat from those observed in a recent experiment with in-person testing. In the current study, 63% of left-handers showed a right ear advantage and 76% of right-handers did. In contrast, Karlsson et al. (Citation2019) found that 78% of non-right handers and 84% of right-handers had a right ear advantage. Karlsson et al. (Citation2019) also completed a meta-analysis on 57 studies that administered a dichotic listening task. Across all 57 studies, the proportions of those in each handedness group with a right ear advantage were similar to those observed in the current study, with 81% (76% in current study) of right-handers and 65% (63% in current study) of non-right handers showing a right ear advantage. It is important to note that the way in which hand preference was categorized varied between the two studies. In fact, suggestions on how hand preference should be categorized vary widely; for example, Papadatou-Pastou et al. (Citation2020) suggests a three-group approach (left-handers, mixed-handers, and right handers) and Annett (Citation2002) suggests using a continuum instead of categories. This variation may explain the discrepancy between the proportions of those with a right ear advantage within each hand preference group across studies. As the online Dichotic Listening Task was lateralized in the predicted direction, we concluded that the task was a valid measure of receptive language laterality.
We predicted that there would be a right visual-half field advantage on the vVHF task at population level, suggesting left hemispheric dominance for speech production. This was based on Van der Haegen et al. (Citation2011) who found a right VHF advantage on two naming tasks among left- and right-handers. Although the vVHF was lateralized in the predicted direction (right VHF advantage) at population level and within each hand preference group, the proportion of participants with a right VHF advantage was lower than expected. In the current sample, 67% of left-handers and 75% of right-handers showed a right visual half-field advantage. In contrast, overt speech production has been shown to be left-lateralized in around 70% of left-handers and 95% of right-handers (e.g., Knecht et al., Citation2000).
The less satisfactory reliability and more questionable validity of the vVHF task may have been caused, in part, by its dependency on tightly controlled viewing conditions. The vVHF is dependent on presenting lateralized visual stimuli that project to one hemisphere. To achieve this optimally, participants must sit directly facing the screen, at a certain viewing distance and preferably in a chin rest. Although participants were instructed to sit at the correct viewing distance and avoid head movements, it was hard to ensure they were compliant given the nature of online testing. Van der Haegen and Brysbaert (Citation2018) reported that visual half-field paradigms using pictures can yield test-retest reliability of r = .77 when administered in-person. This is likely due to the more closely controlled environment with in-person testing. The lack of control over viewing conditions on this task may also explain the unexpectedly low proportion of participants with a right VHF advantage. If images are not equally displaced in parafoveal vision due to head movements, then one image may be processed more efficiently because of this. This would result in laterality indices reflecting viewing conditions rather than a hemispheric processing advantage. Future work may wish to present images in peripheral vision, as this is less likely to be impacted by slight movements of the head or changes in viewing conditions.
Relationship between motor laterality and language laterality
In contrast to findings reported by Flowers and Hudson (Citation2013), we found no association between sequence-based motor laterality and language laterality. There are several differences between the design of the current study and that of Flowers and Hudson (Citation2013). Firstly, a non-clinical sample was used here, and a clinical sample was used in the earlier study. The lateralization of the epilepsy cohort in Flowers and Hudson (Citation2013) may have been impacted by their lesions, and this could have been a confounding factor. Secondly, the task used here to measure speech production laterality (the vVHF task) showed relatively poor reliability, whereas Flowers and Hudson (Citation2013) used the highly accurate and reliable Wada test (Wada, Citation1949). Additionally, Flowers and Hudson (Citation2013) placed participants’ into motor laterality groups using arbitrary cutoffs, whereas the z-score method was used in the current study.
The absence of an association between speech production laterality and motor laterality in the current study is still surprising given the presence of one in several other studies (e.g., Hodgson et al., Citation2019; Hodgson et al., Citation2021). However, all three studies discussed (Flowers & Hudson, Citation2013; Hodgson et al., Citation2019; Hodgson et al., Citation2021) employed the pegboard task, which differs in demands to the Complex Sequence Tapping Task. Firstly, it is worth noting the greater degree of precision required on the pegboard task compared to the Complex Tapping Task. This is because the size of the targets is not consistent between the tasks, with keyboard keys being much larger than the holes on the pegboard task. Investigating whether the degree of precision required on motor tasks influences their association with language laterality is an interesting avenue for future research. This could be done using online mouse-based tasks in which participants “hit” targets of various sizes. However, as the design of mice tend to vary widely, it may be difficult to control for variation in the equipment used by participants.
Another difference between the Complex Tapping Task and the pegboard task is that there is a requirement to grip and release the pegs on the pegboard task, which is not present on the task used here. It may be that the sequencing contained within this component is driving the pegboard's common processing requirements with speech. This is supported by evidence that grip and release tasks, both sequential and non-sequential, show increased left hemispheric activation in neurotypical individuals compared to tasks without this element (Hayashi et al., Citation2008). Hodgson et al. (Citation2021) also demonstrated that when the grip and release component was removed from the pegboard task, it no longer correlated with speech laterality, reinforcing the notion that this is the key component driving the relationship. Future research may wish to focus on why this component may be a key factor when explaining the links between motor laterality and language laterality, as this remains somewhat unclear. Designing an online task with a grip and release element would be almost impossible due to the need for additional equipment. This would make testing the importance of such a component very difficult to achieve with online testing. It is, however, interesting that within our sample, the Complex Sequence Tapping Task did elicit similar motor laterality patterns to the pegboard task used by Flowers and Hudson (Citation2013). It is also worth highlighting that the current study was well powered and sample size far exceeded that of previous studies highlighting an association between sequence-based motor laterality and speech production laterality.
It is also possible that language lateralization may not be entirely a unitary domain; whereby different functions are lateralized independently (e.g., Woodhead et al., Citation2019). If functions are independently lateralized, more thoughtful selection of tasks may be required to ensure that common components exist if we are to replicate key findings. Although we report a null association between vVHF laterality and motor laterality, we acknowledge that this may be a consequence of measurement error. We also ran exploratory analyses to test for an association using the highly reliable Dichotic Listening Task; although this task does not involve speech generation, it is weakly correlated with language production laterality (Parker et al., Citation2022). This too showed a null association with sequence-based motor laterality. This is less surprising given the receptive nature and lack of sequencing required for successful execution of the Dichotic Listening Task (Hickok & Poeppel, Citation2016). This finding is in line with Flowers and Hudson’s suggestion that sequential control in either speech or praxis is the key factor driving associations across domains.
Motor laterality and midline crossing
Steenhuis (Citation1999) concluded that the nature of the preference and performance measures one uses must be carefully considered before a clear description of handedness can be provided. One interesting finding reported in the same paper and taken from an earlier study (Bryden et al., Citation1994) was that when completing modified elongated motor tasks, participants showed more willingness to use their preferred hand compared to their non-preferred hand when operating across their midline. However, little research has focussed on how midline crossing impacts the ability of the hands. We found that those with a left-hand preference showed a significantly larger preferred hand advantage when operating across the midline; yet this effect was not observed in those with a right-hand preference. Further inspection revealed that left-handers’ change in motor laterality when operating across their midline was driven by an improvement in left hand performance, suggesting that they are more skilled at using their preferred hand in contralateral space. This is a novel finding which extends theoretical accounts of motor laterality.
A practical explanation of this observation is that many tools and devices are built with right-handers in mind. This results in left-hander’s being more practised in operating in contralateral space compared to right-handers (Masud & Ajmal, Citation2012). Another possibility is that the ability of the hands is influenced by the location of the visual information required to execute the task. Testing this suggestion is beyond the scope of the current study but future work could examine how motor laterality is impacted by an interaction between location of task (left visual field vs right visual field) and the hand being tested.
Further observations on motor laterality measures
Studies investigating the concordance between preference- and performance-based measures of hand preference have produced mixed results. Findings range from strong concordance (Steenhuis, Citation1999), to weak correlations (Groen et al., Citation2013), to no difference in performance between preference groups (Bishop et al., Citation1996). Our exploratory analyses revealed that laterality indices on the Simple Sequence Tapping Task and midline variation of that task correlated well with EHI scores. There was a moderate correlation between Complex Sequence Tapping laterality indices and EHI scores. This suggests that task difficulty may influence agreement between performance-based measures and preference measures, with more difficult tasks correlating less well with preference measures. This may reflect the depth of processing involved in each task, with simpler tasks requiring less support for successful execution. Most of the tasks on the EHI are all relatively simple tasks (e.g brushing teeth) and are more closely matched in processing demands to the Simple Tapping Tasks than the Complex Tapping Task which may explain the observed correlations.
One limitation of the motor tasks was the lack of control over hand usage and keyboard position. One way to improve this would be to monitor participants via online video calls or video recordings. However, this would be time consuming and could slow down data collection. Future online research measuring motor laterality should aim to strike a balance between improving online experimental control without jeopardizing the benefits of online testing (e.g., large samples and accessibility).
Conclusion
The current study compiled an online battery of tasks that can be used to examine language and motor laterality. We highlighted excellent validity and reliability for most tasks, which should allow researchers to administer the online battery with confidence. Not only are these tasks made available online for reuse, but they are also simple to programme across a variety of web-based platforms. Furthermore, we found no evidence of an association between motor laterality and language laterality. While this lack of association could capture measurement error for the relationship between motor laterality and speech production laterality, this is less likely for receptive language laterality. This emphasizes the importance of using a battery of reliable tasks which assess a range of language and fine motor skill components when investigating the link between language and motor laterality.
Data availability
This study was preregistered on the Open Science Framework (OSF) before the start of data collection. The registration form can be found on the OSF at https://osf.io/3ungd. Working versions of the speech production task and all three motor tasks can be found on Gorilla Open Materials at https://app.gorilla.sc/openmaterials/278292.
The analysis scripts and anonymised data that support the findings of this study are openly available in “Motor study data and scripts” at https://osf.io/eazdy/.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Annett, M. (1970). The growth of manual preference and speed. British Journal of Psychology, 61(4), 545–558. https://doi.org/10.1111/j.2044-8295.1970.tb01274.x
- Annett, M. (2002). Handedness and brain asymmetry: The right shift theory. Psychology Press.
- Annett, M., Hudson, P. T. W., & Turner, A. (1974). The reliability of differences between the hands in motor skill. Neuropsychologia, 12(4), 527–531. https://doi.org/10.1016/0028-3932(74)90083-9
- Anwyl-Irvine, A. L., Massonnié, J., Flitton, A., Kirkham, N., & Evershed, J. K. (2020). Gorilla in our midst: An online behavioral experiment builder. Behavior Research Methods, 52(1), 388–407. https://doi.org/10.3758/s13428-019-01237-x
- Bishop, D. V. M., Ross, V. A., Daniels, M. S., & Bright, P. (1996). The measurement of hand preference: A validation study comparing three groups of right-handers. British Journal of Psychology, 87(2), 269–285. https://doi.org/10.1111/j.2044-8295.1996.tb02590.x
- Bless, J. J., Westerhausen, R., Arciuli, J., Kompus, K., Gudmundsen, M., & Hugdahl, K. (2013). “Right on all occasions?” – On the feasibility of laterality research using a smartphone dichotic listening application. Frontiers in Psychology, 4, 42. https://doi.org/10.3389/fpsyg.2013.00042
- Bless, J. J., Westerhausen, R., Torkildsen, J. V. K., Gudmundsen, M., Kompus, K., & Hugdahl, K. (2015). Laterality across languages: Results from a global dichotic listening study using a smartphone application. Laterality: Asymmetries of Body, Brain and Cognition, 20(4), 434–452. https://doi.org/10.1080/1357650X.2014.997245
- Bradshaw, A. R., Thompson, P. A., Wilson, A. C., Bishop, D. V., & Woodhead, Z. V. (2017). Measuring language lateralisation with different language tasks: a systematic review. PeerJ, 5, e3929. https://doi.org/10.7717/peerj.3929
- Bruckert, L., Thompson, P. A., Watkins, K. E., Bishop, D. V. M., & Woodhead, Z. V. J. (2021). Investigating the effects of handedness on the consistency of lateralization for speech production and semantic processing tasks using functional transcranial Doppler sonography. Laterality, 26(6), 680–705. https://doi.org/10.1080/1357650X.2021.1898416
- Bryden, M. P., Singh, M., Steenhuis, R. E., & Clarkson, K. L. (1994). A behavioral measure of hand preference as opposed to hand skill. Neuropsychologia, 32(8), 991–999. https://doi.org/10.1016/0028-3932(94)90048-5
- Brysbaert, M. (1994). Interhemispheric transfer and the processing of foveally presented stimuli. Behavioural Brain Research, 64(1-2), 151–161. https://doi.org/10.1016/0166-4328(94)90127-9
- Carey, D. P., & Johnstone, L. T. (2014). Quantifying cerebral asymmetries for language in dextrals and adextrals with random-effects meta analysis. Frontiers in Psychology, 5, 1128. https://doi.org/10.3389/fpsyg.2014.01128
- Champely, S. (2020). pwr: Basic functions for power analysis [R package version 1.3-0]. https://cran.r-project.org/web/packages/pwr/index.html.
- Egeler, P. (2017). samplesizeCMH: Power and sample size calculation for the cochran-mantel-haenszel Test [R package version 0.0.0]. https://cran.r-project.org/web/packages/samplesizeCMH/samplesizeCMH.pdf.
- Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47(6), 381. https://doi.org/10.1037/h0055392
- Flinker, A., Korzeniewska, A., Shestyuk, A. Y., Franaszczuk, P. J., Dronkers, N. F., Knight, R. T., & Crone, N. E. (2015). Redefining the role of Broca’s area in speech. Proceedings of the National Academy of Sciences, 112(9), 2871–2875. https://doi.org/10.1073/pnas.1414491112
- Flowers, K. A., & Hudson, J. M. (2013). Motor laterality as an indicator of speech laterality. Neuropsychology, 27(2), 256. https://doi.org/10.1037/a0031664
- Frost, J. A., Binder, J. R., Springer, J. A., Hammeke, T. A., Bellgowan, P. S., Rao, S. M., & Cox, R. W. (1999). Language processing is strongly left lateralized in both sexes: Evidence from functional MRI. Brain, 122(2), 199–208. https://doi.org/10.1093/brain/122.2.199
- Grimme, B., Fuchs, S., Perrier, P., & Schöner, G. (2011). Limb versus speech motor control: A conceptual review. Motor Control, 15(1), 5–33. https://doi.org/10.1123/mcj.15.1.5
- Groen, M. A., Whitehouse, A. J., Badcock, N. A., & Bishop, D. V. (2013). Associations between handedness and cerebral lateralisation for language: A comparison of three measures in children. PLoS One, 8(5), e64876. https://doi.org/10.1371/journal.pone.0064876
- Haaland, K. Y., & Harrington, D. L. (1996). Hemispheric asymmetry of movement. Current Opinion in Neurobiology, 6(6), 796–800. https://doi.org/10.1016/S0959-4388(96)80030-4
- Hayashi, M. J., Saito, D. N., Aramaki, Y., Asai, T., Fujibayashi, Y., & Sadato, N. (2008). Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: A functional magnetic resonance imaging study. Cerebral Cortex, 18(12), 2932–2940. https://doi.org/10.1093/cercor/bhn053
- Häberling, I. S., Corballis, P. M., & Corballis, M. C. (2016). Language, gesture, and handedness: Evidence for independent lateralized networks. Cortex, 82, 72–85. https://doi.org/10.1016/j.cortex.2016.06.003
- Hickok, G., & Poeppel, D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. https://doi.org/10.1038/nrn2113
- Hickok, G., & Poeppel, D. (2016). Neural basis of speech perception. Neurobiology of Language, 299–310. https://doi.org/10.1016/B978-0-12-407794-2.00025-0
- Hoaglin, D. C., & Iglewicz, B. (1987). Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association, 82(400), 1147–1149. https://doi.org/10.1080/01621459.1987.10478551
- Hodgson, J. C., & Hudson, J. M. (2018). Speech lateralization and motor control. Progress in Brain Research, 238, 145–178. https://doi.org/10.1016/bs.pbr.2018.06.009
- Hodgson, J. C., Richardson, D., & Hudson, J. M. (2021). The relationship between lateralization patterns from sequence based motor tasks and hemispheric speech dominance. Neuropsychology, 35(2), 157. https://doi.org/10.1037/neu0000702
- Hodgson, J. C., Tremlin, R., & Hudson, J. M. (2019). Disrupting the speech motor network: Exploring hemispheric specialization for verbal and manual sequencing using a dual-task approach. Neuropsychology, 33(8), 1101. https://doi.org/10.1037/neu0000589
- Hunter, Z. R., & Brysbaert, M. (2008). Visual half-field experiments are a good measure of cerebral language dominance if used properly: Evidence from fMRI. Neuropsychologia, 46(1), 316–325. https://doi.org/10.1016/j.neuropsychologia.2007.07.007
- Karlsson, E. M., Johnstone, L. T., & Carey, D. P. (2019). The depth and breadth of multiple perceptual asymmetries in right handers and non-right handers. Laterality: Asymmetries of Body, Brain and Cognition, 24(6), 707–739. https://doi.org/10.1080/1357650X.2019.1652308
- Knecht, S., Dräger, B., Deppe, M., Bobe, L., Lohmann, H., Flöel, A., Ringelstein, E. B., & Henningsen, H. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123(12), 2512–2518. https://doi.org/10.1093/brain/123.12.2512
- Lemhöfer, K., & Broersma, M. (2012). Introducing LexTALE: A quick and valid lexical test for advanced learners of English. Behavior Research Methods, 44(2), 325–343. https://doi.org/10.3758/s13428-011-0146-0
- Masud, Y., & Ajmal, M. A. (2012). Left-handed people in a right-handed world: A phenomenological study. Pakistan Journal of Social and Clinical Psychology, 10(1), 49–60.
- McManus, I. C., Van Horn, J. D., & Bryden, P. J. (2016). The Tapley and Bryden test of performance differences between the hands: The original data, newer data, and the relation to pegboard and other tasks. Laterality: Asymmetries of Body, Brain and Cognition, 21(4-6), 371–396. https://doi.org/10.1080/1357650X.2016.1141916
- Ocklenburg, S., & Gunturkun, O. (2017). The lateralized brain: The neuroscience and evolution of hemispheric asymmetries. Academic Press.
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. https://doi.org/10.1016/0028-3932(71)90067-4
- Packheiser, J., Schmitz, J., Arning, L., Beste, C., Güntürkün, O., & Ocklenburg, S. (2020). A large-scale estimate on the relationship between language and motor lateralization. Scientific Reports, 10(1), 1–10. https://doi.org/10.1038/s41598-019-56847-4
- Papadatou-Pastou, M., Ntolka, E., Schmitz, J., Martin, M., Munafò, M. R., Ocklenburg, S., & Paracchini, S. (2020). Human handedness: A meta-analysis. Psychological Bulletin, 146(6), 481.
- Parker, A. J., Woodhead, Z. V., Carey, D. P., Groen, M. A., Gutierrez-Sigut, E., Hodgson, J., Hudson, J., Karlsson, E. M., MacSweeney, M., Payne, H., & Simpson, N. (2022). Inconsistent language lateralisation – testing the dissociable language laterality hypothesis using behaviour and lateralised cerebral blood flow. Cortex, 154, 105–134. https://doi.org/10.1016/j.cortex.2022.05.013
- Parker, A. J., Woodhead, Z. V., Thompson, P. A., & Bishop, D. V. (2021). Assessing the reliability of an online behavioural laterality battery: A pre-registered study. Laterality: Asymmetries of Body, Brain and Cognition, 26(4), 359–397. https://doi.org/10.1080/1357650X.2020.1859526
- Parsons, S., Kruijt, A. W., & Fox, E. (2019). Psychological science needs a standard practice of reporting the reliability of cognitive-behavioral measurements. Advances in Methods and Practices in Psychological Science, 2(4), 378–395. https://doi.org/10.1177/2515245919879695
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. URL https://www.R-project.org/.
- Richards, G., Medland, S. E., & Beaton, A. A. (2021). Digit ratio (2D:4D) and handedness: A meta-analysis of the available literature. Laterality: Asymmetries of Body, Brain and Cognition, 26(4), 421–484. https://doi.org/10.1080/1357650X.2020.1862141
- Roux, F., Armstrong, B. C., & Carreiras, M. (2017). Chronset: An automated tool for detecting speech onset. Behavior Research Methods, 49(5), 1864–1881. https://doi.org/10.3758/s13428-016-0830-1
- Roy, E. A., Bryden, P., & Cavill, S. (2003). Hand differences in pegboard performance through development. Brain and Cognition, 53(2), 315–317. https://doi.org/10.1016/S0278-2626(03)00133-7
- Schulze, K., Lüders, E., & Jäncke, L. (2002). Intermanual transfer in a simple motor task. Cortex, 38(5), 805–815. https://doi.org/10.1016/S0010-9452(08)70047-9
- Steenhuis, R. E. (1999). The relation between hand preference and hand performance: What you get depends on what you measure. Laterality: Asymmetries of Body, Brain and Cognition, 4(1), 3–26. https://doi.org/10.1080/713754324
- Tapley, S. M., & Bryden, M. P. (1985). A group test for the assessment of performance between the hands. Neuropsychologia, 23(2), 215–221. https://doi.org/10.1016/0028-3932(85)90105-8
- Van der Haegen, L., & Brysbaert, M. (2018). The relationship between behavioral language laterality, face laterality and language performance in left-handers. PloS one, 13(12), e0208696. https://doi.org/10.1371/journal.pone.0208696
- Van der Haegen, L., Cai, Q., Seurinck, R., & Brysbaert, M. (2011). Further fMRI validation of the visual half field technique as an indicator of language laterality: A large-group analysis. Neuropsychologia, 49(10), 2879–2888. https://doi.org/10.1016/j.neuropsychologia.2011.06.014
- Wada, J. (1949). A new method of determining the side of cerebral speech dominance: A preliminary report on the intracarotid injection of sodium amytal in man. Igaku to Seibutsugaku, 14, 221–222.
- Woodhead, Z. V. J., Bradshaw, A. R., Wilson, A. C., Thompson, P. A., & Bishop, D. V. (2019). Testing the unitary theory of language lateralization using functional transcranial Doppler sonography in adults. Royal Society Open Science, 6(3), 181801. https://doi.org/10.1098/rsos.181801
- Woodhead, Z. V. J., Thompson, P. A., Karlsson, E. M., & Bishop, D. V. M. (2021). An updated investigation of the multidimensional structure of language lateralization in left- and right-handed adults: A test–retest functional transcranial Doppler sonography study with six language tasks. Royal Society Open Science, 8(2), 200696. https://doi.org/10.1098/rsos.200696