ABSTRACT
Objectives: Both advanced age and depression are characterized by changes in sleep patterns. Light exposure is one of the main synchronizers of circadian cycles and influences sleep by inhibiting melatonin secretion, which is mostly sensitive to light of low wavelengths (blue). Blue-blocking (yellow) intraocular lenses (IOLs) have supplanted the usual UV-blocking (clear) IOLs during cataract surgery to prevent age-related macular degeneration, however, the impact of yellow IOLs on sleep and mood is unclear. The purpose of this study was to compare the effects of yellow and clear IOLs on sleep and mood in aged patients undergoing bilateral cataract surgery.
Methods: A randomized controlled superiority study was conducted within three ophthalmic surgical wards in France. A total of 204 subjects (mean age 76.2 ± 7.5 years) were randomized into yellow or clear IOLs groups. Patients completed a sleep diary, the pictorial sleepiness scale and the Beck Depression Inventory (BDI) one week before and eight weeks after the last surgical procedure.
Results: According to an Intent To Treat (ITT) analysis, no significant difference was found between yellow and clear IOLs groups regarding sleep time, sleep latency, total sleep duration, quality of sleep and BDI scores. The rate of patients whose BDI score increased at the cutoff score of ≥5 after surgery was significantly higher in the yellow IOL group (n = 11, 13.1%) compared with the clear IOL group (n = 4; 4.7%); p = 0.02.
Conclusions: Using yellow IOLs for cataract surgery doesn't significantly impact sleep but may induce mood changes in aging.
Introduction
Depression is one of the most frequently encountered psychiatric conditions in patients of advanced age, with an estimated prevalence in the primary care setting of 2%–4% for major depression and up to 10%–15% for sub-syndromal depression (Beekman, Copeland, & M.J., Citation1999). Depression in advanced age is associated with increased mortality (Penninx et al., Citation1999), functional impairment (Lyness, King, Cox, Yoediono, & Caine, Citation1999), increased health care use (Pickett et al., Citation2014). Several risk factors have been identified as being associated with depression among patients of advanced age: age greater than 85, female gender, a past history of depression, existing sleep disturbances and impaired functional status (Cole & Dendukuri, Citation2003) as well as smoking and other vascular risk factors (Rozanski, Blumenthal, Davidson, Saab, & Kubzansky, Citation2005; Weyerer et al., Citation2008). Visual impairment also appears to be a significant risk factor for depression among patients of advanced age (Court, McLean, Guthrie, Mercer, & Smith, Citation2014). Cataracts, which are the most prevalent cause of visual impairment among aging individuals, are also associated with changes in circadian rhythms and mood (Kim, Jung, & Song, Citation2012).
During cataract surgery, the darkened natural lens is removed and replaced with an artificial intra ocular lens (IOL), usually placed into the capsular bag. IOLs typically block UV light, but IOLs that also filter blue or purple light are now routinely available. These yellow IOLs were initially developed to improve contrast sensitivity, protect the retina from damage resulting from short wavelength light and prevent age-related macular degeneration (Davison, Patel, Cunha, Schwiegerling, & Muftuoglu, Citation2011; Mainster, Citation2006; Mainster & Turner, Citation2010). However, yellow IOLs exert a decreased effect of photo-entrainment potential but to a significantly lesser extent that the aging of natural lenses (Brøndsted, Lundeman, & Kessel, Citation2013). Several studies have already demonstrated that UV and blue light filtering IOLs do not negatively impact the quality of vision and quality of life (Espindle et al., Citation2005; Mester, Holz, Kohnen, Lohmann, & Tetz, Citation2008). It has been suggested that blue light exposure could exhibit detrimental effects in aged individuals, in particular on parameters that can affect mood such as sleep-wake cycles. For example, evening exposure to the blue light of the screens of electronic devices induces a delayed phase shift, a decreased duration of Rapid Eye Movement (REM) sleep and an altered quality of sleep (Chang, Aeschbach, Duffy, & Czeisler, Citation2015). Similarly, a brief evening exposure (2H00) to blue light induces changes in the non REM Electro-Encephalogram (EEG) power density of frontal regions, which can be interpreted as an alerting effect of short wavelength light (Chellappa et al., Citation2013). Using yellow IOLs during cataract surgery may consequently counteract these potentially negative effects. To our knowledge, only three non-randomized studies have compared the effects of clear and yellow IOLs on circadian rhythms with a focus on sleep. One study involved 34 patients (Leruez et al., Citation2015), another study included 49 patients (Landers, Tamblyn, & Perriam, Citation2009), and another larger study included 961 patients (Alexander et al., Citation2014). None of these studies reported differences regarding quality of life or quality of sleep between patients in the clear or yellow IOLs groups. Only few prospective and randomized trials have been published, and these studies did not detect any difference between clear and yellow IOLs regarding sleep patterns (Brøndsted et al., Citation2015; Schmoll et al., Citation2014). However, no studies have simultaneously assessed sleep and mood changes before and after the surgical procedure. Taken together, these data support the rationale to evaluate the effects of yellow IOLs on sleep and mood changes among elderly subjects undergoing cataract surgery. Consequently, the aim of the present study was to demonstrate the superiority of yellow IOLs as compared with clear IOLs on sleep and mood changes before and after bilateral cataract surgery.
Methods
Setting
The study was performed in two French university hospitals (CHU de Tours, CHU de Brest) and a general hospital (Loches) where the data were collected. The study was funded by the French Ministry of Health under grand number 2009-API-10-47) sponsored by the CHRU de Tours (Tours University Hospital) and approved by the French National Drug Safety Agency (Agence National de Sécurité du médicament- ANSM) and the local ethics committee in accordance with bioethics laws (Comité de Protection des Personnes—CPP of Tours, France). The study was registered in the French Ministry of Health database of institutional prospective study (ID Number: PHRI09-PJP / IOL et sommeil, EudraCT registration number RCB 2009-A00719-48) and in the ClinicalTrials.gov database (NCT02304900).
Patients and study population
To be included in the study, patients were required to be 60–90 years old, speak and read French, and have bilateral cataracts (to be eligible for surgery). Patients were first pre-screened by an ophthalmologist for the diagnosis of cataracts and an assessment of surgical criteria. Patients with a best-corrected visual acuity of ≤20/32 in each eye were diagnosed as having symptomatic visual loss. Visual acuity was measured with the Monoyer chart at 5 m and converted to Snellen acuity. Patients with another ophthalmologic disorder, sleep complaints or disorders, or patients who received continuous and stable psychotropic treatment (benzodiazepine or Z drug) were not excluded. Patients underwent the initial protocol procedure 7 days after providing written informed consent.
Surgical procedures
Patients were randomized with an internet-based program while in the operating room and were divided into two groups depending on the IOL used: the ‘yellow’ (blue-blocking IOL) group and the ‘clear’ (UV-blocking IOL) group. Patients (but not surgeons) were blind to the elected intervention. Other assessors, and the authors of this study, were also blinded to the group assignment. The statistician was not blinded. Each center chose implants that were available, and these IOLs were handled according to standard practices in each center. The only restriction in the choice of implant was the version of implant. The implant was required to exist in various sizes with at least one version (3-pieces) over 12.5 mm in diameter for cases of sulcus implantation (without changing the IOL light filter). For clear IOLs, surgeons used: Hoya AF-1VA60BB, AF-1 VA65BB, Alcon SN 60WF or MN60AC. For yellow IOLs, surgeons used: Hoya PY 60AD, AF-1 YA 65BB, Alcon SA 60AT or MA50BM. Experienced surgeons performed the cataract surgeries and IOL insertions. The procedure consisted of phacoemulsification and aspiration followed by the fixation of an intra-capsular IOLs under topical, regional or general anesthesia. The delay between the first and second procedures was between one to three months ().
Data collection and outcome measures
For all pre-screened patients, initial information included: age, gender, ophthalmologic history and comorbidities (such as glaucoma, age-related macular degeneration [AMD] or diabetic retinopathy), best corrected visual acuity for both eyes, biometric data to calculate the required refractive power of the IOL, and results from the biomicroscopic and fundus examinations.
Sleep was evaluated with a pictorial sleepiness scale (Maldonado, Bentley, & Mitchell, Citation2004) and a sleep diary as recommended by the American Academy of Sleep Medicine (Morgenthaler et al, Citation2007). The pictorial sleepiness scale is based on drawings and rates the quality of sleep, ranked from 0 (not sleepy, not tired) to 4 (really sleepy, tired). Each patient was instructed to complete a sleep diary every day for one week for two time periods. The first period occurred one week before the first surgery, and the second period occurred 2 months after the second surgery. Each day, sleep time, sleep latency, total sleep duration and quality of sleep were evaluated by the patient. Changes prior to and following surgery were calculated for each patient from the average values after surgery and the average values before surgery. The primary outcome variable was the change in average sleep time.
The level of depressive symptomatology was assessed with the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock, & Erbaugh, Citation1961), which is a 13-item self-administered questionnaire that is reliable in aged patients and has been previously used to test the effect of cataract surgery on mood and depression (Ishii, Kabata, & Oshika, Citation2008). The highest possible score for the questionnaire is 39, and a cutoff score of 5 or higher is consistent with a high probability of having mild to moderate depression (Beck & Beamesderfer, Citation1974).
Statistical analyses
The study was designed as a randomized superiority trial. Considering the sleep time as the primary outcome variable, a sample size of 400 patients (200 in each arm) would have provided 90% power to detect a 30 min difference in the change of average sleep time between the two groups (considering a standard deviation (SD) of 92 min).
The randomization sequence was created by a statistician using SAS software and was stratified by center with a 1:1 allocation using random block sizes.
Quantitative variables are described as the means and SD. The Intent To Treat (ITT) population comprised all randomized patients except those who withdrew their consent to participate in the study (as required by French legislation). In cases of missing outcome data, we performed a multiple imputation using baseline features, medical center and treatment group. We used a linear regression model to evaluate the difference in means for primary and secondary outcomes. The BDI score was transformed into a binary variable. During the post-surgery assessment, the patients were considered as ‘depressed’ when the BDI score was 5 or higher (or ‘not depressed’ if less than 5). A patient was considered ‘improved’ when moving from the ‘depressed’ group to the ‘non-depressed’, ‘deteriorated’ when moving from the ‘non-depressed’ to the ‘depressed’ group, and ‘unchanged’ otherwise. A Fisher's exact test was performed on the changes in patient categorization with regard to the BDI scores, to compare values between the two IOL groups.
No interim analysis was performed; therefore, all analyses were performed with a type-I error of 0.05. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina, USA) and R 2.15.2 (R Development Core Team. R: A language and environment for statistical computing R).
Results
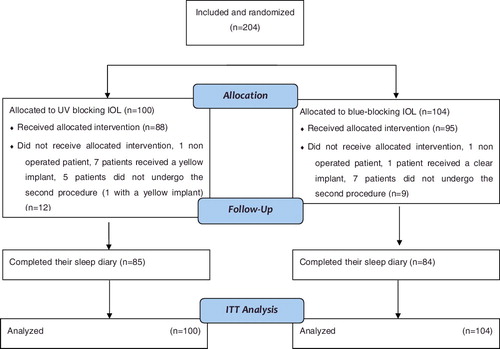
Overall, 204 participants were randomized among the three centers: 104 in the yellow IOL group and 100 in the clear IOL group () from 1 February 2010 to 31 March 2011. Considering that the rate of inclusion was twice as low as expected and that the investigator (OZ) that included most of the recruited patients left the institution, the recruitment was ended at the end of March 2011. Baseline characteristics did not differ between groups (). A total of 169 patients filled out the sleep diary every day for a week with the sleep time information included. The demographic characteristics of the participants who did not complete the study were similar to those who completed the study. The primary sleep and mood characteristics in pre- and post-surgical periods in both the clear and yellow groups are reported in . The results of the linear regression model comparing the yellow group to the clear group (ITT analysis) are reported in .
Table 1. Characteristics of the patients at baseline. Qualitative variables are expressed as n (%) and quantitative variables as the mean and SD.
Table 2. Baseline and post-surgery characteristics of sleep and mood patterns in clear and yellow IOL groups (mean +/– SD).
Table 3. Mean changes in sleep and mood scores and linear regression model comparing two groups (ITT analysis).
Mean change of average sleep time after surgery was –4.5 ± 27.1 min for the clear group and –4.6 ± 30.2 min for the yellow group. In the intention-to-treat analysis of the sleep time defined as the primary outcome measure based on a linear regression model, change in average sleep time did not differ significantly between the two groups δyellow vs clear = 0.17 [–8.1; 8.5]; p = 0.98. The median change of average total sleep latency after surgery was 5.3 ± 50.5 min in the clear group and 6.7 ± 44.8 min in the yellow group. There were no differences in changes between the two groups, δyellow vs clear = 1.7 [–12.2; 15.5]; p = 0.81. The mean change in sleep latency was –3.6 ± 22.1 min for the clear group and –2.5 ± 25.9 min for the yellow group. There were no differences in the change between the yellow and clear group, δyellow vs clear = 0.5 [–7.5; 6.5]; p = 0.8.
Regarding psychotropic drug consumption, 58 patients (28.4%) were regular users, mostly of a benzodiazepine or a z-drug alone (45 among these 58 patients). Psychotropic drug consumption remained unchanged before and after surgery in the yellow (n = 98) and clear (n = 91) IOL groups (Fischer test, p = 0.64).
The BDI score was similar before and after surgery in both groups (mean change was 0.2 ± 2.5 in the clear group and 0.5 ± 2.3 in the yellow group). No differences were detected between the two groups, δyellow vs clear = 0.35 [–0.57; 0.80]; p = 0.75. When using a cutoff score of ≥5 for the BDI to classify patients as not depressed or depressed, the rate of patients who were classified as not depressed at baseline whose BDI score increased at the cutoff score of ≥5 after surgery was significantly higher in the yellow IOL group (n = 11, 13.1%) compared to the clear IOL group (n = 4; 4.7%); p = 0.02 ().
Table 4. Evolution of BDI score after surgery (improved means that a patient who had a BDI score ≥5 at baseline had a score < 5 after surgery; deteriorated means that a patient who had a BDI score < 5 at baseline had a score ≥5 after surgery).
Discussion
Unexpectedly, no significant differences were detected in terms of sleep among aged patients undergoing bilateral cataract surgery with regard to the type of IOLs the patients received. This is in line with the results of a recent study that included more than 1000 patients and failed to find any differences in terms of overall quality of sleep between UV-blocking and blue filtering IOLs (Alexander et al., Citation2014). In this study, the overall wake-related daytime function was improved in both groups one month after surgery and remained stable for over 12 months. However, this study was not randomized. Another recent prospective non randomized study included a similar number of 200 subjects and reported a higher sleep latency score at the Pittsburgh Sleep Questionnaire Inventory (PSQI) in yellow IOLs group (Ayaki, Negishi, Suzukamo, & Tsubota, Citation2015). A few other randomized studies that evaluated changes in sleep and circadian rhythms with regard to the type of IOL filter were recently published. The sample size was limited, respectively, to 76 (Brøndsted et al., Citation2015) and 80 (Schmoll et al., Citation2014) patients, and none of these studies found any differences between yellow and clear IOLs on circadian rhythms while using less subjective endpoints, such as post-illumination pupil response (PIPR) to blue light after light exposure, actimetry, and salivary melatonin concentration.
Surprisingly, the number of patients whose BDI scores reached the usual cutoff score of ≥5 after surgery was higher in the yellow IOL group than in the clear IOL group. This suggests that patients receiving yellow IOLs could be at higher risk to develop depression or at least, rather than being improved, mood may have been altered in patients who received yellow IOLs. This is in contradiction with a previous study in which patients were less depressed 2 months after cataract surgery compared to before surgery, regardless of the type of IOLs used (Ishii et al., Citation2008). However, in this non-randomized study, changes in BDI scores were mostly correlated with changes in the Mini–Mental State Examination (MMSE) score, an endpoint we did not assess in the present study.
Rather than improving sleep and mood, our results suggest that blue-blocking IOLs may exert a detrimental effect on mood. As a possible explanation, the mood change may be the consequence of altered circadian rhythms that have been shown to have a significant impact on mood (Albrecht, Citation2017). Circadian cycles are synchronized by a pacemaker localized in the supra-chiasmatic nucleus (SCN) of the anterior hypothalamus (Klein et al., Citation1983), which main input comes from the photosensitive retinal ganglion cells (pRGCs) that are localized within the retina (Berson, Dunn, & Takao, Citation2002). Light exposure suppresses the daytime pineal secretion of melatonin, which is triggered and maintained by darkness (Lewy, Wehr, Goodwin, Newsome, & Markey, Citation1980). The circadian cycle of melatonin secretion is partially desynchronized in blind individuals (Sack, Lewy, Blood, Keith, & Nakagawa, Citation1992) but can be restored by a daily administration of exogenous melatonin (Skene & Arendt, Citation2007). Photosensitive cells of the retinal ganglion being mostly sensitive to low wavelengths of 480 nm (blue light) (Berson et al., Citation2002; Brainard et al., Citation2001), the daytime level of melatonin suppression may be altered by filtering the light in the blue spectrum by the use of yellow IOLs. Considering that both advanced age and depression are characterized by an advanced phase shift (Myers & Badia, Citation1995), blue-light exposure rather than blue-blocking could counteract these changes, as suggested by recent data demonstrating that a diurnal prolonged (not limited to the pre-bed period) exposure to blue-enriched clear light is effective in improving clinical symptoms of seasonal affective disorder (Meesters, Dekker, Schlangen, Bos, & Ruiter, Citation2011), induces a delayed phase shift (Munch et al., Citation2011), increases the quality of sleep and improves behavior of nursing home residents with depressive symptoms and Alzheimer's disease (Figueiro et al., Citation2014). By blocking the light in the blue spectrum, yellow IOLs could limit this potential correction and the beneficial effect of light exposure in the blue spectrum.
One of the strengths of this study is the superiority of the randomized controlled design and the choice of clinical endpoints assessing both mood and sleep patterns. Moreover, to reflect real life conditions, patients with known or treated sleep disorders (or those receiving psychotropic treatments) were not excluded. The rate of 28% of patients (equally distributed in both groups and in the pre- and post-surgical periods) having at least one psychotropic treatment, mostly a hypnotic drug, is consistent with previous data from the aged population in France (Ohayon, Caulet, & Lemoine, Citation1996).
A possible explanation of our failure to find any difference in terms of sleep time, sleep duration and quality of sleep is that we used patient reported outcomes as sleep assessment endpoints (a diary and a self-administered pictorial scale) without getting any objective information on circadian rhythms, such as actimetry parameters or melatonin concentrations. A recent study nevertheless, demonstrated that subjective assessment of sleep time is well correlated to actimetry (Ritter, Sauer, Pfeiffer, Bauer, & Pfennig, Citation2016). In addition, having failed to reach the initial target of participant's inclusion, which underpowered the statistical analysis, is another weakness of the study. Moreover, the only significant impact we found on mood has to be cautiously interpreted, as a score higher that 5 at the BDI does not imply patients suffer from significant depression, and many confounding factors we were unable to control for, such as seasonality, the post-surgical complications and other serious adverse events, could explain this slight change. The lack of any information regarding the cognitive status of patients is another major weakness of this study, as impaired vision may be associated with lower cognitive performance among patients of advanced age, and age-related cognitive decline could also be associated with sleep changes (Harrabi et al., Citation2015). Finally, we cannot exclude that as surgical procedures were undertaken during the all year span, seasonality could have affected the sleep-wake cycle and may have constituted a potential covariate.
Taken together, data from the literature and the results of the present study suggest that the use of yellow IOLs is not superior to white IOLs with regard to sleep and mood. Instead, our results suggest that yellow IOLs may exert a detrimental effect on mood, but these results need to be further replicated.
Acknowledgments
Authors thank Jean Rateau MD, Yannick Nochez MD, Samuel Majzoub MD, Charles Seguier MD, Ismet Bekhechi MD, Anne Favard MD, Jeremy Halfon MD, Marie Pisella, Marie Coudray Leclerc, Elody Marnat, for their support to clinical investigation, and Bruno Giraudeau PHD, Brigitte Lucas, MD for methodological support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albrecht, U. (2017). Molecular mechanisms in mood regulation involving the circadian clock. Front Neurologica, 8, 30. doi:10.3389/fneur.2017.00030
- Alexander, I., Cuthbertson, F. M., Ratnarajan, G., Safa, R., Mellington, F. E., Foster, R. G., … Wulff, K. (2014). Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Investigative Ophthalmology & Visual Science, 55(8), 4999–5004. doi:10.1167/iovs.14-14054
- Ayaki, M., Negishi, K., Suzukamo, Y., & Tsubota, K. (2015). Color of intra-ocular lens and cataract type are prognostic determinants of health indices after visual and photoreceptive restoration by surgery. Rejuvenation Research, 18(2), 145–152. doi:10.1089/rej.2014.1613
- Beck, A. T., & Beamesderfer, A. (1974). Assessment of depression: The depression inventory. Modern Problems of Pharmacopsychiatry, 7(0), 151–169.
- Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
- Beekman, A. T., Copeland, J. R. M., & M.J., P. (1999). Review of community prevalence of depression in later life. British Journal of Psychiatry, 174, 307–311.
- Berson, D. M., Dunn, F. A., & Takao, M. (2002). Phototransduction by retinal ganglion cells that set the circadian clock. Science (New York, N.Y.), 295(5557), 1070–1073. doi:10.1126/science.1067262
- Brainard, G. C., Hanifin, J. P., Greeson, J. M., Byrne, B., Glickman, G., Gerner, E., & Rollag, M. D. (2001). Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(16), 6405–6412.
- Brøndsted, A. E., Lundeman, J. H., & Kessel, L. (2013). Short wavelength light filtering by the natural human lens and IOLs – implications for entrainment of circadian rhythm. Acta Ophthalmologica, 91(1), 52–57. doi:10.1111/j.1755-3768.2011.02291.x
- Brøndsted, A. E., Sander, B., Haargaard, B., Lund-Andersen, H., Jennum, P., Gammeltoft, S., & Kessel, L. (2015). The effect of cataract surgery on circadian photoentrainment: A randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology, 122(10), 2115–2124. doi:10.1016/j.ophtha.2015.06.033
- Chang, A. M., Aeschbach, D., Duffy, J. F., & Czeisler, C. A. (2015). Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proceeding of National Academy of Sciences of the United States America, 112(4), 1232–1237. doi:10.1073/pnas.1418490112
- Chellappa, S. L., Steiner, R., Oelhafen, P., Lang, D., Gotz, T., Krebs, J., & Cajochen, C. (2013). Acute exposure to evening blue-enriched light impacts on human sleep. Journal of Sleep Research, 22(5), 573–580. doi:10.1111/jsr.12050
- Cole, M. G., & Dendukuri, N. (2003). Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. American Journal of Psychiatry, 160, 1147.
- Court, H., McLean, G., Guthrie, B., Mercer, S. W., & Smith, D. J. (2014). Visual impairment is associated with physical and mental comorbidities in older adults: A cross-sectional study. BMC medicine, 12, 181. doi:10.1186/s12916-014-0181-7
- Davison, J. A., Patel, A. S., Cunha, J. P., Schwiegerling, J., & Muftuoglu, O. (2011). Recent studies provide an updated clinical perspective on blue light-filtering IOLs. Graefe's Archive for Clinical and Experimental Ophthalmology, 249(7), 957–968. doi:10.1007/s00417-011-1697-6
- Espindle, D., Crawford, B., Maxwell, A., Rajagopalan, K., Barnes, R., Harris, B., & Hileman, K. (2005). Quality-of-life improvements in cataract patients with bilateral blue light-filtering intraocular lenses: Clinical trial. Journal of Cataract and Refractive Surgery, 31(10), 1952–1959. doi:10.1016/j.jcrs.2005.03.060
- Figueiro, M. G., Plitnick, B. A., Lok, A., Jones, G. E., Higgins, P., Hornick, T. R., & Rea, M. S. (2014). Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer's disease and related dementia living in long-term care facilities. Clinical Interventions in Aging, 9, 1527–1537. doi:10.2147/CIA.S68557
- Harrabi, H., Kergoat, M. J., Rousseau, J., Boisjoly, H., Schmaltz, H., Moghadaszadeh, S., … Freeman, E. E. (2015). Age-related eye disease and cognitive function. Investigative Ophthalmology Visual Science, 56(2), 1217–1221. doi:10.1167/iovs.14-15370
- Ishii, K., Kabata, T., & Oshika, T. (2008). The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. American Journal of Ophthalmology, 146(3), 404–409. doi:10.1016/j.ajo.2008.05.014
- Kim, Y. H., Jung, K. I., & Song, C. H. (2012). The effect of cataract on sleep time and quality in late adulthood. Aging Clinical and Experimental Research, 24(6), 663–668. doi:10.3275/8501
- Klein, D. C., Smoot, R., Weller, J. L., Higa, S., Markey, S. P., Creed, G. J., & Jacobowitz, D. M. (1983). Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic leads to spinal cord circuit in the melatonin rhythm generating system. Brain Research Bulletin, 10(5), 647–652.
- Landers, J. A., Tamblyn, D., & Perriam, D. (2009). Effect of a blue-light-blocking intraocular lens on the quality of sleep. Journal of Cataract and Refractive Surgery, 35(1), 83–88. doi:10.1016/j.jcrs.2008.10.015
- Leruez, S., Annweiler, C., Gohier, B., Beauchet, O., Ebran, J.-M., Gohier, P., & Milea, D. (2015). Blue light-filtering intraocular lenses and post-operative mood: A pilot clinical study. International Ophthalmology, 35(2), 249–256. doi:10.1007/s10792-014-9944-6
- Lewy, A. J., Wehr, T. A., Goodwin, F. K., Newsome, D. A., & Markey, S. P. (1980). Light suppresses melatonin secretion in humans. Science (New York, N.Y.), 210(4475), 1267–1269.
- Lyness, J. M., King, D. A., Cox, C., Yoediono, Z., & Caine, E. D. (1999). The importance of subsyndromal depression in older primary care patients: Prevalence and associated functional disability. Journal of the American Geriatrics Society, 47(6), 647–652.
- Mainster, M. A. (2006). Blue-blocking intraocular lenses and pseudophakic scotopic sensitivity. Journal of Cataract and Refractive Surgery, 32(9), 1403–1404; author reply 1404-1405; discussion 1406. doi:10.1016/j.jcrs.2006.06.014
- Mainster, M. A., & Turner, P. L. (2010). Ultraviolet-B phototoxicity and hypothetical photomelanomagenesis: Intraocular and crystalline lens photoprotection. American Journal of Ophthalmology, 149(4), 543–549. doi:10.1016/j.ajo.2009.11.028
- Maldonado, C. C., Bentley, A. J., & Mitchell, D. (2004). A pictorial sleepiness scale based on cartoon faces. Sleep, 27(3), 541–548.
- Meesters, Y., Dekker, V., Schlangen, L. J., Bos, E. H., & Ruiter, M. J. (2011). Low-intensity blue-enriched white light (750 lux) and standard bright light (10,000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry, 11, 17. doi:10.1186/1471-244X-11-17
- Mester, U., Holz, F., Kohnen, T., Lohmann, C., & Tetz, M. (2008). Intraindividual comparison of a blue-light filter on visual function: AF-1 (UY) versus AF-1 (UV) intraocular lens. Journal of Cataract and Refractive Surgery, 34(4), 608–615. doi:10.1016/j.jcrs.2007.11.049
- Morgenthaler, T. I., Lee-Chiong, T., Alessi, C., Friedman, L., Aurora, R. N., Boehlecke, B., Brown, T., Chesson, A. L. Jr, Kapur, V., Maganti, R., Owens, J., Pancer, J., Swick, T. J., Zak, R; Standards of Practice Committee of the American Academy of Sleep. Medicine. Practice parameters for the clinical evaluation and treatment of circadian rhythmsleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007 Nov; 30 (11) 1445–59.
- Munch, M., Scheuermaier, K. D., Zhang, R., Dunne, S. P., Guzik, A. M., Silva, E. J., … Duffy, J. F. (2011). Effects on subjective and objective alertness and sleep in response to evening light exposure in older subjects. Behavioural Brain Research, 224(2), 272–278. doi:10.1016/j.bbr.2011.05.029
- Myers, B. L., & Badia, P. (1995). Changes in circadian rhythms and sleep quality with aging: Mechanisms and interventions. Neuroscience & Biobehavioral Reviews, 19(4), 553–571.
- Ohayon, M., Caulet, M., & Lemoine, P. (1996). [The elderly, sleep habits and use of psychotropic drugs by the French population]. L'Encéphale, 22(5), 337–350.
- Penninx, B. W., Geerlings, S. W., Deeg, D. J., van Eijk, J. T., van Tilburg, W., & Beekman, A. T. (1999). Minor and major depression and the risk of death in older persons. Archives of General Psychiatry, 56, 889–895.
- Pickett, Y. R., Ghosh, S., Rohs, A., Kennedy, G. J., Bruce, M. L., & Lyness, J. M. (2014). Healthcare use among older primary care patients with minor depression. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22(2), 207–210. doi:10.1016/j.jagp.2012.08.018
- Ritter, P. S., Sauer, C., Pfeiffer, S., Bauer, M., & Pfennig, A. (2016). Comparison of subjective and objective sleep estimations in patients with bipolar disorder and healthy control subjects. Sleep Disorders, 2016, 4031535. doi:10.1155/2016/4031535
- Rozanski, A., Blumenthal, J. A., Davidson, K. W., Saab, P. G., & Kubzansky, L. (2005). The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: The emerging field of behavioral cardiology. Journal of the American College of Cardiology, 45(5), 637–651. doi:10.1016/j.jacc.2004.12.005
- Sack, R. L., Lewy, A. J., Blood, M. L., Keith, L. D., & Nakagawa, H. (1992). Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. The Journal of Clinical Endocrinology and Metabolism, 75(1), 127–134. doi:10.1210/jcem.75.1.1619000
- Schmoll, C., Khan, A., Aspinall, P., Goudie, C., Koay, P., Tendo, C., … Dhillon, B. (2014). New light for old eyes: Comparing melanopsin-mediated non-visual benefits of blue-light and UV-blocking intraocular lenses. The British Journal of Ophthalmology, 98(1), 124–128. doi:10.1136/bjophthalmol-2013-304024
- Skene, D. J., & Arendt, J. (2007). Circadian rhythm sleep disorders in the blind and their treatment with melatonin. Sleep Medicine, 8(6), 651–655. doi:10.1016/j.sleep.2006.11.013
- Weyerer, S., Eifflaender-Gorfer, S., Köhler, L., Jessen, F., Maier, W., & Fuchs, A. (2008).… German AgeCoDe Study, g. Prevalence and risk factors for depression in non-demented primary care attenders aged 75 years and older. Journal of Affective Disorders, 111 (2–3163. doi:10.1016/j.jad.2008.02.008