Abstract
Purpose: To evaluate differences in key outcomes between younger and older women receiving the oral contraceptive oestradiol valerate/dienogest (E2V/DNG).
Methods: We conducted a pooled post hoc analysis of primary data from 12 studies of E2V/DNG, stratified by age (≤25 [n = 1309] and >25 [n = 2132] years). Outcomes included safety, efficacy, bleeding profile and hormone-withdrawal-associated symptoms (HWAS). Bleeding and HWAS analyses are also presented for women aged ≤20 years (n = 362). Discontinuations were considered a proxy for patient satisfaction.
Results: Results were generally similar for younger and older women. The percentage of women aged ≤25 and >25 years experiencing intracyclic bleeding did not differ between groups (13.4% and 12.8% at cycle 12, respectively), with similar results in women aged ≤20 years (12.7%, cycle 12). Rates of withdrawal bleeding were very similar in women aged ≤25 and >25 years (78.5% and 78.9%, respectively, cycle 12). We also found a similar adjusted Pearl index in the two age groups (0.45 vs 0.57, respectively), similar rates of AEs and HWAS and no difference in discontinuations.
Conclusions: Women aged ≤25 and >25 years have a similar experience with an E2V/DNV oral contraceptive, supporting this as an appropriate contraceptive option in younger and older women.
摘要
目的:评价口服戊酸雌二醇/地诺孕素(E2V/DNG)对中老年女性的主要疗效差异。
方法:我们对12项E2V/DNG研究的原始数据进行了汇总后分析, 按年龄分层(≤25 [n=1309]和>25 [n= 2132]年)。结果包括安全性、有效性、出血情况和与激素退出相关的症状(HWAS)。对≤20岁的妇女(n=362)也进行了出血和HWAS分析。停药认为是病人满意度的代表。
结果:年轻女性和年长女性的结果大致相似。≤25岁的女性和>25岁的女性发生周期性出血的比例在组间无差异(分别为13.4%和12.8%, 12个周期), ≤20岁的女性有类似的结果(12.7%, 12个周期)。≤25岁和>25岁患者的撤退出血率非常相似(分别为78.5%和78.9%, 12个周期)。我们还发现, 在两个年龄组中, 调整后的Pearl指数相似(分别为0.45和0.57), AEs和HWAS的发生率相似, 停药情况也没有差异。
结论:≤25岁和>25岁的妇女应用E2V/DNV口服避孕药有类似的经历, 支持这是一种适合年轻和老年妇女的避孕选择。
Introduction
Women benefit from a wide choice of contraceptive options. Since their introduction nearly 60 years ago, combined oral contraceptives (COCs) have been the most popular form of reversible hormonal contraception, accounting for 44% of the contraception chosen by women in a survey of 12,094 women aged 15–49 years from Brazil, France, Germany, Italy and the USA [Citation1]. Efficacy, impact on health and well-being, and cost influence a woman’s selection of a contraceptive [Citation1].
Oestradiol valerate/dienogest (E2V/DNG, Bayer AG, Germany) delivers oestradiol (E2), the oestrogen identical to endogenously produced 17β-oestradiol [Citation2]. E2 offers certain advantages over ethinylestradiol (EE), the oestrogen component in conventional COCs, including a lower impact on the hepatic system and therefore on haemostatic parameters [Citation3–5]. The progestogen DNG offers potent endometrial suppression and a direct antiandrogenic effect without other hormonal or antihormonal side effects [Citation6]. E2V/DNG has a multiphasic dosing regimen, which comprises four dosing phases (each phase containing a different dose of E2V either alone or in combination with DNG) followed by 2 days of placebo tablets [Citation7].
E2V/DNG provides reliable contraceptive efficacy for women aged 18–50 years, evidenced in both clinical trials and real-world studies [Citation8,Citation9]. Clinical trials of E2V/DNG demonstrate an acceptable bleeding profile, with similar rates of intracyclic bleeding in women treated with EE/LNG and those treated with E2V/DNG [Citation2]. In the real-world CONTENT study, women who switched from using EE-containing pills to using E2V/DNG experienced a lower rate of discontinuation for bleeding (p < 0.0001), shorter, lighter and less painful bleeding, and greater user satisfaction (80.7% vs 64.6%) compared with those who switched to using progestogen-only pills (POPs) [Citation10].
INAS-SCORE, a large, transatlantic, prospective, controlled, non-interventional, long-term cohort study, investigated the occurrence of cardiovascular events between two cohorts: women initiating use of E2V/DNG and women initiating other COCs, including LNG-containing COCs [Citation11]. These real-world data showed that the use of E2V/DNG was associated with a similar or even lower risk of confirmed VTE compared with LNG-containing COCs and other COCs [Citation11].
While these results support benefit for women of all ages, clinical guidelines often recommend E2V/DNG as an option for older women [Citation12]. This may be attributed to several factors: (i) the increased absence of withdrawal bleeding with E2V/DNG versus EE/LNG may be perceived as advantageous by older women, whereas younger women may prefer the reassurance of regular bleeding [Citation2]; (ii) the reduced metabolic impact of E2V/DNG versus EE/LNG may particularly attract older women [Citation13,Citation14]; (iii) there is a perception that the missed pill guidelines for E2V/DNG may be too complicated for younger women [Citation15]; (iv) the favourable cardiovascular risk profile has been considered especially suitable for older women [Citation13].
However, emerging evidence challenges this traditional prescribing of E2V/DNG. A recent study by Merki-Feld et al., which investigated the unmet needs in contraceptive counselling and choice, found that women of all ages (18–49 years) ranked assurance of reliability and a low risk of thrombosis as the most important attributes of a contraceptive [Citation16]. The same study found that women aged <30 years were more likely to forget to take their pill than women aged >30 years (54% vs 47%, p < 0.05). Younger women may benefit from a regimen with a shortened and non-pill-free hormone-free interval (HFI) as regimens in which placebo tablets are taken during the HFI have been associated with fewer pill omissions [Citation17].
Given the large amount of high-quality clinical data and real-world experience currently available on the E2V/DNG oral contraceptive, we now have sufficient data to compare the clinical outcomes of E2V/DNG in women aged 25 years and younger. This age threshold was based on the World Health Organisation (WHO) definition for ‘young people’ [Citation18].
To address the suitability of the E2V/DNG regimen in younger women, we completed a re-analysis of prior studies to compare outcomes in specific age groups and ascertain the clinical utility of and satisfaction with E2V/DNG in younger and older women. We hypothesised that bleeding, efficacy, tolerability and satisfaction outcomes between older and younger users would not differ.
Methods
We pooled data from 12 phase II–IIIb studies investigating E2V/DNG and performed analyses on safety, bleeding outcomes, efficacy and hormone-withdrawal-associated symptoms (HWAS). Please see Supplementary material (1. Study information, and 2. Methods) for further detail on the individual studies and analysis methodology. The safety analysis set is defined as all women who took at least one dose of study drug.
The study populations included women aged ≥18 years (except one study including women aged ≥14 years). Two studies had no upper age limit; the others had a limit of 35 or 50 years. While most studies enrolled healthy female volunteers, some specifically enrolled women suffering from dysfunctional uterine bleeding, HWAS, acquired oral contraceptive (OC)-associated female sexual dysfunction or primary dysmenorrhoea.
Due to the fact that studies have not been planned and powered for a comparison between age groups and because the analysis has been done post hoc, results are of exploratory nature only. We did not conduct a power analysis because the sample included all women in the available studies. We calculated stratified cause-specific hazard ratios (HRs) for discontinuation of study medication due to adverse events (AEs) and withdrawal of consent. These analyses only included studies in which E2V/DNG was indicated for contraceptive purposes.
We additionally examined bleeding and HWAS endpoints in very young women (aged ≤20 years, n = 362). This analysis allows us to better understand how these younger women respond to this contraceptive option.
All studies included in the pooled data analyses had relevant Institutional Review Board approval and patient consent.
Bleeding outcomes
Bleeding/spotting episodes are defined as day(s) with bleeding/spotting preceded and followed by at least 2 bleed-free days. ‘Bleeding’ is defined as bleeding that is the same or more than normal menstruation relative to the woman’s experience. ‘Spotting’ is defined as bleeding that is less than that associated with normal menstruation relative to the woman’s experience, with no need for sanitary protection (except panty liners). Bleeding/spotting data are described per 90-day reference period (as recommended by WHO) [Citation19]. The evaluation by reference period in the original studies enables a description of the bleeding pattern irrespective of the treatment regimen, i.e., to allow for comparisons between, for example, an oral contraceptive and an intrauterine device. Data at reference period 4, which represents cycles 9–12, are highlighted here, as this time point represents the pattern of stable use.
Intracyclic bleeding is defined as all bleeding episodes that do not fulfil the criteria for withdrawal bleeding (as described below) and is reported per 28-day cycle. Data were collected for up to 28 cycles, depending on the study; data at cycle 12, that is, 1 year, are highlighted here.
Withdrawal bleeding (scheduled bleeding) is defined as a bleeding episode that began during the progestogen-free period (starting on cycle day 25 with E2V/DNG) or started not more than 4 days before the progestogen withdrawal (i.e., day 21 with E2V/DNG) in any cycle. All other (unexpected) bleeding episodes are classified as intracyclic bleeding. If no bleeding occurred until cycle day 20 with E2V/DNG, we assessed this as an absence of scheduled bleeding in the previous treatment cycle. Withdrawal bleeding is reported per 28-day cycle; data at cycle 12, i.e., about 1 year, are highlighted here. We included bleeding data only for those participants in the safety analysis set.
Hormone-withdrawal-associated symptoms
The HWAS of headache and pelvic pain were assessed using a visual analogue scale (VAS).
Results
The study analysis population included a total of 3441 women who received E2V/DNG, aged ≤25 years (n = 1309) and >25 years (n = 2132). The sample included 362 women under age 20.
Demographics
With the exception of mean age, we found similar demographic characteristics in the ≤25 and >25 years age groups, with a notable difference only in the percentage of current smokers (31.6% and 10.8% in the younger and older groups, respectively; ). Although the proportions of women who had previously used COCs or condoms differed between the studies, within each study we found similar percentages between the age groups.
Table 1. Characteristics of women using E2V/DNG by age group (safety analysis set).
Discontinuations
The analysis on discontinuations due to AEs and due to withdrawal of consent included 3177 women treated with E2V/DNG in OC studies (1291 women aged ≤25 years, 1886 women aged >25 years).
We found no difference between the age groups for discontinuations due to AEs (women aged ≤25 years 109 [8.4%], >25 years 158 [8.4%]; HR 0.92 [95% confidence interval (CI) 0.71–1.18]) or withdrawal of consent (50 [3.9%], 59 [3.1%]; HR 0.86 [95% CI 0.58–1.26]).
Adverse events
We found similar percentages of AEs by primary system organ class between the two age groups (Supplementary material, Table 3.1). We considered reproductive system and breast disorders to be AEs of particular interest and found similar rates in the two age groups except for dysmenorrhoea and metrorrhagia, which were both higher in the younger age group.
Bleeding analyses
Bleeding/spotting
We found a similar mean number of bleeding, spotting or bleeding/spotting days per 90-day reference period between the two age groups (). The number of bleeding/spotting days generally declined over time; in reference period 4, women aged ≤25 and >25 years experienced 12.3 (standard deviation [SD] 8.2) and 12.3 (SD 8.6) days with bleeding/spotting, respectively. The mean number of spotting-only days, a major contributor to the number of bleeding/spotting days reported, was also similar in the two age groups (); in reference period 4, women aged ≤25 years experienced 5.6 (SD 5.8) days of spotting only and women aged >25 years experienced 5.9 (SD 6.2) days.
Figure 1. Bleeding/spotting by age group (safety analysis set). ‘Bleeding’ is defined as bleeding that is the same or more than normal menstruation relative to the woman’s experience. ‘Spotting’ is defined as bleeding that is less than that associated with normal menstruation relative to the woman’s experience, with no need for sanitary protection (except panty liners). An ‘episode’ is defined as any set of one or more bleeding or spotting days (consecutive or separated by only one bleeding-free day). An episode is bounded by at least 2 consecutive bleeding-free days.
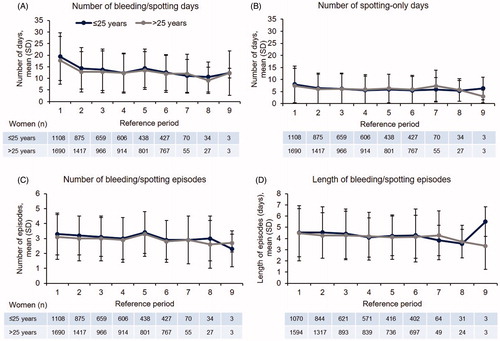
The mean number of bleeding/spotting episodes (defined as day[s] with bleeding/spotting preceded and followed by at least 2 bleed-free days) was similar in the two age groups and remained fairly constant over several reference periods (); in reference period 4, women aged ≤25 years experienced 3.0 (SD 1.4) episodes and women aged >25 years experienced 2.9 (SD 1.5) episodes. The mean length of bleeding/spotting episodes was also very similar in the two age groups (): 4.1 (SD 1.8) and 4.2 (SD 2.1) days in reference period 4 for women aged ≤25 and >25 years, respectively.
When reviewing these bleeding outcomes in very young women aged ≤20 years, we found that the bleeding/spotting profile was very similar to that of the older women (Supplementary material, Figure 4.1). For example, women aged ≤20, >20 to ≤25, and >25 years experienced 13.0 (SD 7.7), 12.1 (SD 8.4) and 12.3 (SD 8.6) days of bleeding/spotting, respectively, in reference period 4.
Intracyclic bleeding
The percentage of women reporting intracyclic bleeding, reported per 28-day cycle, decreased over time for both groups. Younger women aged ≤25 years reported more intracyclic bleeding during the early cycles of use, but the percentage became similar to that of women aged >25 years in later cycles (13.4% and 12.8% at cycle 12; ). The mean number of intracyclic bleeding days was similar in women in both age groups and tended to reduce over time.
Figure 2. Maximum intensity of intracyclic bleeding and percentage of women affected by age group (safety analysis set). Intensity of intracyclic bleeding episodes (B) was graded from 1 to 5, with 1 being no bleeding and 5 being heavy bleeding.
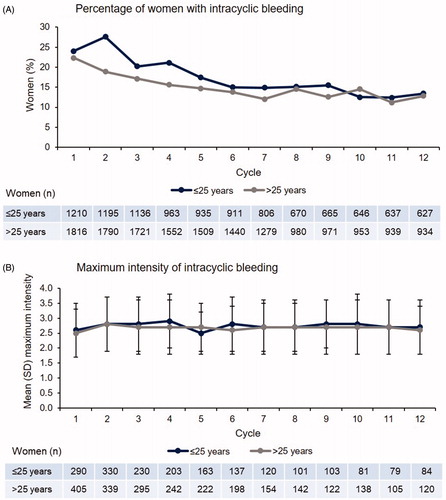
The intensity of intracyclic bleeding episodes was graded from 1 to 5, with 1 being no bleeding and 5 being heavy bleeding. The mean maximum intensity of intracyclic bleeding was similar between the age groups at cycle 12 (2.7 and 2.6 [SD 0.9 and 0.8], respectively; ).
In women aged ≤20 years, the intracyclic bleeding profile was very similar to that of the older age groups; intracyclic bleeding was reported by 12.7% of women aged ≤20 years, 13.7% of women aged >20 to ≤25 years and 12.8% of women aged >25 years at cycle 12 (Supplementary material, Figure 4.2).
Withdrawal bleeding
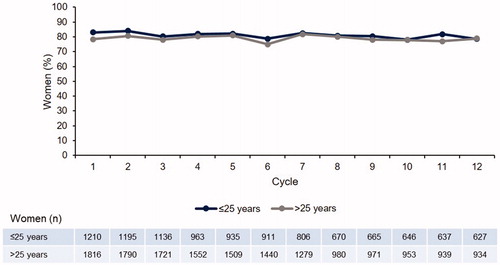
The percentage of women with withdrawal bleeding was very similar in the two age groups () and remained constant over 12 cycles (78.5% and 78.9% of women aged ≤25 years and >25 years experienced withdrawal bleeding at cycle 12, respectively). The reverse of these data is that 21.5% and 21.1% of women, respectively, did not experience withdrawal bleeding each cycle. There was a similar trend up to 28 cycles (data not shown).
The proportion of women aged ≤20 years with withdrawal bleeding was numerically slightly higher than that in the older age groups: 84.9% of women aged ≤20 years at cycle 12 (Supplementary material, Figure 4.3).
Pearl index
Detail on the Pearl index calculation can be found in the Supplementary material (Section 2. Methods). The unadjusted Pearl index was 0.56 (upper limit of 95% CI 1.30) for women aged ≤25 years compared with 1.46 (upper limit of 95% CI 2.50) for women aged >25 to ≤35 years (). The adjusted Pearl index was similar in the two age groups, at 0.45 (upper limit of 95% CI 1.16) vs 0.57 (upper limit of 95% CI 1.33), respectively.
Table 2. Efficacy with E2V/DNG assessed by Pearl index (full analysis set).
Hormone-withdrawal-associated symptoms
Headache intensity was very similar in the two age groups (); women aged ≤25 and >25 years reported mean scores of 20.01 (SD 20.29) and 17.77 (SD 17.77) at cycle 13, respectively. Women aged ≤25 years reported slightly higher levels of pelvic pain (21.50 [SD 22.75]) compared with women aged >25 years (17.94 [SD 19.58]) at cycle 13 (). When looking at improvements in headache and pelvic pain with E2V/DNG, a high percentage of women in both age groups reported VAS decreases of 15 mm from baseline (). Around 40% of women in both age groups reported even greater VAS decreases of 45 mm from baseline.
Figure 4. Hormone-withdrawal-associated pain by visit and age group as determined by averaging the three highest VAS values during days 22 to 28 (full analysis set).
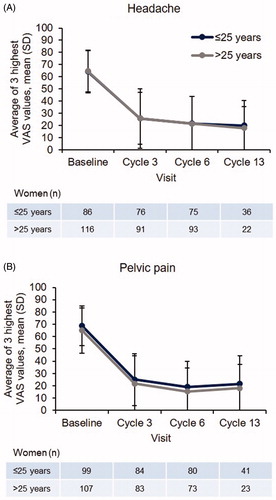
Figure 5. Responder analyses of change in pelvic pain and headache as determined by average of the three highest VAS values during days 22 to 28 from baseline to cycle 6 by age group (full analysis set).
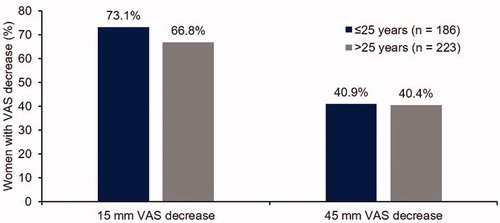
In women aged ≤20 years, rates of headache and pelvic pain intensity were very similar to those in the older age groups (Supplementary material, Figure 4.4), as were 15- and 45-mm VAS decreases from baseline for pelvic pain and headache (Supplementary material, Figure 4.5).
Discussion
Findings and interpretation
This pooled analysis of phase II–IIIb clinical trials of E2V/DNG provides evidence that safety, bleeding profiles, efficacy and HWAS with E2V/DNG are comparable for women aged ≤25 and >25 years. Furthermore, the post hoc analysis of women aged ≤20 years also demonstrates that younger women have a very similar experience of using E2V/DNG compared with older women.
We calculated cause-specific HRs for discontinuation of study medication due to AEs and withdrawal of consent between age groups. This analysis is clinically relevant because discontinuations can be a proxy for women’s satisfaction, quality-of-life measures, tolerability and an acceptable bleeding profile. The HR for discontinuation due to AEs for women aged ≤25 years was 0.92 times that for women aged >25 years (95% CI 0.71–1.18). The HR for discontinuation due to withdrawal of consent for women aged ≤25 years was 0.86 times that for women aged >25 years (95% CI 0.58–1.26). These results do not indicate a difference between the age groups and suggest that younger women are as satisfied as older women with E2V/DNG treatment.
Similarities or differences in comparison with other studies
The efficacy and effectiveness of E2V/DNG have been investigated in clinical and real-world studies [Citation8,Citation9]. In a pooled analysis of three large-scale, multicentre, phase-III trials, E2V/DNG was shown to provide reliable contraceptive efficacy in women aged 18–50 years, with a Pearl index of 0.79 [Citation8]. Furthermore, in the real-world INAS-SCORE study, E2V/DNG had a significantly lower risk of contraceptive failure compared with other COCs (HR 0.5; 95% CI 0.3–0.7), including LNG-containing COCs (HR 0.3; 95% CI 0.2–0.5) [Citation9]. Our pooled analysis showed similar efficacy for younger and older women.
In clinical trials, E2V/DNG showed superiority over EE/LNG with regard to reducing the frequency and intensity of HWAS, including headache and pelvic pain [Citation20]. The tolerability of E2V/DNG is enhanced by its dosing regimen, in which there are only 2 days of placebo tablets [Citation7]. This short HFI ensures the delivery of stable E2 levels throughout the cycle [Citation21,Citation22], minimising HWAS [Citation23]. This may account for the reductions from baseline we observed in headache and pelvic pain across all ages as indicated by a drop in VAS score of 45 mm.
Although not specifically included in this analysis, other advantages of E2V/DNG use have been shown in women of all ages and will benefit younger women, for example, E2V/DNG has a minimal impact on metabolic and haemostatic parameters and a more favourable effect than EE/LNG on lipid markers [Citation14,Citation24]. Furthermore, younger women are likely to appreciate other health benefits of COCs, which include reductions in dysmenorrhoea, heavy menstrual bleeding, premenstrual syndrome and acne [Citation25].
Strength and weaknesses of the study
In this study, we examined data amassed from several robust, well-designed phase II–IIIb clinical trials of E2V/DNG, which individually provide a wealth of data on the efficacy and tolerability of E2V/DNG in several populations of women. However, this also leads to a limitation of this pooled analysis in that the underlying data are from a variety of study designs and populations. Nevertheless, the individual study designs and inclusion criteria applied equally to both age groups of women within the studies, so the results remain meaningful and relevant to clinical practice. We chose to evaluate our main outcomes of intracyclic and withdrawal bleeding at cycle 12, and use reference period 4 for bleeding spotting to compare time intervals with stable bleeding patterns between groups.
An additional limitation is that, due to the fact that this analysis was performed post hoc, i.e., analysis approaches were not prespecified, analyses are of exploratory nature only, and no confirmatory conclusions can be drawn.
Open questions and future research
Further research is recommended to corroborate the findings in this analysis; this would include studies in which the analyses of results between younger and older women were part of the prespecified data analysis. Although presenting data for adolescents under 18 would also have value, the original studies did not include sufficient numbers of very young women for a complete analysis.
Recommendations for clinicians
We undertook this analysis of pooled study data to gain an improved understanding of E2V/DNG in younger women (aged ≤25 years). The results provide evidence and reassurance that E2V/DNG is an appropriate contraceptive option in this age group. Of note, no new safety events were revealed by this pooled analysis.
Supplementary information
Download MS Word (504.6 KB)Acknowledgements
The data collection and analysis were carried out by Bayer AG, Berlin, Germany. The authors wish to thank Darwin Healthcare Communications for writing support.
Disclosure statement
Christiane Ahlers: Is an employee at Bayer AG.
Ralf Bannemerschult: Is an employee at Bayer AG.
Johannes Bitzer: Has received honoraria for participation in Ad Board Meetings and for giving invited lectures from Bayer AG, Merck, Actavis, Exeltis, Teva, Theramex, Effik, Mithra, Gedeon Richter, Natural cycles, Ava.
Jeffrey Jensen: Has received payments for consulting from Abbvie, Cooper Surgical, Bayer Healthcare, Merck, Sebela, and the Population Council. Oregon Health & Science University (OHSU) has received research support from Abbvie, Bayer Healthcare, Daré, Estetra SPRL, Medicines360, Merck, and Sebela. These companies and organisations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU.
Rossella E Nappi: Had a past financial relationship (lecturer, member of advisory boards and/or consultant) with Boehringer Ingelheim, Ely Lilly, Gedeon Richter, HRA Pharma, Pfizer Inc, Procter & Gamble Co, TEVA Women’s Health Inc, Zambon SpA. At present, she has an on-going relationship with Bayer HealthCare AG, Endoceutics, Exceltis, Merck Sharpe & Dohme, Novo Nordisk, Palatin Technologies, Shionogi Limited, Theramex.
Susanne Parke: Is an employee at Bayer AG.
References
- Mansour D. International survey to assess women’s attitudes regarding choice of daily versus nondaily female hormonal contraception. Int J Womens Health. 2014;6:367–375.
- Ahrendt HJ, Makalova D, Parke S, et al. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception. 2009;80:436–444.
- Grandi G, Napolitano A, Cagnacci A. Metabolic impact of combined hormonal contraceptives containing estradiol. Expert Opin Drug Metab Toxicol. 2016;12:779–787.
- Sitruk-Ware R, Nath A. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract Res Clin Endocrinol Metab. 2013;27:13–24.
- Wiegratz I, Lee JH, Kutschera E, et al. Effect of four oral contraceptives on hemostatic parameters. Contraception. 2004;70:97–106.
- Harada T, Taniguchi F. Dienogest: a new therapeutic agent for the treatment of endometriosis. Womens Health (Lond Engl). 2010;6:27–35.
- Qlaira (estradiol valerate/dienogest). [cited 2020 Feb 24] Available from: www.medicines.org.uk/emc/product/6536/smpc.
- Nelson A, Parke S, Makalova D, et al. Efficacy and bleeding profile of a combined oral contraceptive containing oestradiol valerate/dienogest: a pooled analysis of three studies conducted in North America and Europe. Eur J Contracept Reprod Health Care. 2013;18:264–273.
- Barnett C, Hagemann C, Dinger J, et al. Fertility and combined oral contraceptives - unintended pregnancies and planned pregnancies following oral contraceptive use – results from the INAS-SCORE study. Eur J Contracept Reprod Health Care. 2017;22:17–23.
- Briggs P, Serrani M, Vogtlander K, et al. Continuation rates, bleeding profile acceptability, and satisfaction of women using an oral contraceptive pill containing estradiol valerate and dienogest versus a progestogen-only pill after switching from an ethinylestradiol-containing pill in a real-life setting: results of the CONTENT study. IJWH. 2016;8:477–487.
- Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94:328–339.
- Borgelt LM, Martell CW. Estradiol valerate/dienogest: a novel combined oral contraceptive. Clin Ther. 2012;34:37–55.
- Guida M, Bifulco G, Di Spiezio Sardo A, et al. Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill. Int J Womens Health. 2010; Aug 242:279–290.
- Junge W, Mellinger U, Parke S, et al. Metabolic and haemostatic effects of estradiol valerate/dienogest, a novel oral contraceptive: a randomized, open-label, single-centre study. Clin Drug Investig. 2011;31:573–584.
- Micks E, Jensen JT. Estradiol valerate and dienogest: a novel four-phasic oral contraceptive pill effective for pregnancy prevention and treatment of heavy menstrual bleeding. Womens Health (Lond Engl). 2011;7:513–524.
- Merki-Feld GS, Caetano C, Porz TC, et al. Are there unmet needs in contraceptive counselling and choice? Findings of the European TANCO Study. Eur J Contracept Reprod Health Care. 2018;23:183–193.
- MacGregor EA, Guillebaud J. The 7-day contraceptive hormone-free interval should be consigned to history. BMJ Sex Reprod Health. 2018;44:214–220.
- WHO. Adolescent health and development 2018 [cited 2018 July]. Available from: http://www.searo.who.int/entity/child_adolescent/topics/adolescent_health/en/
- Mishell DR, Jr., Guillebaud J, Westhoff C, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception. 2007;75:11–15.
- Macias G, Merki-Feld GS, Parke S, et al. Effects of a combined oral contraceptive containing oestradiol valerate/dienogest on hormone withdrawal-associated symptoms: results from the multicentre, randomised, double-blind, active-controlled HARMONY II study. J Obstet Gynaecol. 2013;33:591–596.
- Zeun S, Lu M, Uddin A, et al. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care. 2009;14:221–232.
- Fruzzetti F, Tremollieres F, Bitzer J. An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest. Gynecol Endocrinol. 2012;28:400–408.
- Jensen JT, Parke S, Mellinger U, et al. Hormone withdrawal-associated symptoms: comparison of oestradiol valerate/dienogest versus ethinylestradiol/norgestimate. Eur J Contracept Reprod Health Care. 2013;18:274–283.
- Klipping C, Duijkers I, Parke S, et al. Hemostatic effects of a novel estradiol-based oral contraceptive: an open-label, randomized, crossover study of estradiol valerate/dienogest versus ethinylestradiol/levonorgestrel. Drugs R D. 2011;11:159–170.
- ESHRE Capri Workshop Group. Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update. 2005;11:513–525.