Abstract
Objective
Primary aim of this study was to investigate endometriosis characteristics of patients with psychiatric conditions or depression. The secondary aim was to study tolerability of dienogest in this context.
Methods
This observational case-control study included endometriosis data from patients visiting our clinic from 2015–2021. We collected information from patient charts and in phone interviews based on a structured survey. Patients with surgical confirmed endometriosis were included.
Results
344 patients fulfilled the inclusion criteria: n = 255 no psychiatric disorder, n = 119 any psychiatric disorder and n = 70 depression. Patients with depression (EM-D, p=.018; p=.035) or psychiatric condition (EM-P, p=.020; p=.048) suffered more often from dyspareunia and dyschezia. EM-P patients had more often primary dysmenorrhoea with higher pain scores (p=.045). rASRM stage or localisation of lesions did not differ. EM-D and EM-P patients discontinued dienogest treatment more often related to worsening of mood (p= .001, p=.002).
Conclusion
EM-D or EM-P had a higher prevalence of pain symptoms. This could not be attributed to differences in rASRM stage or location of endometriosis lesions. Strong primary dysmenorrhoea might predispose to develop chronic pain-based psychological symptoms. Therefore, early diagnosis and treatment are relevant. Gynaecologist should be aware of the potential impact of dienogest on mood.
SHORT CONDENSATION
Women with endometriosis and psychiatric disorders especially have more dyschezia and dyspareunia, independent from rASRM stage, depth of infiltration and localisation of endometriosis lesions. Dienogest has an impact on mood especially in already prone patients.
Trial registration: trial registration number: NCT04816357. https://clinicaltrials.gov/ct2/show/NCT04816357
Date of registration: 22.03.2021, date of enrolment of the first subject: 25.03.2021
摘要
目的:本研究的主要目的是调查患有精神疾病或抑郁症患者的子宫内膜异位症特征。次要目的是研究在这种情况下对地诺孕素的耐受性。
方法:这项观察性病例对照研究包括2015-2021年在我们诊所就诊的子宫内膜异位症患者。我们从患者的病历和基于结构化调查的电话采访中收集信息。手术证实的子宫内膜异位症患者被纳入其中。
结果:344名患者符合纳入标准:n = 255无精神障碍, n = 119任何精神障碍和n = 70抑郁症。抑郁症患者(EM-D, p=.018;p=.035)或精神疾病患者(EM-P, p=.020;p=.048)患有排尿困难和痛经更常见。EM-P患者原发性痛经更常见, 疼痛评分更高(p=.045)。rASRM阶段或病变的定位没有差异。EM-D和EM-P患者因情绪恶化而停止地诺孕素的治疗更常见(p=.001, p=.002)。
结论:EM-D或EM-P的疼痛症状发生率更高。这不能归因于rASRM阶段或子宫内膜异位症病灶位置的差异。强烈的原发性痛经可能容易发展为慢性疼痛的心理症状。因此, 早期诊断和治疗是必要的。妇科医生应该意识到地诺孕素对情绪的潜在影响。
简要概括
患有子宫内膜异位症和精神障碍的妇女更容易出现排泄障碍和排尿困难, 与rASRM阶段、浸润深度和子宫内膜异位症病变的定位无关。地诺孕素对情绪有影响, 特别是在有倾向的病人中。
试验注册:试验注册号:NCT04816357.
https://clinicaltrials.gov/ct2/show/NCT04816357
注册日期:22.03.2021, 第一例的入组日期:25.03.2021
Introduction
Endometriosis (EM) is an inflammatory proliferative chronic disease affecting 5–10% of women of reproductive age [Citation1,Citation2]. A combination of immunological, environmental, anatomical, and emotional factors are discussed as pathomechanisms for the ectopic growth of endometrial tissue [Citation1,Citation2]. Patients can be without symptoms or suffer significantly from dysmenorrhoea, dyspareunia, dyschezia, dysuria, and chronic pelvic pain [Citation3]. Infertility can be a long-term consequence. Comorbidities of EM include migraine, depression, and autoimmune diseases [Citation3,Citation4].
For research purposes, surgery, and confirmation of endometriosis lesions by biopsy is recommended.
The individual and social burden of endometriosis compromises patients’ relationships, physical and mental well-being independent of the endometriosis stage and is estimated to be responsible for 40% of chronic pelvic pain complaints and 35% of female infertility cases [Citation3,Citation5].
Psychiatric disorders, mainly anxiety and depression, are common in women suffering from endometriosis [Citation3,Citation6,Citation7]. It is yet unclear if anxiety and depression in EM patients are a consequence of EM-associated pain or a different condition. A meta-analysis of genome-wide association (GWAS) studies revealed a shared genetic aetiology for endometriosis and depression [Citation4]. Such a genetic connection of both conditions might result in a special phenotype of EM.
The 19-nortestosterone derivative dienogest (DNG) is broadly used for endometriosis treatment [Citation8,Citation9]. It exerts a strong suppressive effect on endometrial growth and modifies the inflammatory microenvironment of endometriotic lesions [Citation8]. Like other progestins, DNG may exert a negative impact on mood in a subset of women (5.1%) [Citation10]. It is relevant to understand, if women with endometriosis and depression or other psychiatric conditions can without concern be treated with this progestin.
The primary aim of the current study was to examine if women with endometriosis and psychiatric conditions on one hand and diagnosed depression on the other hand differ regarding endometriosis symptoms, rASRM stage and localisation from women with endometriosis alone. The second aim was to assess DNG tolerability by analysing the duration of use and reasons for discontinuation. Improved understanding of the comorbidity might contribute to earlier diagnosis, other therapeutic approaches, and reduction of the long-term negative impact on psychiatric well-being.
Methods
For this observational case-control study we identified endometriosis patients who visited our outpatient clinic of the Department of Gynaecology at University Hospital Zurich from 2015 to July 2021. Charts were included if endometriosis was confirmed by operation and biopsy and rASRM score was reported. The severity of endometriosis is classified by the American Society of Reproductive Medicine (rASRM) score and the #ENZIAN classification describing the location, amount, depth, and size of endometriotic lesions, involvement of pelvic structures, the extent of pelvic adhesions, and obstruction of the fallopian tubes [Citation11]. We included premenopausal women aged 18–55 years who, after informed consent, agreed to participate in a telephone interview. The study was part of a larger study investigating the potential impact of comorbidities on endometriosis features.
We excluded patient charts without histologically confirmed endometriosis, and those with adenomyosis or scar endometriosis only. Furthermore, we excluded patients not willing to participate and cases, who could not be interviewed due to insufficient language knowledge.
Patients interested in participating after being contacted and informed by phone received a sheet with detailed study information. After obtaining written or oral consent, we conducted telephone interviews based on a modification of the ‘Women’s Health Symptom Survey Questionnaire’ of the World Endometriosis Research Foundation adapted to our study question [Citation12]. The modification included questions about the general history, medical conditions, use of medications and specific questions about psychiatric symptoms and diagnosis. Furthermore, we collected information on the use of dienogest, its duration and reasons for discontinuation. Before the start of the interview, the survey was validated in 50 test interviews, interviews were conducted by medical staff educated in evaluation of medical history, gynaecological and psychiatric disorders and able to clarify questions during the interviews. Current and past medication was evaluated, excluding a possible co-effect of other drugs on reported symptoms.
For the analyses, participants were divided into three groups: Group 1 (EM-O): patients without a history of psychiatric disorder, group 2 (EM-P): patients with any psychiatric diagnosis in their history, group 3 (EM-D): patients with a history of depression. Patients with a psychiatric disorder were specifically asked for their psychiatric diagnosis and about medical treatment at present and in the past. Patients without psychiatric disorders served as controls.
Endometriosis was staged using the revised American Society of Reproductive Medicine (rASRM) Score in the patient chart [Citation13]. From the surgical records endometriosis localisation, depth of infiltration, and affected compartments were noted. In the interview, we used a pain score from 1–3 to describe the pain intensity, with 1 describing light pain, 2 describing moderate pain, and 3 describing severe pain.
Statistical analysis
Data was analysed using SPSS® statistics (IBM®, Armonk, New York, United States).
Shares in percent were used for categorical variables and means including standard deviation (SD) were used for numeric variables. To compare categorical variables among groups, we used Chi-Square or Fisher’s exact test (expected frequencies <5), depending on expected frequencies.
To compare numeric variables between two groups, we used the independent sample t-test for normally distributed variables and Wilcoxon-Mann-Whitney test for not normally distributed variables.
A two-tailed p-value ≤ 0.05 was considered statistically significant.
The study was approved by the cantonal ethics commission of Zurich (BASEC Nr. 2021-00285) and registered on clinical Trials.gov (NCT04816357).
Results
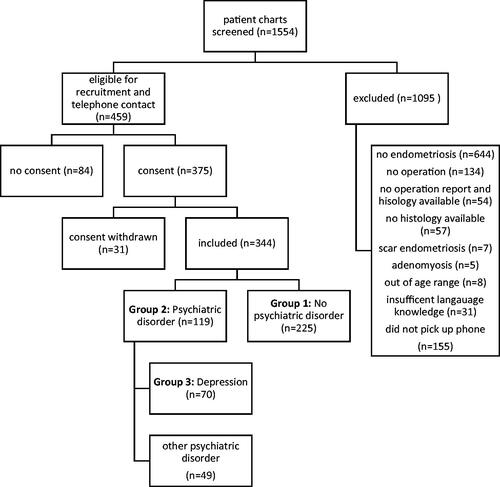
The screening procedure is described in . 344 women fulfilled the inclusion criteria (). 119 patients reported any type of psychiatric condition (group 2, EM-P), 70 women had a history of depression (group 3, EM-D),
Group 2 (any psychiatric disorder) consisted of n = 70 (58.8%) women with depression, n = 21 (17.6%) women with adaptive disorders, n = 18 (15.1%) women with anxiety, n = 10 (8.4%) women with post-traumatic stress disorder and others (9.2%, n = 11). Prescribed psychiatric medication reported were selective serotonin uptake inhibitors (33.6%, n = 40), tricyclic antidepressants (2.5%, n = 3), lorazepam (2.5%, n = 3), monoamine oxidase inhibitors (0.8%, n = 1), norepinephrine–dopamine reuptake inhibitors (0.8%, n = 1).
Altogether, 20.3% (n = 70) of all endometriosis patients suffered from depression. 22.8% (n = 16) were currently taking dienogest (vs 22.2% (n = 50) patients without psychiatric disorder). 55.7% (n = 39) of patients with depression reported a history of dienogest intake (vs 47.5% (n = 107) patients without psychiatric disorders).
Baseline criteria did not differ between groups, except that BMI was higher in group 2 (). The percentage of women with a history of dienogest use did not differ between groups ().
Table 1. Baseline characteristics.
We found significant differences in endometriosis symptoms between women without and those with psychiatric conditions or depression (). Primary dysmenorrhoea tended to be more frequent in EM-P patient (p=.121) and associated with a significantly higher pain score (p=.045). More women with EM-P and EM-D suffered from dyspareunia (EM-P: OR 1.84 (95% CI 1.15 − 2.96); EM-D: OR 2.00 (95% CI 1.12 − 3.58)) and dyschezia (EM-P OR 1.65 (95% CI 1.06 − 2.58); EM-D: OR 1.79 (1.04 − 3.07)).
Table 2. Endometriosis related clinical parameters.
At present the number of women using a pain killer more than 3 days/month was significantly higher in the EM-D group. Both, EM-P and EM-D patients reported more often to not respond to pain medications (p=.011; p=.015).
EM-D patients tended to have experienced more endometriosis surgeries (). Subanalyses revealed that the number of operations was not higher in patients with no response to analgesics. Women stating no response to analgesics (n = 68) did neither experience more surgeries, nor more often more than one surgery (Number of surgeries: EM-O 1.5 (SD 1.1); EM-D 1.9 (SD 1.6), p = 0.288; >1 surgery % (n): EM-O = 26.5 (9); EM-D 40.9 (9), p = 0.259).
Compared to group 1, localisation of the endometriosis lesions was less frequent in the ovaries, and there was a trend to more lesions in the rectovaginal septum in EM-D patients (). No differences were found regarding depth of infiltration, rASRM stage or number of affected compartments (). Although not significant, it might be of relevance that the percentage of women with infiltration >3cm was lower in women with depression (12.9% vs. 20.0%, p=.177), despite more pain.
Table 3. Endometriosis localisation and stage.
Reasons to discontinue treatment with dienogest are presented in . Both, participants with any psychiatric condition and those with depression reported more frequently to have discontinued dienogest treatment because of worsening of mood (p=.002; p= .001) ().
Table 4. Reasons for discontinuation of dienogest.
Discussion
Findings and interpretation
In accordance with existing evidence, that women with endometriosis are prone to psychiatric diseases, the prevalence of depression in our trial was higher (20.3%) than in the general population (5–10%) [Citation6,Citation14]. The percentage of women with onset of more severe dysmenorrhoea at menarche was higher in the EM-P group. Both, women with any psychiatric disorder (EM-P) and those with depression (EM-D) reported significantly more often dyspareunia and dyschezia and responded less to analgesics (). Despite more pain symptoms in both groups, there were no differences in rASRM stages or depth of infiltration, which is the parameter believed to be more associated with endometriosis pain () [Citation15]. In contrast, fewer patients with depression were diagnosed with more than 3 cm depth of infiltration (12.9% vs. 20.0% p=.240). Localisation of endometriosis lesions tended to differ between groups, with more lesions in the rectovaginal septum in EM-D patients (p=.063) and a trend to less lesions at the ovaries (). Within patients with dienogest treatment, those with depression or psychiatric problems mentioned significantly more often depression as reason for discontinuation ().
Results in the context of what is known
Prevalence of depression in our endometriosis patients is slightly higher than reported in previous studies, possibly related to differences in data collection and not histologically verified endometriosis diagnosis in other trials (14–15%) [Citation16,Citation17]. The finding, that rASRM stage is not associated with pain symptoms is in line with current literature [Citation3,Citation16,Citation18]. Pain intensity seems to be more related to the localisation of deep infiltrating endometriosis [Citation15,Citation19].
The dysmenorrhoea rate of approximately 75% in our study is in line with findings in a recent cohort study including 1560 women [Citation18]. We further found a prevalence of primary dysmenorrhoea in the range of the highly varying percentages reported in studies with adolescents ranging from 20–90% [Citation20–22]. The earlier start of dysmenorrhoea in EM-D patients did not affect the mean age of first surgery (30.7 years EM-D vs. 31.6 years EM-O, p=.330). Considering the well-known consequences of long-term pelvic pain, delay of diagnosis could substantially contribute to psychiatric symptoms [Citation16,Citation23–25].
Deep dyspareunia is associated with deep infiltrating endometriosis [Citation15,Citation23,Citation24]. The dyspareunia rate in our trial was higher (60.5%) than reported in survey-based studies (45%) and even higher in women with EM-D (71.4%) and EM-P (68.9%) [Citation16,Citation23,Citation24]. In EM-D patients, this could potentially be attributed to the higher frequency of EM-lesions in the rectovaginal septum (p=.063). Our study does not allow final conclusions, but it might be worth to study deeper in future, if deep dyspareunia might be a special attribute of EM-D patients, as EM-P patients did not differ from EM-O patients regarding localisation. We found no differences in depth of infiltration between groups. Neither did we observe a higher prevalence of EM lesions at the ligaments/pelvic wall, what also has been reported to be associated with dyspareunia [Citation15,Citation23]. Some authors indicate, that dyspareunia is associated with a higher rate of depression, independent of the diagnosis of endometriosis [Citation26–28]. Warzecha et al. found a significant positive correlation between the beginning of dyspareunia and depression in women with endometriosis, collecting data on symptoms with a written survey (n = 246 respondents) [Citation16]. Also painful defaecation was associated with an increased risk of depression (OR = 7.7, 95% CI 1.4–42.3, p=.01) [Citation16]. The prevalence for dyschezia in our trial (46%) is in line with other studies presenting data of patients with histologically confirmed endometriosis (45% to 52%) [Citation16,Citation18]. The OR in our study for dyschezia and depression was 1.65 (1.06 − 2.58). Prevalence of dyschezia was lower (25%) in a small cohort study with 96 patients without histological confirmation of the disease [Citation24].
Endometriosis and depression share specific genetic polymorphisms. Women with the comorbidity might present with another phenotype of EM and more severe pain or respond less to treatment [Citation29,Citation30].
Insofar, pelvic pain might not be the only reason for the development of psychiatric conditions in EM patients, but predisposition might play a role as well [Citation16,Citation31].
Findings like a significant correlation between prevalence of depression and mean age of dysmenorrhoea onset however support the idea that chronic pain generates the development of psychiatric disorders [Citation16]. A meta-analysis by Gambadauro et al. found, that pain is the main factor determining the prevalence of depression in women with chronic pelvic pain with or without confirmed endometriosis [Citation7].
Our findings support the theory proposed by Laganà of a vicious cycle of psychological diseases amplifying endometriosis-related pain, resulting in worsening of psychopathological symptoms [Citation3].
That women in the EM-D group responded less to analgesics could be related to the well-known increased pain perception in patients with depression [Citation32]. EM-D patients also tended to experience more operations (32.4 vs. 44.3% p=.060). Chronic pain results in increased pain sensitivity potentially due to associated alteration in brain structure and function [Citation33]. Central and peripheral hypersensitivity in endometriosis patients is suggested [Citation33].
Clinical Implications
For patients with strong primary dysmenorrhoea, optimised pain management and further evaluation of the course of pain might prevent chronification, neuroplastic changes in pain memory and development of psychiatric comorbidities. Our data suggest, that especially in adolescents without response to medical treatments diagnosis of endometriosis by laparoscopy and specific treatment might contribute to prevent chronification of pain symptoms. Today ultrasound and MRI have improved but limited sensitivity, especially in the case of discrete endometriosis lesions.
Awareness for the potential comorbidity depression in women with EM is relevant, as hormonal treatment, especially progestin-only treatment, is known to negatively affect mood in a subset of women [Citation10]. It is unknown, how women with pre-existing psychiatric conditions tolerate the continuous use of the progestin dienogest. In the VIPOS trial, a higher risk for new depression or worsening of depression could not be excluded [Citation34]. In our trial, six months discontinuation rates with dienogest did not differ between groups. However, EM-P and EM-D women reported significantly more often worsening of mood as a reason for discontinuation (). Consequently, we would recommend close monitoring of patients with pre-existing psychiatric problems.
Research Implications
Patients suffering from endometriosis and depression share increased pain perception. It is suspected, that one of the reasons are shared gene polymorphisms, but further research is needed to confirm this supposition [Citation29,Citation30]. The detected trend of more rectovaginal endometriosis in women with depression needs to be confirmed in larger studies, as well as the trend to more endometriosis surgeries for women with primary dysmenorrhoea. Further studies are also needed to evaluate the correlation of onset of psychiatric diseases and dysmenorrhoea onset at menarche.
Strengths and limitations
Strengths of our study include the histologically confirmed diagnosis and the detailed collection of information related to endometriosis and psychiatric symptoms in a personal interview and not in a survey only. Phone interviews conducted by medical experts allowed clarifications whenever needed. We can however not exclude some recall bias related to questions addressing symptoms in the past. A limitation might be, that we had no access to reports from psychiatrists. However, clarification and distinction of depression from other psychiatric disorders was achieved during the phone interview. Data for the group with all psychiatric disorders probably are influenced by results of the huge group of women with depressions. This was considered when interpreting the results.
Conclusion
Women with endometriosis and depression or other psychiatric conditions have a higher prevalence of dyschezia, and dyspareunia, independent from rASRM stage, depth of infiltration and localisation of endometriosis lesions. They also respond less to analgesics. The onset of stronger pain already at menarche, together with the high delay to first operation, might boost the development of alterations in brain structure and function which results in increased pain sensitivity. It is feasible that some of the psychological conditions observed in our study have evolved from chronic pain. Early diagnosis and treatment of endometriosis, especially strong primary dysmenorrhoea is therefore highly important.
Only women with depression tended to have more often rectovaginal lesions. Further studies are needed to evaluate if this could be an indicator for a shared hereditary trait. Gynaecologists should be aware of the impact of dienogest on mood in women with psychiatric conditions.
Ethical approval
The study was approved by the cantonal ethics commission of Zurich (BASEC Nr. 2021-00285) on 16.03.2021 and registered on clinical Trials.gov (NCT04816357).
Author contributions
HD analysed and interpreted raw data, wrote the article, supervised data collection, performed sample checks for correct coding of data, performed interview training with students and served as a contact person for students. GM conceptualised the study, led the study, co-analysed, and interpreted the data and revised the manuscript. PI provided the patient charts and revised the manuscript. JM, AM, AN and MN added expertise and revised the manuscript. CK and LP collected the data, performed the interviews, and revised the manuscript.
Acknowledgement
To University of Zurich for enabling the funding for Open Access publication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. 2021;397(10276):839–852.
- Abbey A, Halman LJ, Andrews FM. Psychosocial, treatment, and demographic predictors of the stress associated with infertility. Fertil Steril. 1992;57(1):122–128.
- Laganà AS, La Rosa VL, Rapisarda AMC, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–330.
- Adewuyi EO, Mehta D, Sapkota Y, et al. Genetic analysis of endometriosis and depression identifies shared loci and implicates causal links with gastric mucosa abnormality. Hum Genet. 2021;140(3):529–552.
- Lorencatto C, Vieira MJ, Pinto CL, et al. [Evaluation of the frequency of depression in patients with endometriosis and pelvic pain]. Rev Assoc Med Bras (1992). 2002;48(3):217–221.
- Chen LC, Hsu JW, Huang KL, et al. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord. 2016;190:282–285.
- Gambadauro P, Carli V, Hadlaczky G. Depressive symptoms among women with endometriosis: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;220(3):230–241.
- Grandi G, Mueller M, Bersinger NA, et al. Does dienogest influence the inflammatory response of endometriotic cells? A systematic review. Inflamm Res. 2016;65(3):183–192.
- Grandi G, Barra F, Ferrero S, et al. Hormonal contraception in women with endometriosis: a systematic review. Eur J Contracept Reprod Health Care. 2019;24(1):61–70.
- Merki-Feld GS, Apter D, Bartfai G, et al. ESC expert statement on the effects on mood of the natural cycle and progestin-only contraceptives. Eur J Contracept Reprod Health Care. 2017;22(4):247–249.
- Johnson NP, Hummelshoj L, Adamson GD, et al. World endometriosis society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–324.
- Foundation WER. Women’s Health Symptom Survey Questionnaire. 2021. http://www.endometriosisfoundation.org/WERF-WHSS-Questionnaire-English.pdf
- Revised American society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821.
- Cavaggioni G, Lia C, Resta S, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res Int. 2014;2014:786830.
- Fauconnier A, Chapron C, Dubuisson JB, et al. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002;78(4):719–726.
- Warzecha D, Szymusik I, Wielgos M, et al. The impact of endometriosis on the quality of life and the incidence of Depression-A cohort study. Int J Environ Res Public Health. 2020;17(10):3641.
- Friedl F, Riedl D, Fessler S, et al. Impact of endometriosis on quality of life, anxiety, and depression: an Austrian perspective. Arch Gynecol Obstet. 2015;292(6):1393–1399.
- Stochino-Loi E, Millochau JC, Angioni S, et al. Relationship between patient age and disease features in a prospective cohort of 1560 women affected by endometriosis. J Minim Invasive Gynecol. 2020;27(5):1158–1166.
- Montanari E, Dauser B, Keckstein J, et al. Association between disease extent and pain symptoms in patients with deep infiltrating endometriosis. Reprod Biomed Online. 2019;39(5):845–851.
- French L. Dysmenorrhea. Am Fam Physician. 2005;71(2):285–291.
- Smorgick N, As-Sanie S. Pelvic pain in adolescents. Semin Reprod Med. 2018;36(2):116–122.
- Rodrigues AC, Gala S, Neves Â, et al. [Dysmenorrhea in adolescents and young adults: prevalence, related factors and limitations in daily living]. Acta Med Port. 2011;24(Suppl 2):383–388. Decquiz 389-92.
- Porpora MG, Koninckx PR, Piazze J, et al. Correlation between endometriosis and pelvic pain. J Am Assoc Gynecol Laparosc. 1999;6(4):429–434.
- Apostolopoulos NV, Alexandraki KI, Gorry A, et al. Association between chronic pelvic pain symptoms and the presence of endometriosis. Arch Gynecol Obstet. 2016;293(2):439–445.
- Sepulcri RdP, do Amaral VF. Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 2009;142(1):53–56.
- Shum LK, Bedaiwy MA, Allaire C, et al. Deep dyspareunia and sexual quality of life in women With endometriosis. Sex Med. 2018;6(3):224–233.
- Facchin F, Barbara G, Saita E, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36(4):135–141.
- Yong PJ, Williams C, Yosef A, et al. Anatomic sites and associated clinical factors for deep dyspareunia. Sex Med. 2017;5(3):e184–e195.
- Méar L, Herr M, Fauconnier A, et al. Polymorphisms and endometriosis: a systematic review and meta-analyses. Hum Reprod Update. 2020;26(1):73–102.
- Ancelin ML, Norton J, Canonico M, et al. Aromatase (CYP19A1) gene variants, sex steroid levels, and late-life depression. Depress Anxiety. 2020;37(2):146–155.
- Souza CA, Oliveira LM, Scheffel C, et al. Quality of life associated to chronic pelvic pain is independent of endometriosis diagnosis–a cross-sectional survey. Health Qual Life Outcomes. 2011;9:41.
- Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691.
- Brawn J, Morotti M, Zondervan KT, et al. Central changes associated with chronic pelvic pain and endometriosis. Hum Reprod Update. 2014;20(5):737–747.
- Moehner S, Becker K, Lange JA, et al. Risk of depression and anemia in users of hormonal endometriosis treatments: results from the VIPOS study. Eur J Obstet Gynecol Reprod Biol. 2020;251:212–217.