Abstract
Introduction
Postoperative gastroesophageal reflux disease (GERD) can be a consequence of laparoscopic sleeve gastrectomy (LSG). Intrathoracic sleeve migration (ITSM) is a factor contributing to its development. This study aimed to investigate whether the occurrence of ITSM can be prevented by applying a polyglycolic acid (PGA) sheet around the His angle.
Material and methods
In this retrospective analysis, 46 consecutive patients who underwent LSG were divided into two groups: Group A – our standard LSG in the first half (n = 23) and Group B – our standard LSG with PGA sheet covering the angle of His in the second half (n = 23). We compared the two groups for one-year postoperative GERD and the incidence of ITSM.
Results
No significant differences were found between the two groups in terms of patient background, operation time, and one-year postoperative total body weight loss, and no adverse effects related to the PGA sheet were observed. Group B had a significantly lower incidence of ITSM than Group A, and the rate of acid-reducing medicine usage was less pronounced in Group B during follow-up (p < .05).
Conclusion
This study suggests that applying a PGA sheet can be safe and effective in reducing postoperative ITSM and preventing exacerbations of postoperative GERD.
Introduction
Laparoscopic sleeve gastrectomy (LSG) is a popular type of bariatric and metabolic surgery (BMS) worldwide, with 67% of primary procedures being LSG [Citation1]. LSG has been established as a safe and effective procedure; however, one of its complications is postoperative gastroesophageal reflux disease (GERD). The occurrence of de novo or exacerbated GERD after surgery can result in postoperative discomfort, extended use of acid-reducing medication, and potentially revision surgery. Moreover, the potential risk of developing esophageal cancer due to prolonged reflux esophagitis and Barrett’s esophagus (BE) cannot be overlooked [Citation2].
Intrathoracic sleeve migration (ITSM) is considered one of the primary causes of GERD after LSG [Citation3,Citation4]. ITSM refers to a sliding hiatal hernia arising after LSG. Considering the correlation between ITSM and gastroesophageal reflux symptoms post-LSG surgery, preventive measures against ITSM during primary LSG could be highly beneficial. The effectiveness of concomitant hernial repair and fundocrural fixation remains controversial. Performing additional procedures, such as the Nissen procedure or ligamentum teres cardiopexy, only costs a little extra time; however, the lack of long-term efficacy data remains a concern [Citation5].
The NeoveilTM sheet (Gunze Ltd., Kyoto, Japan), composed of polyglycolic acid (PGA), is a bioabsorbable mesh sheet commonly used in surgical suturing or tissue reinforcement [Citation6–8]. The NeoveilTM sheet mechanism for reinforcing tissue is believed to involve fibroblasts infiltrating the PGA sheet and serving as a scaffold for the development of newly formed granulation tissue at the implant site [Citation9]. Considering these mechanisms, we hypothesized that the PGA sheet could not only reinforce the staple line but also potentially prevent ITSM by promoting adhesion between the upper portion of the gastric sleeve and the liver. This study aimed to assess whether the application of a PGA sheet could reduce the risk of ITSM and GERD post-LSG surgery.
Material and methods
Patients and design
This single-center study retrospectively reviewed prospectively collected data from patients who underwent LSG and completed a 12-month postoperative examination. This study was conducted in accordance with the ethical standards of our institutional committee (approval number IRB#S21023_S18061). A total of 46 consecutive patients (24 males and 22 females; mean age, 45.9 years) who underwent LSG at Toho University Sakura Medical Center between April 2020 and February 2022 were included in the study. The inclusion criteria were body mass index (BMI) >35 kg/m2 with one or more comorbidities (type 2 diabetes, dyslipidemia, hypertension, and obstructive sleep apnea syndrome). Each patient was preoperatively screened and evaluated by a multidisciplinary team. The study excluded five patients who did not attend the postoperative one-year follow-up visit and those who underwent other types of bariatric and metabolic surgery, including 11 cases of LSG with duodenojejunal bypass and one case of laparoscopic Roux-en-Y gastric bypass.
The patients were divided into two groups based on the timing of LSG and the use of the PGA sheet: Group A - our standard surgical procedure, as outlined below, consisted of performing LSG with reinforcement of the staple line using a continuous suture technique. Group B – our standard LSG with PGA sheet covering the angle of His.
Patients in Group A underwent surgery from April 2020 to February 2021, while patients in Group B were operated on between March 2021 and February 2022.
Laparoscopic sleeve gastrectomy
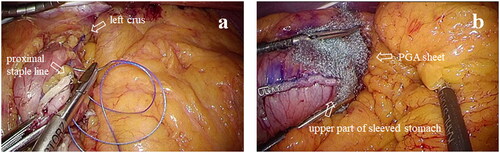
Between April 2020 and February 2022, the same surgical team performed standardized LSG procedures at our hospital. LSG was performed by the use of a six-trocar technique. We did not routinely check the hiatus because preoperative upper gastrointestinal (UGI) endoscopy excludes the presence of symptomatic hiatal hernia and severe reflux esophagitis (grade C or D according to the Los Angeles classification). After dissection of the gastric greater curvature and complete mobilization of the fundus with exposure of the left crus using energy devices such as LigaSure™ (Covidien, Boulder, CO, USA) or Harmonic HD 1000i® (Ethicon, Cincinnati, OH, USA), the stomach was vertically transected. Gastric transection was initiated 5 cm proximal to the pylorus to create a narrow gastric sleeve against a 36-Fr gastric calibration tube. For the first staple fire (Endo-GIA™ (Covidien, Boulder, CO, USA) or Echelon Flex™ (Ethicon, Cincinnati, OH, USA)), we always use black loads and then purple or gold loads for further stapling. The stapler was fired in sequence along with the calibration tube until it reached the angle of His, at which we maintained a distance of approximately 1 cm from the gastroesophageal junction. In all cases, the entire staple line was oversewed with 3-0, non-absorbable V-LocTM (Covidien, Dublin, Ireland) while the gastromesenteropexy was used for the fixation of the sleeved stomach. In Group A, a running suture was started by anchoring the most proximal part of the staple line and left crus, as shown in . On the other hand, in Group B, the running suture was performed without fixation to the left crus. In addition, in Group B, each half of a NeoveilTM sheet (10 × 5 cm) was wrapped around the upper staple line and the angle of His, as depicted in .
Figure 1. (a) The diaphragmatic crura is fixed with a proximal staple line. (b) Polyglycolic acid (PGA) sheet wrapped around the upper part of sleeved stomach.
Typically, patients are discharged on postoperative day 4 with appropriate dietary instructions from a dietician. Postoperatively, patients received prophylactic proton pump inhibitors for three months and continued as needed if GERD symptoms persisted. Follow-up visits were scheduled for postoperative months 1, 3, and 6 and every six months thereafter, as part of our postoperative BMS program. Laboratory evaluation and weight measurements were performed at each visit. All postoperative patients underwent mandatory thoracic and abdominal computed tomography (CT) and upper gastrointestinal (UGI) endoscopy one year after surgery. The costs associated with the surgery, such as hospitalization fees, surgical fees, examination fees, and medication costs, are all covered by Japan’s medical insurance system.
Data acquisition
We retrieved preoperative data from medical records, which included patient characteristics such as sex, age, height, weight, and body mass index (BMI), as well as the visceral fat area (VFA), subcutaneous fat area (SFA), usage of acid-reducing medicine (proton pump inhibitor or potassium-competitive acid blocker), and GERD symptoms. The operative time was recorded. For the 12-month postoperative data, we obtained the total weight loss percentage (%TWL) and VFA, SFA, use of acid-reducing medicine, GERD symptoms, and any surgery-related complications.
Evaluation of ITSM and hiatal hernia
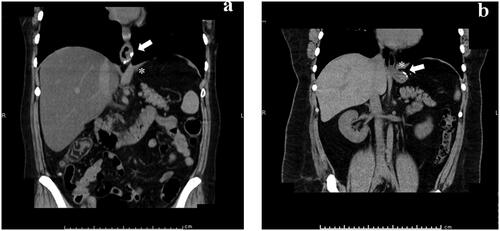
This study evaluated the incidence of ITSM and hiatal hernia after LSG. ITSM is a sliding hernia that can develop after LSG and is defined as the presence of a staple line located above the diaphragmatic crus on coronal CT (). All patients enrolled in the study underwent preoperative and postoperative one-year CT imaging to assess the upper abdomen and thorax. The CT examinations were performed using a multislice spiral 64-detector CT system (Revolution EVO; GE Healthcare, Waukesha, WI, USA) with a slice thickness of 5 mm. Both axial and coronal CT images were acquired and subsequently analyzed on the SYNAPSE (Fuji Medical Systems, Tokyo, Japan) workstation. These images facilitated the measurement of the migration of the proximal end of the staple line in millimeters above the level of the diaphragmatic crus.
Figure 2. ITSM was defined as the presence of staple lines (arrows) above diaphragmatic crus (*) in coronal CT view: (a) ITSM (+), (b) ITSM (−).
Esophageal hiatal hernia type was also assessed using the endoscopic Hill classification (Supplementary Appendix 1). The Hill classification system provides both the anatomical and functional aspects of hiatal hernias and indicates that the higher the grade, the greater the likelihood of a patient having reflux esophagitis [Citation10].
Evaluation of GERD
We defined GERD based on the general definition of the condition as either the presence of an esophageal mucosal lesion confirmed by endoscopy or any symptoms related to gastroesophageal reflux that required treatment with acid-reducing medicine [Citation11].
The Los Angeles Classification system is widely used in clinical practice and research to assess the severity of esophagitis and to guide treatment decisions. This classification system is based on the endoscopic appearance of the esophagus and categorizes esophagitis into four grades: A, B, C, and D [Citation12].
The Frequency Scale for Symptoms of GERD (FSSG) questionnaire was used to evaluate the severity of GERD symptoms [Citation13], also known as the F-scale. We assessed GERD after LSG by scoring the frequency of seven items on the F-scale questionnaire specifically related to acid reflux symptoms. The seven questions are shown in Supplementary Appendix 2. The grading of the items by the patient was based on a Likert scale ranging from 0 (never) to 4 (always). The outcome was the severity score of GERD symptoms, which was calculated by adding the scores of the seven items. The scores ranged from 0 to 28.
Statistical analysis
Continuous variables are shown as the mean and standard deviation (SD) or median and interquartile range, whereas categorical variables are shown as the number and percentage.
Parametric data were compared using a paired Student’s t-test, whereas nonparametric data were analyzed using the Mann–Whitney U test. Categorical variables were compared using the χ2 test. All statistical analyses were performed with R software version 3.2.3 (http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmed.html), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). Values were considered statistically significant at two-sided p-values < .05.
Results
Before surgery, no significant differences were observed between the groups in terms of demographic, clinical, or anthropometric characteristics (). On preoperative CT, the esophagogastric junction was located in the abdominal cavity in all cases. No significant differences were observed between the two groups regarding the preoperative grading of the esophageal hiatal hernia based on the Hill classification. Furthermore, no significant differences were found between the two groups in the preoperative severity of GERD, as assessed by the LA classification and the F-scale score for acid reflux. The preoperative use of acid-reducing medications prior to surgery was similar in both groups ().
Table 1. Pre- and postoperative demographic data and GERD-related variables for the study groups.
No significant differences were observed in the operation time between Group A (175 min) and Group B (163 min). In Group A, two patients underwent endoscopy due to repeated episodes of vomiting at postoperative months 1 and 2. However, no structural stenosis was detected in either patient, and the symptoms resolved spontaneously.
The reduction in VFA and SFA one year post-LSG surgery did not differ significantly between Group A and Group B (89.1 cm2 vs. 81.4 cm2, p = .601 and 227 cm2 vs. 129 cm2, p = .221, respectively). Additionally, %TWL at 1-year post-LSG surgery was not significantly different between Group A and Group B (28.5 ± 9.1% vs. 24.3 ± 11.7%, p = .175). One year after LSG, both groups showed some degree of grade progression and increased scores based on the Hill and LA classification assessed through endoscopic evaluation, as well as the F-scale score evaluated using FSSG. However, our analysis did not reveal any significant differences between the two groups in terms of these evaluations. In Group B, while there was no significant difference in the migration distance towards the proximal side compared to Group A, the incidence rate of ITSM one year post-LSG surgery was significantly lower (Group A: 52% vs. Group B: 13%, p = .01) (). Additionally, the rate of acid-reducing medicine intake one year postoperatively was significantly lower in Group B than in Group A (Group A: 65% vs. Group B: 30%, p = .038) ().
During reoperations for a ventral hernia [case 1] and intussusception caused by a submucosal tumor of the ileum [case 2], we reexamined the upper part of the sleeved stomach 13 and 14 months after LSG in Group B. The NeoveilTM sheet used during LSG contained a green colorant, which allowed us to locate the application site. Mild adhesions were observed between the upper part of the stomach and the sub-hepatic surface ( [case 1]). No adverse effects related to the PGA sheets were observed during the observation period in Group B.
Discussion
One major drawback of LSG is the potential for developing de novo GERD or exacerbating preexisting GERD. A recent meta-analysis by Yeung et al., including 10,718 patients with follow-ups from three to 132 months, found that 19% experienced worsening GERD, while 23% developed de novo GERD after LSG [Citation14]. Moreover, long-term complications of postoperative GERD, such as erosive esophagitis, BE, and esophageal adenocarcinoma (EAC), are concerns [Citation2]. With the high prevalence of GERD in a growing number of obese patients and obesity being a known risk factor for Barrett Esophagus (BE) and esophageal adenocarcinoma (EAC) [Citation2], these concerns might deter LSG as a BMS option in the future.
Several mechanisms have been proposed to explain GERD worsening after LSG, including lower esophageal sphincter (LES) pressure, angle of His disruption, reduced gastric compliance with higher intragastric pressure, decreased gastric emptying, late sleeve dilatation, and postoperative sliding hiatal hernia development [Citation15]. ITSM, an underreported sliding hiatal hernia, can occur after LSG and correlates with GERD symptoms based on migration degree [Citation16,Citation17]. Few studies address ITSM incidence after LSG, but it can occur postoperatively with a reported incidence of 30%–72.5% [Citation3]. In this study, although intraoperative hiatal hernia defect checks were not performed in both groups, the incidence of ITSM one year postoperatively was higher in Group A, with a rate of 52%, consistent with a previous report.
Some authors suggest LSG with concomitant hiatal hernia repair may reduce the negative effects of LSG on GERD [Citation18]. However, data on additional cruroplasty are controversial. A three-dimensional CT-based study on ITSM showed migration presence was not dependent on time after surgery or preoperatively diagnosed axial hernia [Citation19]. The role of aggressive hiatal hernia repair with cruroplasty during LSG in preventing ITSM remains undefined [Citation20]. Furthermore, LSG with additional fundoplication or ligamentum teres cardiopexy have been described as treatment options for preventing GERD caused by ITSM. Preliminary reports show safety, feasibility, GERD symptom improvement, and reduced esophagitis rates at short-term follow-up [Citation21,Citation22]. However, due to additional operating time and unknown long-term results, these procedures are performed selectively [Citation5].
The NeoveilTM sheet, an absorbable PGA degraded by hydrolysis and absorbed after about 15 weeks, is widely used in gastrointestinal, pancreatic, and thoracic surgeries to reinforce weak tissues or staple regions [Citation6–8]. In our practice, PGA sheet covering was originally initiated with the intention of reinforcing the upper gastric staple line, where sleeve leaks commonly occur [Citation23], with less costly reinforcement material. The NeoveilTM sheet, available in Japan, costs $64 per 5 × 10 cm. This research was inspired by the absence of ITSM on abdominal CT one year postoperatively in early cases of Group B. Our analysis revealed that Group B had a significantly lower incidence of ITSM compared to Group A, with a follow-up period of at least one year after LSG. This finding indicates that placing a PGA sheet over the upper part of the sleeved stomach could potentially reduce the risk of ITSM after LSG.
The non-enzymatic degradation of PGA results in mild acidity from the glycolic acid, which could potentially cause chronic inflammation and serves as a scaffold facilitating the infiltration of inflammatory cells and fibroblasts [Citation24–26]. It is hypothesized that this may promote the formation of new granulation tissue and subsequent adhesions that form in the vicinity of the implanted PGA sheet. Consistent with these assumptions, the local findings observed during the second laparoscopic surgery in the two cases of Group B with the PGA sheet in place indicated the presence of tissue adhesion between the liver and the upper part of the stomach.
Despite the statistically significant lower incidence of ITSM in Group B compared to Group A one year after LSG, there were no significant differences between the two groups in terms of the degree of esophagitis based on the LA classification or the flap valve laxity based on the Hill classification. Additionally, although the acid reflux scores on the F-scale were similar between the groups, the lower rate of acid-reducing medication usage in Group B indicates that patients in this group required less medication to manage their acid reflux symptoms compared to those in Group A.
Decreased LES pressure is considered a cause of GERD after LSG, but its direct impact remains debatable [Citation20]. Partial resection of sling fibers during surgery might cause low LES pressure [Citation27]. Conversely, some researchers argued that ITSM significantly decreases LES pressure [Citation3]. LES pressure comprises intrinsic and extrinsic components. The intrinsic component consists of esophageal muscle fibers under neurohormonal control. Extrinsic factors include external compression by the diaphragm, crura, and phrenoesophageal ligament [Citation28]. Proximal LES displacement due to ITSM separates the intrinsic LES from extrinsic compression. Therefore, it is plausible that the ITSM-preventive effect of the PGA sheet helps maintain LES pressure, reducing gastric acid reflux into the distal esophagus, alleviating GERD symptoms, and decreasing acid-reducing medication use.
This study had several limitations. First, it was a single-institution retrospective cohort study. However, all clinicopathological data were prospectively collected using a consistent protocol, and operations were performed by the same surgical team, controlling for potential confounding factors such as surgical techniques and perioperative care protocols. Second, despite the value of the pilot study as a necessary first step in exploring a novel intervention, our study had a small sample size and can only be considered as preliminary work. One of the future tasks is to conduct a randomized controlled trial using the validated definition of ITSM, including the modality of assessment and the length of migration [Citation4,Citation17,Citation29]. Additionally, PGA sheet effectiveness in preventing ITSM was assessed only in the first year after LSG, requiring further investigation to determine long-term effects. Third, the analysis of GERD after LSG was based only on symptoms and endoscopic findings in the present study, so more objective ways of assessing GERD, such as manometry and 24-h impedance-pH monitoring, could be included in future studies. Finally, PGA sheets were effective in preventing ITSM and GERD in patients with mild preoperative hiatal hernia and/or reflux esophagitis, but further studies are needed to determine their effectiveness in patients with severe preoperative conditions.
Therefore, our findings suggest that the application of a PGA sheet in the upper part of the sleeved stomach during LSG may be effective in reducing postoperative ITSM and GERD. The simplicity and safety of this approach make it an attractive option that is worth considering as a preventive measure against the potential risks of ITSM.
Supplemental Material
Download MS Word (511.6 KB)Acknowledgment
The authors would like to express their gratitude to Cactus Communications Pvt. Ltd. (https://www.editage.jp/) for English language editing and Gunze Ltd. for providing a photo of the NeoveilTM sheet.
Declaration of interest
No potential conflict of interest was reported by the author(s).
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13645706.2023.2271348)
Additional information
Funding
References
- IFSO. The seventh IFSO global registry report. 2022. https://www.ifso.com/pdf/ifso-7th-registry-report-2022.pdf.
- Fisher OM, Chan DL, Talbot ML, et al. Barrett’s oesophagus and bariatric/metabolic surgery—IFSO 2020 position statement. Obes Surg. 2021;31(3):915–934. doi: 10.1007/s11695-020-05143-6.
- Choi SJ, Kim SM. Intrathoracic migration of gastric sleeve affects weight loss as well as GERD-an analysis of remnant gastric morphology for 100 patients at one year after laparoscopic sleeve gastrectomy. Obes Surg. 2021;31(7):2878–2886. doi: 10.1007/s11695-021-05354-5.
- Termine P, Boru CE, Iossa A, et al. Transhiatal migration after laparoscopic sleeve gastrectomy: myth or reality? A multicenter, retrospective study on the incidence and clinical impact. Obes Surg. 2021;31(8):3419–3426. doi: 10.1007/s11695-021-05340-x.
- Ferrer JV, Acosta A, García-Alementa EM, et al. High rate of de novo esophagitis 5 years after sleeve gastrectomy: a prospective multicenter study in Spain. Surg Obes Relat Dis. 2022;18(4):546–554. doi: 10.1016/j.soard.2021.11.011.
- Misawa K, Yoshikawa T, Ito S, et al. Safety and feasibility of linear stapling device with bioabsorbable polyglycolic acid sheet for duodenal closure in gastric cancer surgery: a multi-institutional phase II study. World J Surg. 2019;43(1):192–198. doi: 10.1007/s00268-018-4748-x.
- Iwasaki K, Barroga E, Enomoto M, et al. Use of polyglycolic acid sheets for the prevention of pancreatic fistula after laparoscopic gastrectomy: a single-center retrospective study. Am Surg. 2022:31348221146971.
- Kabuto T, Omasa M, Nagata S, et al. The effect of polyglycolic acid sheet in preventing postoperative recurrent pneumothorax: a prospective cohort study. J Cardiothorac Surg. 2023;18(1):13. doi: 10.1186/s13019-023-02111-w.
- Takagi T, Tsujimoto H, Torii H, et al. New polyglycolic acid fabric for the prevention of postoperative pancreatic fistulas. Asian J Surg. 2018;41(1):59–64. doi: 10.1016/j.asjsur.2016.08.001.
- Hansdotter I, Björ O, Andreasson A, et al. Hill classification is superior to the axial length of a hiatal hernia for assessment of the mechanical anti-reflux barrier at the gastroesophageal junction. Endosc Int Open. 2016;4(3):E311–E317. doi: 10.1055/s-0042-101021.
- Tutuian R, Tack J, Bredenoord AJ, et al. Modern diagnosis of GERD: the Lyon consensus. Gut. 2018;67(7):1351–1362. doi: 10.1136/gutjnl-2017-314722.
- Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45(2):172–180. doi: 10.1136/gut.45.2.172.
- Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39(9):888–891. doi: 10.1007/s00535-004-1417-7.
- Yeung KTD, Penney N, Ashrafian L, et al. Does sleeve gastrectomy expose the distal esophagus to severe reflux?: a systematic review and meta-analysis. Ann Surg. 2020;271(2):257–265. doi: 10.1097/SLA.0000000000003275.
- Oor JE, Roks DJ, Ünlü Ç, et al. Laparoscopic sleeve gastrectomy and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Surg. 2016;211(1):250–267. doi: 10.1016/j.amjsurg.2015.05.031.
- Saber AA, Shoar S, Khoursheed M. Intra-thoracic sleeve migration (ITSM): an underreported phenomenon after laparoscopic sleeve gastrectomy. Obes Surg. 2017;27(8):1917–1923. doi: 10.1007/s11695-017-2589-6.
- Karila-Cohen P, Pelletier AL, Saker L, et al. Staple line intrathoracic migration after sleeve gastrectomy: correlation between symptoms, CT three-dimensional stomach analysis, and 24-h pH monitoring. Obes Surg. 2022;32(7):1–9. doi: 10.1007/s11695-022-06074-0.
- Rosenthal RJ, Diaz AA, Arvidsson D, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis. 2012;8(1):8–19. doi: 10.1016/j.soard.2011.10.019.
- Baumann T, Grueneberger J, Pache G, et al. Three-dimensional stomach analysis with computed tomography after laparoscopic sleeve gastrectomy: sleeve dilation and thoracic migration. Surg Endosc. 2011;25(7):2323–2329. doi: 10.1007/s00464-010-1558-0.
- Hutopila I, Ciocoiu M, Paunescu L, et al. Reconstruction of the phreno-esophageal ligament (R-PEL) prevents the intrathoracic migration (ITM) after concomitant sleeve gastrectomy and hiatal hernia repair. Surg Endosc. 2023;37(5):3747–3759. doi: 10.1007/s00464-022-09829-z.
- Rebecch F, Ugliono E, Morino M. Reflux following bariatric surgery. Minim Invasive Surg. 2022;6(5):30. doi: 10.20517/2574-1225.2021.147.
- Runkel A, Scheffel O, Marjanovic G, et al. Augmentation of hiatal repair with the ligamentum teres hepatis for intrathoracic gastric migration after bariatric surgery. Obes Surg. 2021;31(4):1422–1430. doi: 10.1007/s11695-020-05153-4.
- Kim J, Azagury D, Eisenberg D, et al. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11(4):739–748. doi: 10.1016/j.soard.2015.05.001.
- Ceonzo K, Gaynor A, Shaffer L, et al. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Eng. 2006;12(2):301–308. doi: 10.1089/ten.2006.12.301.
- Matoba M, Hashimoto A, Tanzawa A, et al. Prevention of polyglycolic acid-induced peritoneal adhesions using alginate in a rat model. Biomed Res Int. 2015;2015:403413. doi: 10.1155/2015/403413.
- Funai K, Suzuki K, Shimizu K, et al. Ablation of weak emphysematous visceral pleura by an ultrasonically activated device for spontaneous pneumothorax. Interact Cardiovasc Thorac Surg. 2011;12(6):908–911. doi: 10.1510/icvts.2010.264044.
- Braghetto I, Lanzarini E, Korn O, et al. Manometric changes of the lower esophageal sphincter after sleeve gastrectomy in obese patients. Obes Surg. 2010;20(3):357–362. doi: 10.1007/s11695-009-0040-3.
- Rosen RD, Winters R. Physiology, lower esophageal sphincter. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557452/.
- Sabry K, Elmaleh HM, El-Swaify ST, et al. Surgical management algorithm for intrathoracic sleeve migration: a retrospective series and literature review. J Laparoendosc Adv Surg Tech A. 2022;32(10):1078–1091. doi: 10.1089/lap.2022.0298.

![Figure 3. The location where the PGA sheet was applied can be identified by the arrows [case 1].](/cms/asset/2fa11179-c0c9-4ad1-bb14-60e4bfed5ae1/imit_a_2224437_f0003_c.jpg)