Abstract
A key question for evidence-based medicine (EBM) is how best to model the way in which EBM should ‘[integrate] individual clinical expertise and the best external evidence’. We argue that the formulations and models available in the literature today are modest variations on a common theme and face very similar problems when it comes to risk analysis, which is here understood as a decision procedure comprising a factual assessment of risk, the risk assessment, and the decision what to do based on this assessment, the risk management. Both the early and updated models of evidence-based clinical decisions presented in the writings of Haynes, Devereaux and Guyatt assume that EBM consists of, among other things, evidence from clinical research together with information about patients’ values and clinical expertise. On this A-view, EBM describes all that goes on in a specific justifiable medical decision. There is, however, an alternative interpretation of EBM, the B-view, in which EBM describes just one component of the decision situation (a component usually based on evidence from clinical research) and in which, together with other types of evidence, EBM leads to a justifiable clincial decision but does not describe the decision itself. This B-view is inspired by a 100-years older version of EBM, a Swedish standard requiring medical decision-making, professional risk-taking and practice to be in accordance with ‘science and proven experience’ (VBE). In the paper, we outline how the Swedish concept leads to an improved understanding of the way in which scientific evidence and clinical experience can and cannot be integrated in light of EBM. How scientific evidence and clinical experience is integrated influences both the way we do risk assessment and risk management. In addition, the paper sketches the as yet unexplored historical background to VBE and EBM.
1. Introduction
EBM is actually only a reformulation of the motto ‘science and proven experience.’ (Werkö et al. Citation2002, 3478, our translation)
Globally, evidence-based medicine (EBM) is favoured in the public sector. EBM is often concerned with risk assessment and risk management. Consider a 77-year-old woman who is admitted with non-rheumatic atrial fibrillation and her first bout of mild left ventricular failure; ‘What is the risk reduction for stroke from warfarin therapy in such a patient, and what is the risk of harming her with this therapy?’, asks Rosenberg and Donald (Citation1995) and presents EBM in four steps:
| • | Formulate a clear clinical question from a patient’s problem | ||||
| • | Search the literature for relevant clinical articles | ||||
| • | Evaluate (critically appraise) the evidence for its validity and usefulness | ||||
| • | Implement useful findings in clinical practice | ||||
In Sweden a bipartite, partly overlapping standard that is more than 100 years older than EBM applies in the public sector and parts of the private sector as well. This Swedish standard requires decision-making and practice to be based on both science and proven experience, or vetenskap och beprövad erfarenhet (VBE), and indeed leading Swedish physicians often think of EBM as a reformulation of the Swedish standard. Lars Werkö, ‘the icon beyond all comparison in Swedish health care’ (Hont Citation2009) is a clear example (e.g. see Werkö et al. (Citation2002) above). Since its first legal application in Swedish health care in the late 1800s, the VBE standard has been pressed into service in law and public policy in areas as diverse as medicine and health care, education, environmental risk assessment, veterinary care and social work. We shall primarily focus on the application of VBE in medicine. To be strict we should thus be talking about VBE-M. However, in order not to complicate matters unnecessarily we shall in this paper assume that what is true of VBE in medicine is true of VBE in general.
Swedish health law explicitly requires that health care is provided in accordance with VBE. The preparatory works have justified the requirement by stressing the important role that VBE plays in reducing patient risks (Prop. 2009/10:210, 191). Furthermore, legal scholars have pointed out that the VBE requirement itself implies a risk-benefit assessment (cf. Rynning Citation1994, 138; Wahlberg and Sahlin Citation2017).
The Swedish concept of VBE helps us to understand the ways in which scientific evidence and clinical experience both can and cannot be integrated within EBM, and thus the extent to which EBM can be used to understand and reduce risks. The similarities and dissimilarities between VBE and EBM shed light on the capacity of EBM to ‘[integrate] individual clinical expertise and the best external evidence’ (Sackett et al. Citation1996). This most influential and elusive ambition of EBM (Sackett et al. Citation1996) is the primary focus of the current paper. In addition, however, VBE helps to bring out the historical background to EBM.
2. EBM and VBE: similarities and dissimilarities
Prima facie it makes sense to compare EBM and VBE. (1) Both introduce evidentiary standards for decision-making (not a standard for science as such). (2) Each promotes the goal of making more use of science and sound evidence in practical decision-making. But (3) the two approaches diverge, on the surface at least, when it comes to the types of evidence that should be allowed to influence practical decision-making and play a role in risk assessment.
(1) EBM highlights the decision-making context. It is primarily about the professions and the connection between the academic disciplines and the professions; only by implication is it about the disciplines themselves. Several of the leading articles on EBM were published in the British Medical Journal, which has the slogan: helping doctors make better decisions. Rosenberg’s and Donald’s well-known 1995 BMJ paper is entitled ‘Evidence based medicine: an approach to clinical problem-solving’.
Similarly, in Swedish law VBE is the gold standard for decision-making and practice, especially in health care. For example, VBE states that medical practice must be based on science and proven experience. Health care workers who do not provide care in accordance with VBE can be criticized by the Health and Social Care Inspectorate and even be held responsible according to penal law. VBE also helps to define patients’ rights to reimbursement for expenses associated with treatments in other European countries. The legislative use of science and proven experience illustrates that the notion is intended for policy-making, practical decision-making and reducing risks.
(2) To a large extent EBM focuses on the need to make more, and better, use of research findings in clinical decision-making:
Evidence based medicine is the process of systematically finding, appraising, and using contemporaneous research findings as the basis for clinical decisions. For decades people have been aware of the gaps between research evidence and clinical practice, and the consequences in terms of expensive, ineffective, or even harmful decision making. Inexpensive electronic databases and widespread computer literacy now give doctors access to enormous amounts of data. Evidence based medicine is about asking questions, finding and appraising the relevant data, and harnessing that information for everyday clinical practice. (Rosenberg and Donald Citation1995)
The focus, claims (Eddy Citation2005, 14), ‘is on educating physicians to help them bring more research and evidence into their individual decisions about individual patients.’ The same goes for VBE, but there are differences. EBM is primarily about decision-making. VBE is primarily about patient security and avoiding unnecessary risk-taking.
Before we proceed, it should be noted that, in health care at least, the Swedish notion is not explicitly defined in any official documents. One will search in vain for suitable stipulations to guide applications of the laws in which the expression occurs. Hence its characteristics have to be inferred from its intended purpose and many applications, i.e. its use and history. We will therefore present a sketch of the history of VBE (and to a lesser extent EBM).
It was probably no accident that the Swedish concept emerged in health care regulations in the late 1800s. The concept itself is, at least, somewhat older; almost exactly the same formulation occurs in the oath that were taken by those who were awarded the licentiate degree in medicine in Uppsala, Lund, and Stockholm from 1829 and onwards (SFS 1829:12, see also Kock Citation1939). It is sometimes argued that the mid-1800s were dominated by lack of confidence in the therapeutic methods available in Sweden and elsewhere. Scholarly work referring to this period uses terms such as ‘doubt disease’ (Fåhræus Citation1950, 98), ‘therapeutic nihilism’ (Danek Citation1969, 65), ‘the bankruptcy of therapy’, and ‘crisis in medical self-confidence’ (Stolt Citation1994, Chapters 4 and 5). Mid-1800s advances in basic medical science did not reach practitioners and were generally of little therapeutic consequence (Porter Citation1995), and this was especially so, perhaps, for the typical Swedish countryside doctor:
Practitioners in the countryside used trial and error, and as late as 1850 they had little use of medical science in their everyday practice. (Stolt Citation1994, 159, our translation)
Whatever the connection might have been medical science advanced rapidly during the second half of the 1800s: from Louis Pasteur’s 1859 suggestion that micro-organisms may cause many human and animal diseases, Joseph Lister’s 1867 publication ‘On the Antiseptic Principle in the Practice of Surgery’, showing that disinfection reduces post-operative infections, to Robert Koch’s 1882–1883 isolations of the micro-organism responsible for tuberculosis and cholera. In short, the decades before the Swedish regulation was put in place involved several breakthroughs in medical science; it was a time when medical science, finally, advanced to a position from which it could actually prove useful to medical practice. It is indeed interesting that those who explain the emergence of EBM also refer to medical breakthroughs in the late 1800s: ‘The 100-year period between 1885 and 1985 brought amazing medical breakthroughs’ (Howick Citation2011, 11).
It makes sense to ponder what happened in Finland during this period. For centuries – until 1809 – Finland and Sweden were joined. Considerable overlap with regard to the requirement of science and proven experience between the two countries would come as no surprise. The Medical Society of Finland (Finska läkaresällskapet) was founded in 1835 with the dual purpose of developing medical science and health care. It was followed by The Finnish Medical Society (Duodecim) in 1881, which aimed to develop medical science and practice in Finland.Footnote1 Nowadays, the overwhelming majority of Finnish physicians who are members of The Finnish Medical Association (Lääkäriliitto), founded in 1910, are committed to treating patients in accordance with science and proven experience through the code of medical ethics approved by the association’s delegate committee (Lääkäriliitto Citation2014). Moreover, it is a legal requirement in Finland since 1994 that all health care personnel should only apply methods that there is proven experience of (since 2000 the same requirement holds for those in veterinary care).Footnote2 Much of the development leading to the current role of VBE in Finland has happened after 1809. Thus, the overlap is not a mere historical artefact. So what we refer to as the Swedish concept has a perfect match in Finland, although it is much more widespread in its Swedish applications. The motivation behind the requirement in Finland is arguably the same as in Sweden since the two countries had so much in common during the concept’s pre-history. Ideas similar to VBE occur in eighteenth-century writings by, for instance, the father of paediatrics in Sweden, Nils Rosén; moreover, a predecessor from 1733 resembles in some respects the 1829 oath we have referred to above (e.g. Eklöf Citation2000).
In fact, one could claim that VBE is a Nordic concept rather than exclusively Swedish. For instance, since 1998 psychologists in the Nordic countries are committed to VBE through Yrkesetiska principer för psykologer i Norden (Sveriges Psykologförbund Citation1998, 6)
Certainly, it was not only in the Nordic countries that it was acknowledged, around 1890, that medical science ought to guide medical decision-making. Failure to consider medical science in the medical profession was criticized in The Boston Medical and Surgical Journal too:
… medical art without science is not only unprogressive, but almost inevitably becomes quackery. As soon as we treat our patients by rule of thumb, by tradition, by dogmas, or by metaphysical axioms, we do injury to ourselves as well as to them. (Pye-Smith Citation1900, 173)
There is arguably a similar story to be told about the emergence of EBM exactly 100 years later. Here both Guyatt and Sackett report on the need to be sceptical vis-à-vis received medical wisdom (Howick Citation2011, chapter 2).
(3) However, it seems that experience of a specific kind – proven experience – was also identified as important as a result of the arrival of VBE. Somewhat paradoxically, it seems that the development of relevant scientific evidence was accompanied by a corresponding development (or upgrading, or rating-up) in the role of evidence of a certain kind from experience as well. This is the third relevant comparison point between EBM and VBE. Looking at the recent introduction of science and proven experience in the Swedish Education Act (2010:800), we can see that Swedish schools and education authorities have developed a growing interest in proven experience. In particular, these discussions highlight the evidential relevance of experience within the professional collective. A third comparison between EBM and VBE can therefore be based on the way they deal with the relationship between two different types of evidence: evidence that is ‘scientific’ and evidence that is ‘experienced based’.
Whether the prominence of proven experience is to be counted as a similarity between EBM and VBE depends on whether EBM and VBE have the same effect of rating up experience of the proven kind. We are ready to argue that they do, but this assessment depends on an assumption few advocates of EBM endorse. The assumption is that there is no strong link between EBM and science – or rather that the link, such as it is, is no stronger than that connecting EBM and proven experience. For a related observation, see Stoyanov, Machamer, and Shaffner (Citation2012, 150). In other words, the scientific classification here is not straightforwardly guaranteed by the use of certain methods or methodologies recommended by EBM such as, for example, randomized controlled trials (RCT). Much of what is regarded as being at the core of EBM could then equally well be classified as proven experience. To the extent that this assumption is accepted there is reason to think that EBM would simply prioritize experience of a certain kind. Advocates of EBM might be dissatisfied with that implication because they wish to preserve the link between basic science and clinical research:
Evidence-based medicine focuses on these systematic studies simply because they represent the most advanced stages of testing to ascertain whether the innovations of basic science work, how well they work, and for whom they work when applied in the clinical setting. Thus, evidence-based medicine is not in competition with basic science; rather it depends on it and builds on it. (Haynes et al. Citation1996, 196–97)
A further dissimilarity between EBM and VBE can be detected. The meaning of VBE varies with context among medical practitioners. (Persson and Wahlberg Citation2015) reports that the BE (or proven experience) component is sometimes taken to report a property of doctors and sometimes used to refer to a fact about how seriously a therapy has been tested in practice. By contrast, EBM seems fixed. Indeed, if it were not fixed, it would be difficult to understand the need for instruments such as the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. GRADE is a framework for synthesizing and rating the quality of evidence, and for providing clinical practice guidelines addressing alternative management options. It is used by many important actors in health care all over the world, including WHO and the Cochrane Collaboration.
To the extent that EBM introduced a new paradigm – see, for instance, Evidence-Based Medicine Working Group (EBMWG Citation1992) – it ought to follow that something of fundamental importance remains fixed through various applications of EBM. The primary candidate for this static role is the EBM position on evidence (see next section, and Howick Citation2011, 4).
3. EBM and VBE: the question of evidence
There is a clear sense in which medicine must always be based on some kind of evidence. If evidence is merely a ground for belief, there will not be anything new about EBM. The ‘evidence’ in EBM, and in the ‘science/vetenskap’ (V) and ‘proven experience/beprövad erfarenhet’ (BE) of VBE, is all about what medical practitioners, or policy-makers, can justifiably base their decisions and risk analyses on.
EBM is a procedure, or approach, that ensures, or perhaps maximizes, justifiable decisions. However, as we will argue, it is far from clear that it is a good risk analysis approach. VBE, as it stands, is a criterion for evaluating whether a decision or risk analysis is warrantable. This does not entail that VBE is only put to use post hoc. VBE – even its BE-component – can be used prospectively, too, as can be seen from the discussions in the Journal of the Swedish Medical Association cited in Persson and Wahlberg (Citation2015) and in the oath taken by Finnish physicians – which requires that one to tries to advance proven experience in one’s field.
So, what is this evidence that makes EBM different from medicine practised before 1990? There are two suggestions, both present in the prehistory of EBM. Archie Cochrane wrote in 1972:
It is surely a great criticism of our profession that we have not organised a critical summary, by specialty or subspecialty, adapted periodically, of all relevant randomised controlled trials. (quoted from Sønbø Kristiansen and Mooney (Citation2004, 2))
The second is that EBM builds on an idea of levels of evidence which not only identifies but also ranks the relevant kinds of evidence. For Cochrane in 1972, it was randomized controlled trials that constituted the relevant level. In the case of EBM, the Oxford Centre for Evidence-Based Medicine (CEBM) presents a comprehensive list of levels of evidence for different clinical questions. For therapy/prevention these are, from the top down: systematic review of RCTs, individual RCTs (all or none), systematic reviews of cohort studies, individual cohort studies, ‘outcomes’ research, ecological studies, individual case-control studies, case series, and last, expert opinions either without explicit critical appraisal or based on physiology, bench research or ‘first principles’ (CEBM Citation2009).
It is obvious that evidence from basic medical science (physiological processes) and institutional or individual experience are not held in high regard.Footnote3 The evidence on which decisions should be based is that deriving from clinical research. This evidence has high predictive value. An early presentation of EBM from the EBMWG (Citation1992), states that:
Evidence-based medicine de-emphasizes intuition, unsystematic clinical experience, and pathophysiologic rationale as sufficient grounds for clinical decision making and stresses the examination of evidence from clinical research. (EBMWG Citation1992, 2420)
It is certainly plausible to say that EBM cannot be based solely on evidence from clinical research. Results from clinical research are not always there to be had, and where they are unavailable unsystematized clinical experience can be used as evidence:
… systematic attempts to record observations in a reproducible and unbiased fashion markedly increase the confidence one can have in knowledge about patient prognosis, the value of diagnostic tests, and the efficacy of treatment. In the absence of systematic observation one must be cautious in the interpretation of information derived from clinical experience and intuition, for it may at times be misleading. (EBMWG Citation1992, 2421)
This is not the situation with regard to VBE. Good therapeutic decision-making rests, according to VBE, on two evidential sources – science and proven experience. In EBM, by contrast, acknowledgement of the importance of clinical experience as evidence seems to be limited to cases where there are no relevant research findings.
This way of conceiving of EBM might not be shared by Swedish doctors. Trained as they are in thinking about VBE, it is natural for Swedish practitioners to assume that EBM has a place for BE as evidence, too (EBM replaces, as it were, the older idea of science (V), in VBE):
It is a misunderstanding to assume that EBM no longer involves what we have called ‘proven experience’ … The right way to use personal experience is to contrast experience against the literature when a current problem is analysed. (Werkö et al. Citation2002, 3478–3479, our translation)
However, as we shall argue in the next section, the combination of EBM and BE is sometimes problematic. EBM’s take on evidence, in what appears to be the most common version of EBM internationally (the A-views subsection, see below), is too restrictive to allow for full incorporation of BE.
4. EBM and VBE: integrating science and experience
In an influential statement of EBM by Sackett et al. (Citation1996), it is clear from the subtitle of the paper that individual clinical expertise is also important in EBM:
Evidence based medicine: what it is and what it isn’t
It’s about integrating individual clinical expertise and the best external evidence
4.1. A-views
(A) is clearly the more common version of EBM. A number of introductions to EBM present flow charts like that in Figure :
Figure 1. The A-view, adapted from http://guides.mclibrary.duke.edu/c.php?g=158201&p=1036021 (downloaded 1 February 2016).
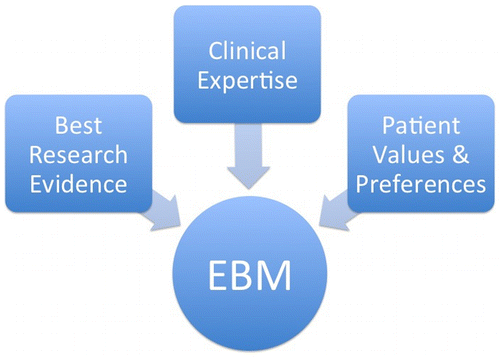
An influential position paper remarks that:
Initially, evidencebased medicine focused mainly on determining the best research evidence relevant to a clinical problem or decision and applying that evidence to resolve the issue. This early formulation deemphasised traditional determinants of clinical decisions, including physiological rationale and individual clinical experience. (Haynes, Devereaux, and Guyatt Citation2002)
Evidence based medicine is the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research. (Haynes, Devereaux, and Guyatt Citation2002)
It is said that the ‘concepts of evidence-based medicine are evolving as limitations of earlier models are addressed’ (Haynes, Devereaux, and Guyatt Citation2002). However, in our view this has not led to the abandonment of the A-view. That view is perhaps even more clearly relied on in later formulations of EBM – e.g. in what is often presented as the ‘contemporary definition’:
… the integration of the best research evidence with clinical expertise and patient values. (Sackett et al. Citation2000, 71)
Evidence-based medicine recognises that such evidence is not “created equal” and provides detailed guides for finding the most rigorous and pertinent evidence for a specific clinical decision.
Incorporating the findings of valid, important and applicable research with your patient values and preferences and your clinical expertise to arrive at the right decision about their individual health care. (CEBM Citation2016)
The evidence, by itself, does not make the decision, but it can help support the patient care process. The full integration of these three components into clinical decisions enhances the opportunity for optimal clinical outcomes and quality of life. (http://guides.mclibrary.duke.edu/c.php?g=158201&p=1036021 [downloaded 1 February 2016])
We title this component of clinical decisions ‘research evidence’ to distinguish it from other forms of information that have always been part of clinical decisions, such as the patient’s history, physical findings, diagnostic tests, circumstances, and stated preferences. (Haynes, Devereaux, and Guyatt Citation2002)
4.2. B-views
The B-view, where EBM is one part of the total decision situation, also has advocates. It is perhaps not surprising that Swedish perspectives sometimes express B-views, since these are much easier to interpret in terms of VBE. For example, in a much quoted passage in a letter to a physician, the Swedish National Board of Health and Welfare explains:
In the exercise of her profession, the medical doctor must take account of both science and proven experience. […] When a new method is introduced, proven experience of it is trivially lacking, and the scientific evidence can suffice for acceptance […]. At other times, long clinical experience might be the dominating evidence in favour of accepting the medical treatment whereas theoretical and/or experimental evidence for its effectiveness might be lacking. (quoted from SOU 1989:60, our translation)
The B-view is not a uniquely Swedish phenomenon. For instance, Haynes et al. (Citation1996, 196), define evidence-based medicine thus:
Evidence-based medicine is the conscientious and judicious use of current best evidence from clinical care research in the management of individual patients.
At first blush this definition seems very similar to those we have referred to as A-views, and that is probably how it was intended to be presented. Here too, however, it might be argued that EBM is but one part of a larger decision context, the management of individual patients and risks associated with that management. But this is not normally how proponents of EBM picture it. They distinguish between early and updated models (Haynes, Devereaux, and Guyatt Citation2002), and this would be one of the earliest – a model that ‘deemphasised traditional determinants of clinical decisions, including physiological rationale and individual clinical experience.’ The alternative B-view reading would be that the early models present EBM as one component of the decision.
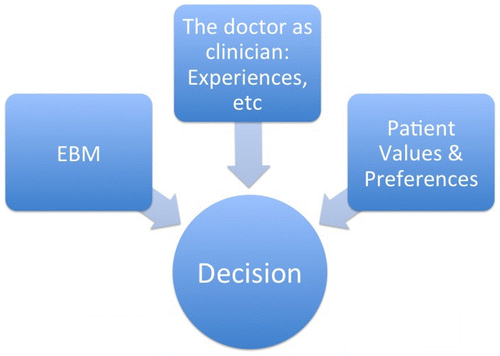
On a B-view, it is much easier to understand EBM and its evidential levels. ‘The doctor as clinician’ (see Figure ) can bring evidence into the decision as well, but it is not the type of evidence EBM speaks of, which concerns research findings only. On the A-view, the clinician’s expertise is only a means of applying research evidence to a particular case (expertise has a role only in risk management, not in risk assessment). That expertise does not provide any additional evidence. On the B-view, by contrast, the individual and collective clinical experience that the clinician adds to the decision basis qualifies as relevant evidence too. Clinical experience underwrites risk assessment as well as risk management. Consequently, according to the B-view EBM does not really set a standard for decision-making. Its capacity to help doctors make better decisions is clearly weakened. EBM becomes much more of a partial tool for decision-making than advocates of EBM normally assume.
Figure 2. The B-view, modified from Asplund Citation(2001).
4. Concluding remarks
Advocates of EBM struggle to model the way evidence-based medicine should ‘[integrate] individual clinical expertise and the best external evidence’ (Sackett et al. Citation1996). We have argued that the formulations and models available in the literature today are variations on a common theme. On these A-views, EBM describes all that goes on in a specific justifiable medical decision. For matters of risk, this entails that clinical experience can be relevant for risk management but not for risk assessment. A-views inevitably create tensions in the concept of evidence they require.
For that reason alone B-views are of interest. On a B-view, EBM describes just one component of the risk assessment (a component usually based on evidence from clinical research). Together with other types of evidence, EBM leads to a justifiable clincial risk assessment, but it does not describe the decision itself. The B-view is inspired by a 100-years older version of EBM, a Swedish standard that requires medical decision-making and practice to be consistent with ‘science and proven experience’.
In sum, the Swedish concept of ‘science and proven experience’ clearly resonates with several characteristics of evidence-based medicine. Like EBM it focuses on evidence (rather than opinion), on science, and on the need for integration. However, the Swedish concept also differs from the concept of evidence-based medicine in that it clearly identifies two sources of evidence as special: science (vetenskap) and proven experience (beprövad erfarenhet). Comparing EBM and VBE, one is struck by the relative clarity of the Swedish notion.
Finally, a version of evidence-based medicine, modelled on the Swedish notion of science and proven experience, would be much more in line with contemporary risk assessment, focusing on the integration of ‘hard’ and ‘soft’ data. The official version of EBM, the A-view, has to rely on that clinical experience informs risk management not risk assessment. Whereas it is an open question whether it is preferable that patients’ values should enter risk assessment, it is difficult to see the rationale for why certain kinds of evidence – i.e. clinical experience – should be excluded from the risk assessment.
Funding
This work is supported by the Swedish Foundation for Humanities and Social Sciences [grant number M14-0138:1] (to J.P., N.V., A.W., L.W., and N.-E.S.) and by The Swedish Research Council Formas [grant number 259-2008-1718] (to J.P.).
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors are grateful for valuable comments from Sten Anttila, Gunnar Broberg, Thomas Kaiserfeld, Peter Machamer, John D. Norton, Sandy Åkerblom, and Haldor Øvreeide and participants at seminars in Lund – notably the 8th Quadrential Fellows Conference – where previous versions of the text have been discussed. Many thanks are due also to the colleagues in the VBE consortium (vbe.lu.se) who commented on earlier drafts of this article. The members of the VBE consortium (apart from the present authors) are: Sten Anttila (SBU), Wändi Bruine de Bruin, Leeds University Business School (UK), Johan Brännmark, Malmö University (Sweden), Alex Davis, Carnegie Mellon University (US), Baruch Fischhoff, Carnegie Mellon University (US), Charlotta Levay, Lund University (Sweden), and Barbara McNeil, Harvard Medical School (US).
Notes
1. The Medical Society of Finland was set up specifically for the Swedish speaking community of practice, whereas the explicit aim of Duodecim was to promote medical practice and uptake of medical science in Finnish. A third society, Suomen Lääkäriliitto (The Finnish Medical Association), was established in 1910. Many thanks to Matti Sintonen for generously helping us to navigate the various medical associations of Finland.
2. Lag om yrkesutbildade personer inom hälso- och sjukvården (1994/559) section 15; Lag om utövning av veterinäryrket (2000/29) section 13; Lääkäriliitto (Citation2014).
3. Holly Andersen provides an interesting argument explaining why this is the case (Andersen Citation2012). In general the fact that our bodies are complex evolved systems makes it likely that relevant variables will be masked.
References
- Education Act (2010:800)
- Lag om yrkesutbildade personer inom hälso- och sjukvården (28.6.1994/559)
- Lag om utövning av veterinäryrket (21.1.2000/29)
- Patient Safety Act (2010:659)
- Prop. 2009/10:210
- SOU (1989:60)
- SFS (1829:12)
- Andersen, Holly. 2012. “Mechanisms: What are they Evidence for in Evidence-based Medicine?” Journal of Evaluation in Clinical Practice 18: 992–999.10.1111/j.1365-2753.2012.01906.x
- Asplund, Kjell. 2001. “Den evidensbaserade medicinen är nödvändig men inte tillräcklig: Bör kompletteras inom områden där det vetenskapliga underlaget är svagt.” Läkartidningen 98 (37): 3898–3901.
- Cartwright, Nancy, and Jeremy Hardie. 2012. Evidence-based Policy – A Practical Guide to Doing it Better. New York: Oxford University Press.10.1093/acprof:osobl/9780199841608.001.0001
- CEBM (Centre for Evidence-Based Medicine). 2009. “Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009).” Accessed February 25, 2016. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/
- CEBM (Centre for Evidence-Based Medicine). 2016. “Making a Decision.” Accessed February 25, 2016. http://www.cebm.net/category/ebm-resources/tools/making-a-decision/
- Danek, K. 1969. “Towards a History of Health.” In Nordisk medicinhistorisk årsbok, 60–72. Stockholm: Medicinhistoriska muséet.
- EBMWG (Evidence-Based Medicine Working Group). 1992. “Evidence-based Medicine. A New Approach to Teaching the Practice of Medicine.” JAMA 268 (17): 2420–2425.
- Eddy, David M. 2005. “Evidence-Based Medicine: A Unified Approach.” Health Affairs 24 (1): 9–17.10.1377/hlthaff.24.1.9
- Eklöf, Motzi. 2000. Läkarens Ethos: Studier i den svenska läkarkårens identiteter, intressen och ideal 1890–1960. Vol. 216. Linköping: Linköping Studies in Arts and Science.
- Fåhræus, Robin. 1950. Läkekonstens historia, del III. Stockholm: Bonniers.
- Haynes, R. Brian, David L. Sackett, J. A. Muir Gray, D. J. Cook, and Gordon Guyatt. 1996. “Transferring Evidence from Research into Practice: 1. The Role of Clinical care Research Evidence in Clinical Decisions.” Evidence-Based Medicine 1 (7): 196–198.
- Haynes, R. Brian, P. J. Devereaux, and Gordon H. Guyatt. 2002. “Clinical expertise in the era of evidence-based medicine and patient choice.” Evidence-Based Medicine 7 (2): 36–38.10.1136/ebm.7.2.36
- Hont, Gabor. 2009. “Svensk sjukvårds nestor Lars Werkö död.” Läkartidningen 106 (42): 3672.
- Howick, Jeremy. 2011. The Philosophy of Evidence-Based Medicine. Chicester: Wiley-Blackwell.10.1002/9781444342673
- Kock, Wolfram. 1939. “Svenska Läkareeder.” Medicinska Föreningens Tidskrift 11: 276–283.
- Lääkäriliitto. 2014. Läkarens etiska regler. Finland: Lääkäriliitto.
- Ling, Sofia. 2004. Kärringmedicin och vetenskap. edited by Torkel Jansson, Jan Lindegren, and Maria Ågren. Vol. 212, Studia Historica Upsaliensa. Uppsala: Acta Universitatis Upsaliensis.
- Persson, Johannes, and Lena Wahlberg. 2015. “Vår erfarenhet av beprövad erfarenhet: några begreppsprofiler och ett verktyg för precisering.” Läkartidningen 112 (49): 2230–2232.
- Porter, Roy. 1995. Disease, Medicine and Society in England, 1550–1860. 2nd ed. Cambridge: Cambridge University Press.10.1017/CBO9780511608117
- Psykologförbund, Sveriges. 1998. Yrkesetiska principer för psykologer i Norden. Antagna av Sveriges Psykologförbunds kongress 1998. http://www.psykologforbundet.se/Documents/OmForbundet/Yrkesetiska-principer-för-psykologer-i-Norden.pdf.
- Pye-Smith, Philip H. 1900. “Medicine as a Science and Medicine as an Art.” The Boston Medical and Surgical Journal 143 (8): 173–177. doi:10.1056/NEJM190008231430801.
- Rosenberg, William, and Anna Donald. 1995. “Evidence Based Medicine: An Approach to Clinical Problem-Solving.” BMJ 310: 1122–1126.10.1136/bmj.310.6987.1122
- Rynning, Elisabeth. 1994. Samtycke till medicinsk vård och behandling: en rättsvetenskaplig studie. Uppsala: Iustus.
- Sackett, David L., William M. C. Rosenberg, J. A. Muir Muir Gray, R. Brian Haynes, and W. Scott Richardson. 1996. “Evidence Based Medicine: What it is and What it isn’t.” BMJ 312: 71–72.10.1136/bmj.312.7023.71
- Sackett, David L., Sharon E. Straus, W. Scott Richardson, William Rosenberg, and R. Brian Haynes. 2000. Evidence-Based Medicine: How to Practice and Teach EBM. Edinburgh: Churchill Livingstone.
- Sønbø Kristiansen, Ivar, and Gavin Mooney. 2004. Evidence-Based Medicine – In its Place. London: Routledge.10.4324/SE0717
- Stolt, Carl-Magnus. 1994. Den beprövade erfarenheten: Medicinsk idéhistoria och läkekonst i Boråsbygden 1780–1900. Borås: Norma.
- Stoyanov, Drozdstoj St., Peter K. Machamer, and Kenneth F. Shaffner. 2012. “Rendering Clinical Psychology an Evidence-based Scientific Discipline: A Case Study.” Journal of Evaluation in Clinical Practice 18: 149–154.10.1111/jep.2012.18.issue-1
- Wahlberg, Lena, and Nils-Eric Sahlin. 2017. “Om icke vedertagna behandlingsmetoder och kravet på vetenskap och beprövad erfarenhet.” Förvaltningsrättslig tidskrift 1: 45–66.
- Werkö, Lars, Kjell Asplund, Peter Aspelin, Mona Britton, Mats Eliasson, Jean-Luc af Geijerstam, and Sten Thelander. 2002. “Två år med EBM i Läkartidningen: Klinisk forskning och rutinsjukvård har närmat sig varandra.” Läkartidningen 99 (36): 3478–3482.
