Abstract
Antimicrobial resistance (AMR) is increasing and spreading throughout the world. Many academic publications address the human health care, veterinary, food safety and environmental aspects of this cross-border challenge. This paper focuses on the broader issue of the governance of AMR at multiple levels, from local to global. The paper provides a literature overview on the full complexity of the governance of the risk. A structured search strategy for the period 2002–2016 was applied using online databases. The literature is analyzed and presented along five themes that were distilled: levels, sectors, responsibilities, uncertainties, and values. On top of the medical-technical dimension of AMR, these five themes need to be taken into account for the governance of AMR risks.
Introduction
Antimicrobial resistance (AMR) risks develop when bacteria adapt and grow in the presence of antibiotics. Drug-resistant bacteria can circulate in populations of human beings and animals, through food, water and the environment, and transmission is influenced by trade, travel and both human and animal migration, as well as healthcare systems (Dorado-García et al. Citation2018, Hendriksen et al. Citation2019, Arcilla et al. Citation2014). Because bacteria circulate within and between different reservoirs, research and policy have been found to require a “One Health” approach – which means that since resistant bacteria are transmitted from one reservoir (for example animals) to another reservoir (for example humans) they must therefore be tackled in both. AMR risks can be framed differently by different actors and decisions on managing these risks – however framed – need to be taken under uncertainty. While it is abundantly clear that AMR is a growing global health threat (WHO Citation2012, OECD Citation2018), the risks also play out differently at different levels and in different sectors and there are still many unknowns. Furthermore, different actors have responsibilities for tackling the risks. Hence, the multilevel governance of these uncertain AMR risks has become hugely complex and attention needs to be paid to the evaluation and improvement of AMR risk governance.
To ensure that antibiotics can also be used effectively in the future, action is required from all countries (and governance levels above and below the national level) (WHO, OIE and FAO, 2015). While some countries, such as the Netherlands and Scandinavian countries, may have been able to manage the threat of AMR relatively well within their own boundaries, it is questionable how long this situation will last. In the future, AMR may become ‘normal’ everywhere and the question then becomes how different actors should respond, individually and collectively, to this situation.
Given that the issue of AMR involves different sectors – including human health (encompassing public health, cure and care), animal health, the environment, the food chain and other sectors – the current threat levels require an interdisciplinary discussion between health care professionals, veterinarians, policy-makers, academics and other relevant actors (Holmes et al. Citation2016, Wernli, Jørgensen, Harbarth et al. Citation2017). In order to gather the required information to govern AMR risks, existing institutionalized boundaries need to be bridged, and ways need to be developed for institutions and individuals to cope with the inevitable uncertainties in the knowledge base and with divergences in their framing of AMR risks.
While different elements of AMR risk governance are addressed in various scientific literatures, an overview is still lacking of the multilevel, multisector governance challenges to deal with AMR risks and attendant uncertainties and values. The present article aims to provide such an overview, which can be used to assess gaps in existing research as well as to provide focus for policy-makers on issues they should tackle in their governance of AMR risks. It is important to make clear from the outset that one of the problems that both academics and policy-makers have to grapple with is that there is no single agreed framing (or ‘ontology’) of the AMR risk. For instance, Wernli, Jørgensen, Morel et al. (Citation2017) have identified five different ‘policy frames’ from global health in policy discourses on AMR governance (see ), and within each of these frames AMR is defined differently. These policy frames each stem from different academic disciplines.
Table 1. Summary of policy frames on AMR. Source: Wernli, Jørgensen, Morel et al. (Citation2017).
The approach followed in this article for the review of a corpus of peer-reviewed literature was similar to that followed by Wernli, Jørgensen, Morel et al. (Citation2017) for their review of policy documents, with as main difference the fact that we have followed an inductive approach for arriving at our themes, where they followed a deductive approach (starting from policy frames already identified in the literature). In the discussion section, we compare our results.
Before we present our review, we first summarize where the governance of AMR risks stands in the context of the ‘Risk Governance Framework’ of the International Risk Governance Council (IRGC), originally published in 2005, updated in 2017 and shown in .
Figure 1. Visual representation of the IRGC Risk Governance Framework (IRGC Citation2017).
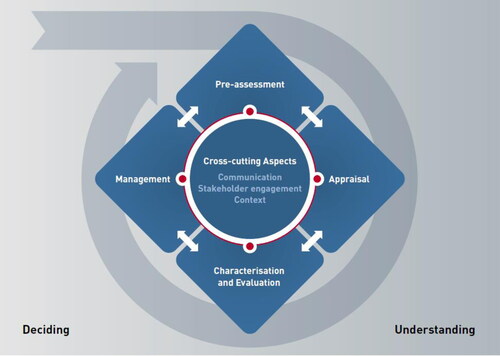
We use the IRGC Risk Governance Framework to briefly describe our preliminary assessment of the status of ‘understanding’ AMR risks, which led to the design and execution of the present literature review. For AMR risks already much knowledge has been generated (largely along different professions), which is relevant for the pre-assessment (e.g. early warning) of the risks as well as the risk assessment (e.g. more precise characterization of the risks). In our experience, a running assumption behind present AMR risk governance is that AMR is primarily a medical-technical issue, particularly a microbiological one, instead of a wider societal problem involving inequality. This is confirmed by the fact that the concern assessment (risk perceptions, social concerns, and socio-economic impacts) has received less attention in academic research. Accordingly, the knowledge characterization (an aspect in the Risk Governance Framework that constitutes the bridge from “understanding” to “deciding”) has largely been truncated to the medical-technical realm. Given the multiple policy frames that have been observed a wider set of values should be brought to bear on judging the profile and seriousness of the risks as they are studied by academics. Allowing for a serious widening of the framing of AMR risks to more than a healthcare problem makes dealing with these risks more complex: academics from different “silos” may generate and evaluate knowledge differently (Spruijt et al. Citation2019), and they often feed their knowledge evaluations separately into the decision-making process.
With regards to deciding on AMR risks we can briefly say the following: with respect to dealing with uncertainties (despite the fact that much knowledge exists, there are still major gaps), there are not sufficient feedback loops from implementation to decision-making, via monitoring and re-evaluation of the risk (cf. Marchau et al. Citation2019). In making decisions on managing the risk of AMR, the fragmentation in specialist understanding of the risk poses a challenge. Generally, the risk is evaluated from a human health perspective. However, causes may originate in other sectors (e.g., agricultural and environmental reservoirs).
While the framing of AMR as a “risk” is widespread in the literature and media (e.g., WHO Citation2012, Davies Citation2013, Economist Citation2016), and detailed attention is typically only given to the medical and technical challenges and not to the multilevel governance dimension of AMR risks, still, the importance of the governance challenges are regularly mentioned. Davies (Citation2013), for instance, after picturing AMR as a threat (an “inconvenient truth”) in which bugs are fighting back and “we are losing the battle”, emphasizes that we have to take responsibility now and that the response needs to be global and multifaceted. She says: “we can manage and mitigate the risk of antimicrobial resistance, which is just as important and deadly as climate change and international terrorism” (Davies, xiii). Davies furthermore flags that for the governance of AMR risks an international framework is needed.
To sum up, in this article, we aim to deal with the full complexity of the AMR risk governance problem. We provide an overview of published academic work on the governance of antimicrobial resistance. The main research question is: Which broader themes can be distilled from published work on the governance of antimicrobial resistance?
Methods
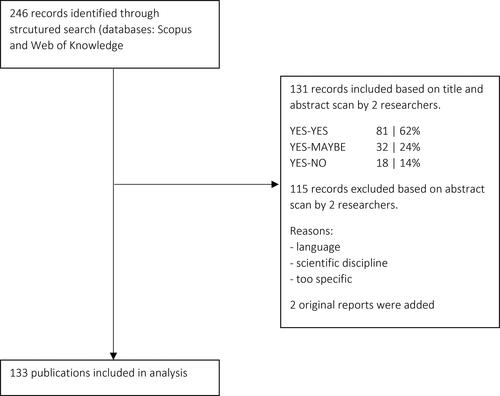
We conducted the literature search using two digital search engines: Scopus and Web of Knowledge. Window 1 outlines the search strategy with key words used. Two researchers simultaneously performed the manual refinement by scanning titles and abstracts. Differences in the assessment by the two researchers were discussed and led in most cases to dismissal of the publication. The three main reasons for dismissal were a language other than English, irrelevant content (e.g., similar keywords but too specific content such as one intervention in one hospital while we were looking for broad themes) and scientific discipline (e.g., just involving a technical or pharmaceutical discipline).
We reviewed work published between 2002 and 2016 to obtain a workable number of papers. This period of more than 14 years is assumed to be long enough to include both recent ideas as well as influential ideas from older literature. shows a flow diagram of the literature selection process. The final selection of publications was then subjected to a qualitative review. For two references, original reports were added: UK Government (Citation2013) and WHO (2015). In total, 133 publications were included in the qualitative review. The full list of publications is included as supplementary material.
The qualitative review process consisted of reading all 133 publications to uncover common denominators with regard to the governance of AMR. While reading, all publications were coded using a list of pre-defined codes (see ), which were arrived at following an inductive approach. The codes were determined on the basis of conversations on the governance of AMR with staff members of an AMR unit within a national public health research institute, and were judged by the staff members involved in the research for this article to capture the most relevant dimensions of the governance of AMR. Based on the analysis of the content of the coded citations five overarching themes were formulated by the authors. These themes form the structure of the results section ().
Table 2. Codes used in coding selected articles.
Window 1: Structured search strategy
((TITLE(AMR OR antibiotic* OR antimicrobial (resistance w/3 policy OR policies OR governance))))
AND
((((TITLE-ABS-KEY(measure* OR proportional* OR flexib* OR adapt* OR regulation* OR risk* OR scenario* OR threat OR precaution)))
AND/OR
((((TITLE-ABS-KEY(veterinair* OR environ* OR medic* OR food OR care OR cure OR human OR public health OR health OR travel* OR migration))))
AND (LIMIT-TO(SUBJAREA,"SOCI") OR LIMIT-TO(SUBJAREA,"ENVI") OR LIMIT-TO(SUBJAREA,"PSYC") OR LIMIT-TO(SUBJAREA,"ARTS") OR LIMIT-TO(SUBJAREA,"SOCI") OR LIMIT-TO(SUBJAREA,"ENVI") OR LIMIT-TO(SUBJAREA,"PSYC") OR LIMIT-TO(SUBJAREA,"ARTS") OR LIMIT-TO(SUBJAREA,"DECI") OR LIMIT-TO(SUBJAREA,"MULT") OR LIMIT-TO(SUBJAREA,"MULT") )
PUBYEAR > 2001
Language: English
Results
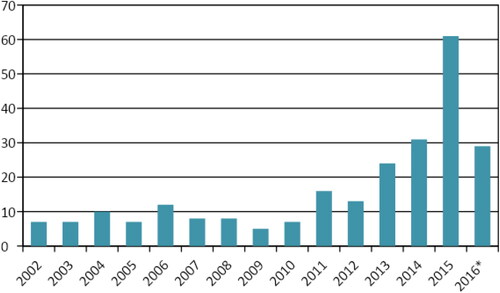
Since 2011, we see a yearly increase in the number of articles published that address the governance of antimicrobial resistance risks (see ). On the basis of coding of selected articles we formulated five broader themes. For the governance of AMR risks we have to deal with all the different levels (institutional, subnational [local networks], national, regional, global); all the different sectors ([including subsectors of] human health care [cure/care, long-term care, public health], veterinary sector, food chain, environment, production/industry, travel); all the different responsibilities (for different levels and sectors and including the role of the government versus that of other actors); all the different uncertainties; and all the different values (held by different actors and countries and affecting political economy, ethics, proportionality of measures, inequality and equity). Next, we present an overview of published work according to the classification of the five themes. The sentences used are the direct result of our review, either using coded text from the articles literally or closely paraphrasing it.
Levels
International
Bacteria are omnipresent and spread worldwide. The spread is accelerated by international travel, trade, tourism and population migration (Memish, Venkatesh, and Shibl Citation2003). These trends lead to a global threat that respects no borders (Smith and Coast Citation2002; Struwe Citation2008). Therefore, a governance response must be international (Struwe Citation2008; Woolhouse et al. Citation2015). There is however a lack of coordination for such a global response. In the literature, there is a call for an international legal agreement (treaty) as well as for funding to support global collective action (Behdinan, Hoffman, and Pearcey Citation2015; Podolsky et al. Citation2015; Årdal et al. Citation2016). Specifically, authors address the need for global standards and policies for comparability of data – within and between sectors (Okeke et al. Citation2005; MacPherson et al. Citation2009; Berendonk et al. Citation2015). Between-country comparisons are mentioned as major political driver for change and an increased focus on antimicrobial resistance control (Dar et al. Citation2016).
National
Health policies are often formulated at the national level. Usually it is easier to formulate and implement policies nationally than internationally (Dye Citation2012). There is an inequality between countries in terms of availability of and access to healthcare. The global infectious disease burden and antimicrobial resistance is disproportionately high in low- and middle- income countries (Laxminarayan et al. Citation2013; Bebell and Muiru Citation2014). Besides, regulatory policies tend to be more common in high-income countries (Bebell and Muiru Citation2014). Research shows that differences in use are related to the regulations and enforcement of antimicrobial use (Rushton Citation2015). Insight in antimicrobial use differs between countries: there is a large difference between countries in available data, from national surveillance systems (Denmark) to insufficient studies in developing countries (Okeke et al. Citation2005; Mathew, Cissell, and Liamthong Citation2007).
Subnational/local networks
Implementation of health care policies ultimately takes place at local level, with the general practitioner and in health care institutions such as hospitals and long-term care facilities. Transmission of AMR takes place through the transfer of patients, among other routes. Patient mobility can be within a country but also via cross-border transfers. The timely exchange of patient information could help preventing spread. Currently, sustainable networks at subnational or national level are absent in many parts of the world (WHO Citation2012). According to the literature local action is needed (Pruden et al. Citation2013), for example through subnational regimes for agricultural and clinical use. Examples of countries that created local health care networks are: Sweden, United Kingdom, and France (Struwe Citation2008; Mölstad, Cars, and Stuwe Citation2008; UK Government Citation2014; Wang et al. Citation2015). The UK and Sweden have subnational antibiotic use and resistance data (UK: England divided into five regions; Sweden: 21 counties) (Struwe Citation2008; UK Government Citation2014; Johnson Citation2015). Sweden also has locally adapted guidelines (Struwe Citation2008). The Netherlands mentions subnational health care networks as ideal (Dik et al. Citation2016).
Sectors
Antibiotics are used in human healthcare and the veterinary sector (including pets). Resistant bacteria are found in humans, animals, food, and the environment. This makes antimicrobial resistance a multi-sectoral issue. Therefore, the governance of antimicrobial resistance should be multi- and cross-sectoral. Different sectors are interdependent, for example, because animal-to-human transfers of resistant genes is confirmed using whole genome sequencing (Laxminarayan et al. Citation2013) (Laxminarayan et al. Citation2013; Woolhouse et al. Citation2015). However, many stakeholders are aligned with one ministry or government agency, often another than the Ministry of Health (Balkhy et al. Citation2016). As a first step, many countries have developed national action plans across ministries (typically the departments of health and agriculture) (Elder, Kuentz, and Holm Citation2016). An example of an integrated animal-human surveillance program is DANMAP (Dar et al. Citation2016). Most antibiotics produced are destined for the agricultural, horticultural, and veterinary sectors (Laxminarayan et al. Citation2013). All adjacent natural environments – of both production and use of antibiotics – are environmental niches to be taken into consideration in the dynamics of AMR (Caniça et al. Citation2015).
Funding agencies have begun to allocate more spending to One Health initiatives, but the global effects of this policy shift in funding antimicrobial resistance control specifically remain to be examined (Dar et al. Citation2016). The availability of resources differs greatly within and between sectors. Resources in the long-term care sector are shrinking and it is hard to devote adequate resources to infection prevention and control (Moro and Gagliotti Citation2013). In the literature, there is a call for integrative, interdisciplinary collaboration between different healthcare specialists (Dik et al. Citation2016).
Responsibilities
Different actors can be expected to have different responsibilities in tackling AMR risks, but responsibilities have not always been clearly defined. Obviously, different government levels and sectors are involved, but who is responsible for what? Coordinated action is largely absent (Laxminarayan et al. Citation2013). No formal global mechanism for harmonizing individual national efforts exists at this time. While global discourse and efforts are slowly developing, their state-centered origins are still visible (Podolsky et al. Citation2015). For instance, the existing International Health Regulations (IHR) are intended for outbreaks of acute infectious diseases and therefore constitute a mismatch with the relatively slow spread of AMR. IHR, however, do provide at present the legal framework for early detection and outbreak control for AMR (Wernli et al. Citation2011; Dar et al. Citation2016).
With the absence of global regulation, leadership currently has to come from national governments and their public health agencies. Public health agencies will need to take a more active role in organizing and coordinating multi-centre AMR surveillance networks; integrating surveillance programmes with Antibiotic Stewardship Programmes is a logical next step (WHO Citation2012). National governments have a critical role in prioritization and provision of public services, such as information, surveillance, cost-effectiveness analysis and cross-sector coordination (WHO Citation2012). There is a need for strong political will to make difficult regulatory decisions. This could be organized through national task forces with a broad intersectoral coordinating role, including all relevant stakeholders, with governmental mandate. We have already seen that regulatory interventions have been crucial in changing antibiotic prescribing practices and policies. One example is the Swedish governmental bill “Strategy to prevent antibiotic resistance and healthcare associated infections” (Struwe Citation2008). Strategies and practical measures that work are well known. Mobilizing the necessary expertise and resources depends on the commitment of policy decision-makers (WHO Citation2012).
With sufficient resources, available governmental responsibility is translated into a variety of actions. For example, in the veterinary sector the Danish and Dutch governments took different responsibilities: the Danish government itself carried out the measures to reduce veterinary antimicrobial use, while the Dutch government adopted a facilitating role (self-regulation) (Speksnijder, Mevius, et al. Citation2015). Developing the evidence base is important work which will inform decisions (UK Government Citation2013). There is however a suboptimal allocation of resources. Poverty-driven practices are found when resources are inadequate. For example, the regulation of sales differs between countries from strict to absent. Policies often exist but enforcement is insufficient or lacking (Bebell and Muiru Citation2014; Zaidi, Dreser, and Figueroa Citation2015). By law over the counter sale is prohibited in many countries, but it is poorly enforced and results in unlicensed distribution (Pruden et al. Citation2013; Premanandh, Samara, and Mazen Citation2015). This leads to a situation referred to as tragedy of the commons: a shared-resource system where individual users/countries act independently according to their own self-interest and contrary to the common good of all users by depleting that resource through their collective action. Another example is infection control, which is an important public health strategy that is costly and that yields significant benefits for parties other than the payer (Behdinan, Hoffman, and Pearcey Citation2015). This touches on the issue of balancing competing interests and needs. The balance between cost and benefit is also an issue in the lobby and choices made by the pharmaceutical industry for the development of new antibiotics.
Uncertainties
With respect to the category of uncertainty, we focus on a broad notion of uncertainty, ranging from statistical expressions to recognized ignorance; this also includes issues of methodological reliability. While there are some well-established facts, uncertainty on AMR risk is such that it requires adaptiveness in policies and governance: the ability to collect data (medical, socio- economic, governance) and making adjustments based on evidence.
Already a large amount of knowledge is available on AMR and research is ongoing. The One Health approach to AMR entails that different sectors are involved. These sectors each have built up their own body of knowledge on AMR. There are, however, numerous knowledge gaps. When preparing policies this happens against a background of uncertainty. In the literature, phrases like “incomplete data”, “absence of evidence”, and “little high-quality evidence” are used to signal this. The WHO (Citation2012) points out that both health and economic consequences of AMR are difficult to quantify and that there is insufficient information on health care-associated as well as societal costs of interventions and on the savings due to their impacts. Remaining uncertainty clears the road for controversy, for example over the relative contribution made to AMR by antimicrobial use in animal agriculture (Scott, Midgley, and Loneragan Citation2015).
There are ways to make uncertainty explicitly a part of the approach to AMR, which in the literature is often referred to as the “flexibility” or “adaptativeness” of policy and regulation. Countries need to adopt measures to make them context specific; through proper surveillance and monitoring, countries need to assess progress, contribute to an expanding knowledge base and help to improve accountability (Dar et al. Citation2016). No single intervention will ever be sufficient and only the use of multiple, wide-ranging associations of interventions might be able to produce an overall synergistic effect. Building feedback loops into complex systems is a way to organize flexibility and adaptation (Wallinga, Rayner, and Lang Citation2015).
Values
When thinking about measures to limit the negative effects of resistant bacteria all previous topics should be considered: levels, sectors, responsibilities and uncertainties. We identified a fifth theme that is highlighted in the literature: values. We grouped literature that addresses values and AMR into three clusters: security, proportionality and expert’s views on their own roles.
The topic of AMR is sometimes addressed as a security issue. Especially Anglo-Saxon literature refers to the issue with words such as “national security” and “global stability” (Smith and Coast Citation2002; Memish, Venkatesh, and Shibl Citation2003; UK Government Citation2013). War metaphors such as “weapons” and “combat” are used to explain the situation and possible actions (Planta Citation2007). There is also literature that critically addresses the use of these words when discussing AMR (Birnbaum Citation2015; Wallinga, Rayner, and Lang Citation2015), stating that it is not a battle that can be won and that focusing on suppressing (instead of eliminating) the development of AMR is more appropriate (Birnbaum Citation2015). Furthermore, there is literature on food safety and biosecurity measures (Rushton Citation2015; Speksnijder, Jaarsma et al. Citation2015; Speksnijder, Mevius, et al. Citation2015; Wielinga et al. Citation2014). All these words give an idea of the framing of the issue.
Another part of the literature explicitly addresses proportionality, defined here as the principle to find a balance between competing values. An example of a question that arises is: in which situations is the use of antibiotics considered justified? Individual decisions to use antimicrobials often ignore the societal perspective of depleting a “common good” (WHO Citation2012). Another question relates to wanting to avoid the development of resistance and/or spread: what barrier precautions are available and useful? Should all patients be screened? And if you screen patients, do you isolate colonized and infected patients? And for all the different options there is the question pertaining to their cost effectiveness? In the EU, the issue of proportionality needs to be addressed with respect to the application of the “precautionary principle”. The precautionary principle implies that when human activities may lead to morally unacceptable harm that is scientifically plausible but uncertain, actions shall be taken to avoid or diminish that harm. However, applying the principle still requires an examination of benefits and costs of action or lack of action.
The third group of literature addresses values of experts, in particular values that pertain to the perception of their own roles vis-à-vis policy. This body of literature is concerned with the way in which multiple and varied stakeholder experts’ values should be considered, for example when thinking about consensus statements versus divergent perspectives in advisory committees (Wallinga, Rayner, and Lang Citation2015). With complex issues such as AMR the debate is sometimes more on values than science (Scott, Midgley, and Loneragan Citation2015). For example, when two scholars examine the same data they can draw different conclusions. At the same time, it seems apparent that moving forward science will continue to play a valued and expanding role by informing debates and policy decisions over the design of the most appropriate regulations.
Discussion
There are some obvious limitations to our results. First of all, other themes might have emerged if we had used older and/or more recent years than in the time period chosen (2002–2016), but we do believe that the themes that emerged in this study are quite robust for adding more years; also note that the analysis that we performed was qualitative, not quantitative. Secondly, the process that was used to arrive at the themes was inductive and another approach might have led to a different set of themes. Again, we believe that the five themes we came up with are relatively robust also for variations in the research design other than the time period. As we announced in the introduction, we here further discuss our results using Wernli, Jørgensen, Morel et al. (Citation2017) policy frames as reference (see ).
Levels
From what we found, there appears to be a clear difference in dominance of policy frames at the different levels (international, national, and subnational/local). At the international level, while recently One Health has been hailed as a new organizing principle (see the discussion below under ‘sectors’), we also can see security functioning as a particularly dominant discourse at times, and calls for funding often include a focus on innovation. How different countries view the governance of AMR risks for themselves depends much on their income category. High-income countries (HICs) focus on a combination of healthcare and security: through regulations, enforcement of antibiotic use, and surveillance, with the aim to keep AMR under control; only a limited number of countries have implemented One Health policies. Low-and-middle-income countries (LMICs) emphasize development: access to medicine is a prerequisite for development, and keeping AMR under control is not their first priority. For the local level, the literature mainly discusses the promise of subnational healthcare networks in HICs, with again a focus on healthcare and security (stewardship and surveillance).
Sectors
Our results confirm that different policy frames are associated with the various sectors involved in the governance of AMR. The healthcare, development, innovation and security policy frames are all focused on different sectors, and the One Health policy frame aims to tie the healthcare, agriculture and environment sectors together. In order for the governance of AMR to be multi- and cross-sectoral an understanding and integration of these policy frames is needed. The fact that stakeholders are typically aligned with different ministries and that the resources differ greatly between sectors indicate serious barriers to an integrated governance. National action plans then have the task to both integrate the different policy frames and make concrete steps in implementation. Examples of integration have been shown possible in some countries in the areas of health, agriculture and environment (One Health), but the global effect of this integration remains to be seen. Within the policy frame of healthcare, our results indicate that work is still needed to promote integrative, interdisciplinary collaboration between different healthcare subsectors.
Responsibilities
Actors who adhere to different policy frames also have different views on who is responsible for doing what. In all policy frames there are roles to play for government (actually, most frames are centered on the nation state), but again what is expected from government differs. In the healthcare frame, it is expected that public health agencies, backed up by governmental mandates, take the leading role in the national governance of AMR and in the coordination of the global governance response. In the development frame, it becomes clear that the governments of LMICs see it as their responsibility to serve the direct short-term interest of their inhabitants who need improved access to antibiotics, at the expense of a stricter regulation of sales, enforcement of rules, and prevention of a “tragedy of the commons”. In the innovation frame, the government is expected to step in to create a more attractive distribution between costs and benefits for the pharmaceutical industry in order for them to develop new antibiotics. In the security frame, there is a clear role for governments to provide for early detection and outbreak control for AMR, in the context of international agreements. And in the One Health frame, there is a clear role for governments to organize the necessary cross-sector coordination.
Uncertainties
The different policy frames lead to the acknowledgment of different knowledge gaps. In the healthcare and security frames, the emphasis is proper surveillance and monitoring, which in many countries is still lacking. In the One Health frame, there is still remaining uncertainty on the relative contribution of antibiotic use in food animals to AMR risks in humans. In the development and innovation frames, the amounts governments are willing to invest in the governance of AMR depend on the health and economic consequences of AMR that could be avoided; these consequences are still difficult to quantify and differ between HICs and LMICs.
Values
The values dimension as far as we found it discussed in the academic literature relates to three specific issues in particular: the pros and cons of the security policy frame, questions about proportionality in the healthcare policy frame, and the expert’s views on their own roles (including their reflection on the different policy frames). First, while the security frame is found powerful to address the issue and mobilize state power, it is questionable whether governing AMR risks is a battle that can be won. Second, in the healthcare frame, the proportionality of measures is a matter of consideration, the question being how cost effectiveness, individual rights and the precautionary principle should be balanced. And third (relevant for all frames), it is signalled that experts should reflect on their own values, in particular values that pertain to the perception of their own roles vis-à-vis policy and their own embeddedness in particular policy frames.
Conclusion
The overall aim of this literature review was to distill an overview of broader themes from published academic work on the governance of antimicrobial resistance. Based on a structured search through published literature five themes could be appointed: levels, sectors, responsibilities, uncertainty and values. Level refers to the administrative stratification in the international, national and sub-national level. Sector refers to the wide array of involved sectors: human healthcare, veterinary sector and others. Responsibilities refers to the question who is responsible for what given the involvement of multi-levels and multi-sectors. A broad notion of uncertainty was used, ranging from statistical expressions to recognized ignorance. Finally, values point to the different ways in which experts assess the situation and the eventual balance between competing values. In addition, different values were shown by synonyms used when discussing the issue, for example war metaphors.
Against the background of five existing policy frames (Wernli, Jørgensen, Morel et al. Citation2017) these five themes show the full complexity of the governance of the risk of AMR. Next to medical technical knowledge, all themes and policy frames should be taken into account when thinking about regulatory measures. Given the complex nature of the governance of AMR we suggest a longlist of evidence-based actions that could be taken. There is no one size fits all governance solution to the risk of AMR given that countries have different healthcare systems, resources and priorities. Consequently, with limited time and resources, it is necessary to prioritize what actions to take first while realizing that governing AMR in the end needs attention on all five themes.
rjrr_a_1779784_sm3160.docx
Download MS Word (31.1 KB)References
- Arcilla, Maris S., Jarne M. van Hattem, Martin C. J. Bootsma, Perry J. van Genderen, Abraham Goorhuis, Constance Schultsz, Ellen E. Stobberingh, et al. 2014. “The Carriage of Multiresistant Bacteria after Travel (COMBAT) Prospective Cohort Study: methodology and Design.” BMC Public Health 14 (1): 410. doi:10.1186/1471-2458-14-410.
- Årdal, C., K. Outterson, S. J. Hoffman, A. Ghafur, M. Sharland, N. Ranganathan, R. Smith, et al. 2016. “International Cooperation to Improve Access to and Sustain Effectiveness of Antimicrobials.” The Lancet 387 (10015): 296–307. doi:10.1016/S0140-6736(15)00470-5.
- Balkhy, Hanan H., Abdullah M. Assiri, Haifa Al Mousa, Seif S. Al-Abri, Huda Al-Katheeri, Huda Alansari, Najiba M. Abdulrazzaq, Awa Aidara-Kane, and Didier Pittet, 2016. “The Strategic Plan for Combating Antimicrobial Resistance in Gulf Cooperation Council States.” Journal of Infection and Public Health 9 (4): 375–385. doi:10.1016/j.jiph.2016.03.003.
- Bebell, L. M., and A. M. Muiru. 2014. “Antibiotic Use and Emerging Resistance: How Can Resource-Limited Countries Turn the Tide?” Global Heart 9 (3): 347–358. doi:10.1016/j.gheart.2014.08.009.
- Behdinan, A., S. J. Hoffman, and M. Pearcey. 2015. “Some Global Policies for Antibiotic Resistance Depend on Legally Binding and Enforceable Commitments.” The Journal of Law, Medicine & Ethics 43 (3_suppl): 68–73. doi:10.1111/jlme.12277.
- Berendonk, T. U., C. M. Manaia, C. Merlin, D. Fatta-Kassinos, E. Cytryn, F. Walsh, H. Bürgmann, et al. 2015. “Tackling Antibiotic Resistance: The Environmental Framework.” Nature Reviews. Microbiology 13 (5): 310–317. doi:10.1038/nrmicro3439.
- Birnbaum, D. 2015. “Antimicrobial Resistance and Stewardship.” Clinical Governance: An International Journal 20 (1): 33–39. doi:10.1108/CGIJ-01-2015-0001.
- Caniça, M., V. Manageiro, D. Jones-Dias, L. Clemente, E. Gomes-Neves, P. Poeta, E. Dias, and E. Ferreira. 2015. “Current perspectives on the dynamics of antibiotic resistance in different reservoirs.” Research in microbiology 166 (7): 594–600.
- Dar, O. A., R. Hasan, J. Schlundt, S. Harbarth, G. Caleo, F. K. Dar, J. Littmann, et al. 2016. “Exploring the Evidence Base for National and Regional Policy Interventions to Combat Resistance.” The Lancet 387 (10015): 285–295. doi:10.1016/S0140-6736(15)00520-6.
- Davies, S. C. 2013. The Drugs Don’t Work: A Global Threat. London: Penguin Books.
- Dik, J.-W H., R. Hendrix, R. Poelman, H. G. Niesters, M. J. Postma, B. Sinha, and A. W. Friedrich. 2016. “Measuring the Impact of Antimicrobial Stewardship Programs.” Expert Review of anti-Infective Therapy 14 (6): 569–575. doi:10.1080/14787210.2016.1178064.
- Dorado-García, Alejandro, Joost H. Smid, Wilfrid van Pelt, Marc J. M. Bonten, Ad C. Fluit, Gerrita van den Bunt, Jaap A. Wagenaar, et al. 2018. “Molecular Relatedness of ESBL/AmpC-Producing Escherichia coli from Humans, Animals, Food and the Environment: A Pooled Analysis.” The Journal of Antimicrobial Chemotherapy 73 (2): 339–347. doi:10.1093/jac/dkx397.
- Dye, C. 2012. “National and International Policies to Mitigate Disease Threats.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 367 (1604): 2893–2900. doi:10.1098/rstb.2011.0373.
- Economist, 2016. “When the Drugs Don’t Work: The Rise of Antibiotic Resistance.” The Economist 419 (8990): 9, 17–19.
- Elder, D. P., M. Kuentz, and R. Holm. 2016. “Antibiotic Resistance: The Need for a Global Strategy.” Journal of Pharmaceutical Sciences 105 (8): 2278–2287. doi:10.1016/j.xphs.2016.06.002.
- Hendriksen, Rene S., Patrick Munk, Patrick Njage, Bram van Bunnik, Luke McNally, Oksana Lukjancenko, Timo Röder, et al. 2019. “Global Monitoring of Antimicrobial Resistance Based on Metagenomics Analyses of Urban Sewage.” Nature Communications 10 (1): 1124. doi:10.1038/s41467-019-08853-3.
- Holmes, Alison H., Luke S. P. Moore, Arnfinn Sundsfjord, Martin Steinbakk, Sadie Regmi, Abhilasha Karkey, Philippe J. Guerin, and Laura J. V. Piddock. 2016. “Understanding the Mechanisms and Drivers of Antimicrobial Resistance.” The Lancet 387 (10014): 176–187. doi:10.1016/S0140-6736(15)00473-0.
- IRGC 2017. Introduction to the IRGC Risk Governance Framework. Revised version. Lausanne: EPFL International Risk Governance Center.
- Johnson, A. P. 2015. “Surveillance of Antibiotic Resistance.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370 (1670): 20140080doi:10.1098/rstb.2014.0080.
- Laxminarayan, R., A. Duse, C. Wattal, A. K. M. Zaidi, H. F. L. Wertheim, N. Sumpradit, E. Vlieghe, et al. 2013. “Antibiotic Resistance: The Need for Global Solutions.” The Lancet. Infectious Diseases 13 (12): 1057–1098. doi:10.1016/S1473-3099(13)70318-9.
- MacPherson, D. W., B. D. Gushulak, W. B. Baine, S. Bala, P. O. Gubbins, P. Holtom, and M. Segarra-Newnham. 2009. “Population Mobility, Globalization, and Antimicrobial Drug Resistance.” Emerging Infectious Diseases 15 (11): 1727–1732. doi:10.3201/eid1511.090419.
- Marchau, V. A. W. J., W. E. Walker, P. J. T. M. Bloemen, and S. W. Popper, eds. 2019. Decision Making under Deep Uncertainty: From Theory to Practice. Cham, Switzerland: Springer.
- Mathew, A. G., R. Cissell, and S. Liamthong. 2007. “Antibiotic Resistance in Bacteria Associated with Food Animals: A United States Perspective of Livestock production.” Foodborne Pathog. Dis 4 (2): 115–133. doi:10.1089/fpd.2006.0066.
- Memish, Z. A., S. Venkatesh, and A. M. Shibl. 2003. “Impact of Travel on International Spread of Antimicrobial Resistance.” International Journal of Antimicrobial Agents 21 (2): 135–142. doi:10.1016/S0924-8579(02)00363-1.
- Mölstad, S., O. Cars, and J. Stuwe. 2008. “Strama: A Swedish Working Model for Containment of Antibiotic Resitance.” Eurosurveillance 13 (46): 8–11.
- Moro, M. L., and C. Gagliotti. 2013. “Antimicrobial Resistance and Stewardship in Long-Term Care Settings.” Future Microbiology 8 (8): 1011–1025. doi:10.2217/fmb.13.75.
- OECD 2018. Stemming the Superbug Tide: Just a Few Dollars More, OECD Health Policy Studies. Paris: OECD Publishing. 10.1787/9789264307599-en.
- Okeke, Iruka N., Keith P. Klugman, Zulfiqar A. Bhutta, Adriano G. Duse, Philip Jenkins, Thomas F. O'Brien, Ariel Pablos-Mendez, and Ramanan Laxminarayan. 2005. “Antimicrobial Resistance in Developing Countries. Part II: Strategies for Containment.” The Lancet Infectious Diseases 5 (9): 568–580. doi:10.1016/S1473-3099(05)70217-6.
- Planta, M. B. 2007. “The Role of Poverty in Antimicrobial Resistance.” Journal of the American Board of Family Medicine: JABFM 20 (6): 533–539. doi:10.3122/jabfm.2007.06.070019.
- Podolsky, Scott H., Robert Bud, Christoph Gradmann, Bård Hobaek, Claas Kirchhelle, Tore Mitvedt, María Jesús Santesmases, Ulrike Thoms, Dag Berild, and Anne Kveim Lie. 2015. “History Teaches us That Confronting Antibiotic Resistance Requires Stronger Global Collective Action.” The Journal of Law, Medicine & Ethics 43 (3_suppl): 27–32. doi:10.1111/jlme.12271.
- Premanandh, J., B. S. Samara, and A. N. Mazen. 2015. “Race against Antimicrobial Resistance Requires Coordinated Action - An Overview.” Frontiers in Microbiology 6: 1536. doi:10.3389/fmicb.2015.01536.
- Pruden, A., J. D. G. Larsson, A. Amézquita, P. Collignon, K. K. Brandt, D. W. Graham, J. M. Lazorchak, et al. 2013. “Management Options for Reducing the Release of Antibiotics and Antibiotic Resistance Genes to the environment.” Environ. Health Perspect 121 (8): 878–885. doi:10.1289/ehp.1206446.
- Rushton, J. 2015. “Anti-Microbial Use in Animals: How to Assess the Trade-Offs.” Zoonoses and Public Health 62 (S1): 10–21. doi:10.1111/zph.12193.
- Scott, H. M., G. Midgley, and G. H. Loneragan. 2015. “Antimicrobials in Animal Agriculture: Parables and Policy.” Zoonoses and Public Health 62 (S1): 3–9. doi:10.1111/zph.12191.
- Smith, R. D., and J. Coast. 2002. “Antimicrobial Resistance: A Global Response.” Bulletin of the World Health Organization 80 (2): 126–133.
- Speksnijder, D. C., A. D. C. Jaarsma, A. C. van der Gugten, T. J. M. Verheij, and J. A. Wagenaar. 2015. “Determinants Associated with Veterinary Antimicrobial Prescribing in Farm Animals in The Netherlands: A Qualitative Study.” Zoonoses and Public Health 62 (S1): 39–51. doi:10.1111/zph.12168.
- Speksnijder, D. C., D. J. Mevius, C. J. M. Bruschke, and J. A. Wagenaar. 2015. “Reduction of Veterinary Antimicrobial Use in The Netherlands: The Dutch Success Model.” Zoonoses and Public Health 62 (S1): 79–87. doi:10.1111/zph.12167.
- Spruijt, P., A. B. Knol, A. C. Petersen, and E. Lebret. 2019. “Expert Views on Their Role as Policy Advisor: Pilot Study for the Cases of Electromagnetic Fields, Particulate Matter, and Antimicrobial Resistance.” Risk Analysis: An Official Publication of the Society for Risk Analysis 39 (5): 968–974. doi:10.1111/risa.13224.
- Struwe, J. 2008. “Fighting Antibiotic Resistance in Sweden-past, present and future.” Wiener klinische Wochenschrift 120 (9-10): 268–279. doi:10.1007/s00508-008-0977-6.
- UK Government 2013. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. London: UK Department of Health & Department for Environment Food and Rural Affairs.
- UK Government 2014. UK 5 Year Antimicrobial Resistance (AMR) Strategy 2013–2018: Annual Progress Report and Implementation Plan, 2014. London: UK Department of Health & Department for Environment Food and Rural Affairs.
- Wallinga, D., G. Rayner, and T. Lang. 2015. “Antimicrobial Resistance and Biological Governance: Explanations for Policy Failure.” Public Health 129 (10): 1314–1325. doi:10.1016/j.puhe.2015.08.012.
- Wang, S., C. Pulcini, C. Rabaud, J.-M. Boivin, and J. Birgé. 2015. “Inventory of Antibiotic Stewardship Programs in General Practice in France and Abroad.” Medecine et Maladies Infectieuses 45 (4): 111–123. doi:10.1016/j.medmal.2015.01.011.
- Wernli, Didier, Thomas Haustein, John Conly, Yehuda Carmeli, Ilona Kickbusch, and Stephan.Harbarth. 2011. “A Call for Action: The Application of the International Health Regulations to the Global Threat of Antimicrobial Resistance.” PLoS Medicine 8 (4): e1001022 doi:10.1371/journal.pmed.1001022.
- Wernli, Didier, Peter S. Jørgensen, Stephan Harbarth, Scott P. Carroll, Ramanan Laxminarayan, Nicolas Levrat, John-Arne Røttingen, and Didier Pittet. 2017. “Antimicrobial Resistance: The Complex Challenge of Measurement to Inform Policy and the Public.” PLoS Medicine 14 (8): e1002378. doi:10.1371/journal.pmed.1002378.
- Wernli, D., P. S. Jørgensen, C. M. Morel, S. Carroll, S. Harbarth, N. Levrat, and D. Pittet. 2017. “Mapping Global Policy Discourse on Antimicrobial Resistance.” BMJ Global Health 2 (2): e000378. doi:10.1136/bmjgh-2017-000378.
- WHO 2015. Global Action Plan on Antimicrobial Resistance. Geneva: Word Health Organization.
- WHO 2015. WHO, FAO, and OIE unite in the fight against Antimicrobial Resistance - Joint AMR Flyer. https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/amr_tripartite_flyer.pdf?ua=1
- WHO. 2012. The Evolving Threat of Antimicrobial Resistance: Options for Action. Geneva: World Health Organization.
- Wielinga, P. R., V. F. Jensen, F. M. Aarestrup, and J. Schlundt. 2014. “Evidence-Based Policy for Controlling Antimicrobial Resistance in the Food Chain in Denmark.” Food Control 40 (1): 185–192. doi:10.1016/j.foodcont.2013.11.047.
- Woolhouse, M., M. Ward, B. van Bunnik, and J. Farrar. 2015. “Antimicrobial Resistance in Humans, Livestock and the Wider environment.” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 370 (1670): 20140083. doi:10.1098/rstb.2014.0083.
- Zaidi, M. B., A. Dreser, and I. M. Figueroa. 2015. “A Collaborative Initiative for the Containment of Antimicrobial Resistance in Mexico.” Zoonoses and Public Health 62 (S1): 52–57. doi:10.1111/zph.12166.