Abstract
In men with erectile dysfunction, venous leakage is a common condition among non-responders to medical management and is attributed to penile smooth muscle atrophy. Androgens play a role in regulating trabecular smooth muscle growth and function. Further, androgens stimulate differentiation of progenitor cells into smooth muscle cells and inhibit their differentiation into adipocytes. We postulate that androgens exert a direct effect on penile tissue to maintain erectile function, and that androgen deficiency produces metabolic and structural imbalances in the corpus cavernosum, resulting in venous leakage and erectile dysfunction. To date, research efforts on the mechanisms by which androgens regulate penile erectile physiology have mainly focused on investigating the role of the NO/cGMP pathway. However, androgen-dependent mechanisms that regulate tissue remodeling have been poorly defined. Characterization of the molecular and cellular mechanisms by which androgens regulate corpus cavernosum structural and functional integrity would provide significant gains in knowledge and understanding of an important pathogenic process. In this review, we discuss the potential role of androgen in maintaining differentiation of progenitor cells into smooth muscle lineage and inhibition of differentiation into adipocytes. Androgen deficiency promotes differentiation into adipogenic lineage, and accumulation of adipocytes in the corpus cavernosum may contribute to erectile dysfunction.
Introduction
Erectile function, a complex neurophysiological process, is dependent upon the health of the penile vascular tissues, nerves and the perineal and ischiocavernosus muscles that support the proximal penis. Adequate arterial inflow and trapping of blood within the cavernosal bodies (veno-occlusion) is critical for the development of increasing pressure and volume expansion. In addition to arterial blood pressure, contraction of the perineal and ischiocavernosus muscles enhances penile rigidity. The veno-occlusive mechanism depends on the integrity of neural, vascular and endocrine systems, as well as the fibro-elastic properties of the cavernosal tissue. It has been noted that cavernosal tissue from men with erectile dysfunction of various etiologies, whether hormonal, neurological or vascular, exhibited reduced smooth muscle content and concomitant increase in connective tissue deposition Citation[1],Citation[2]. It is likely that such changes in penile tissue structure contribute to veno-occlusive dysfunction.
Clinical studies have suggested that surgical or medical castration results in loss of libido and erectile function Citation[3-10]. Aversa et al. Citation[11],Citation[12]noted a strong direct correlation between resistive index values and free testosterone, a relationship that was maintained after adjusting for age, sex hormone binding globulin and estradiol. They concluded that men with erectile dysfunction and low free testosterone may have impaired relaxation of penile smooth muscle, thus providing clinical evidence for the importance of androgen in regulating erectile function Citation[11-13]. In addition, hypogonadal men with confirmed lack of response to sildenafil monotherapy showed greater improvement in erectile function after testosterone treatment Citation[14]. A relationship between restoration of serum testosterone concentrations and improvement in sexual function has been proposed by Seftel et al. Citation[15]. In severe hypogonadal men, testosterone treatment for 6 months induced normalization of nocturnal penile tumescence activity Citation[16]. It is suggested that testosterone plays a key role in the central and peripheral modulation of erectile function, even if the specific threshold concentration of plasma testosterone remains unknown. Interestingly, a recent study demonstrated a dose-dependent effect of testosterone on changes in overall sexual function and self-reported waking erections in men between the ages of 60–75 years Citation[17].
In animal studies Citation[18-21], androgens are deemed critical for maintaining erectile function and support the responsiveness of the vascular smooth muscle to vasoactive drugs. Intracavernosal pressure decreased significantly in castrated animals (vs control), both after pelvic nerve stimulation and intracavernosal papaverine injection Citation[22],Citation[23]. More importantly, testosterone replacement restored penile hemodynamics. Testosterone is critical for maintaining the mass of skeletal muscles in the perineum, as well as neuron size Citation[24-26]. In addition to these trophic effects, which are presumed to be due to mechanisms involving transcriptional regulation, androgens may also regulate vascular smooth muscle contractility by non-genomic mechanisms. While these mechanisms have not been investigated in penile cavernosal tissue, testosterone has been shown to relax coronary arteries by activating potassium channels and inhibiting calcium channels Citation[27-29]. One of the least understood aspects of erectile function is the role of androgens in maintaining penile structural and functional integrity. To date, research efforts on the mechanisms by which androgens regulate penile erectile physiology have mainly focused on the role of the NO/cGMP pathway. However, androgen-dependent mechanisms that regulate tissue remodeling have been poorly defined.
Androgens are essential for maintaining penile trabecular smooth muscle structure and function
We have demonstrated that androgen deprivation results in a significant reduction in trabecular smooth muscle content and a marked increase in connective tissue deposition, in surgically or medically castrated animals Citation[30],Citation[31]. These structural alterations are associated with loss of erectile function. Histological studies in human tissues have shown an association between vasculogenic erectile dysfunction and altered connective tissue deposition Citation[32-36], and the severity of erectile dysfunction has been correlated with a reduced smooth muscle/connective tissue ratio Citation[36-38]. Interestingly, Rogers et al. Citation[39] noted that there was no significant difference in the smooth muscle content between intact, castrated, and testosterone-treated castrated rats when assessed by alpha smooth muscle actin staining, but did observe marked differences in the morphology of the smooth muscle cells by transmission electron microscopy. In sham-operated rats, the smooth muscle cells were arranged in clusters and separated by fine strands of fibro-connective tissue. The intercellular spaces between myocytes were usually quite narrow, with many gap junctions connecting individual cells, and the cytoplasm contained abundant contractile myofilaments and dense bodies. In contrast, myocytes in cavernosal tissue from castrated rats exhibited larger intercellular spaces and decreased amounts of cytoplasmic myofilaments. Tissue from testosterone-treated castrated rats had myocytes that were similar in appearance to those of sham-operated animals. We have also observed marked differences in the trabecular smooth muscle from orchiectomized rabbits compared to those of intact (sham-operated) animals by transmission electron microscopy Citation[40]. In castrated animals, the smooth muscle appeared disorganized, with a large number of cytoplasmic vacuoles, whereas in the intact animals, the smooth muscle cells exhibited normal morphology and were arranged in clusters. These results suggest that androgens have a profound effect on the ultrastructure of the corpus cavernosum, and these alterations may contribute to the loss of physiological function.
Androgen deficiency, in addition to inducing changes in smooth muscle cells that pertain to their morphology, orientation and organelle content/function, may also lead to programmed cell death in cavernosal tissue. In androgen-dependent prostate cancer cells, androgens have been shown to inhibit apoptosis by blocking the activation of caspases, a large family of proteases that play a central role in apoptosis Citation[41]. In other studies using cell lines that express androgen receptor, androgens activate pathways involving phosphatidylinositol 3-kinase (PI3K) and Akt, which can inhibit apoptosis Citation[42]. However, the effects of androgens on these pathways in penile corpus cavernosum have not been examined.
Androgen deficiency produces accumulation of adipocyte in the corpus cavernosum
In addition to the changes in connective tissue and smooth muscle, we have observed accumulation of fat-containing cells in penile tissue sections from orchiectomized animals. Careful histological examination of penile cavernosal tissue from orchiectomized rabbits and rats revealed the presence of cells resembling adipocytes. Two weeks post-orchiectomy, plasma testosterone levels and penile wet weight decreased significantly in rabbits. In rats, plasma testosterone, and penile and prostate wet weights, were significantly reduced at 4 weeks post-orchiectomy. When paraffin embedded penile tissue was processed for either Masson's trichrome or hematoxylin and eosin staining, orchiectomized animals exhibited clusters of “empty” cellular structures in the subtunical region of the corpus cavernosum that were distinct from cavernous spaces. Since normal processing of paraffin embedded tissue with organic solvents results in removal of fat droplets, we fixed penile tissue in glutaraldehyde and post-fixed with osmium tetroxide, which binds to unsaturated lipids and results in a brown or gray-black stain. Tissues were then embedded in epoxy resin to verify that the hollow cells did indeed contain lipid. Epoxy embedded tissue sections were then stained with toluidine blue to visualize the remaining cellular structures. While not all cells retained their lipid content, this fixation and staining procedure confirmed the presence of fat globules in a majority of the hollow cells that were observed in paraffin embedded tissue sections. In addition, cells with large lipid droplets, displaced nuclei and thin areas of cytoplasm (characteristic of adipocytes) were observed with transmission electron microscopy. Interestingly, there were always a few fat cells present in the penile cavernosal tissue of control animals. However, the quantity and distribution of fat-containing cells was greatly increased in tissue sections from orchiectomized animals. In rats, testosterone replacement restored normal cavernosal tissue appearance (Traish et al., unpublished observations). Equally interesting, fat-containing cells were never observed in the corpus spongiosum of intact or orchiectomized animals.
These alterations in cavernosal tissue composition and structure are accompanied by a reduced erectile response to pelvic nerve stimulation Citation[43]. It is interesting to speculate that the presence of fat cells in the corpus cavernosum may contribute to venous leak in the orchiectomized animal.
Estrogens promote adipogenic lineage and accumulation of adipocyte in the corpus cavernosum
Previous studies in the intact rabbit have noted that administration of bisphenol A and tetrachlorodibenzodioxin (TCDD), weak estrogens that act as endocrine disrupters, resulted in an abnormal deposition of fat-containing cells in the subtunical region of the corpus cavernosum Citation[44],Citation[45]. Organ bath studies using cavernosal tissue from bisphenol A- and TCDD-treated animals showed a reduced relaxation response to nitroprusside and acetylcholine. In addition, bisphenol A has also been shown to accelerate terminal differentiation of 3T3L1 fibroblasts into adipocytes through the PI3 kinase pathway Citation[46]. It remains to be determined if the effects of bisphenol A and TCDD are mediated via estrogen receptor action or by interfering with androgen action.
Goyal et al. Citation[47-50], in a series of elegant and well executed studies, have documented that neonatal rats exposed to the estrogen receptor agonists, estradiol or diethylstilbestrol, showed loss of smooth muscle and accumulation of fat-containing cells in the penile corpus cavernosum, whereas animals treated with vehicle exhibited no fat-containing cells Citation[47-50]. The authors showed that such treatment resulted in low plasma androgen levels. It is possible that estrogens are acting as anti-androgens in this tissue and affecting cellular differentiation. Alternatively, estrogens may cause reduction of plasma androgen levels due to alteration in androgen biosynthesis, and in turn promote the differentiation of progenitor cells into adipocytes instead of muscle cells. The changes in penile morphology are associated with infertility and probably erectile dysfunction Citation[47-50]. Since estrogens are known to act as anti-androgens, these studies point to the potential role of androgens in maintaining penile corpus cavernosum structural integrity.
Potential mechanisms by which androgens modulate smooth muscle and adipocyte differentiation in the corpus cavernosum
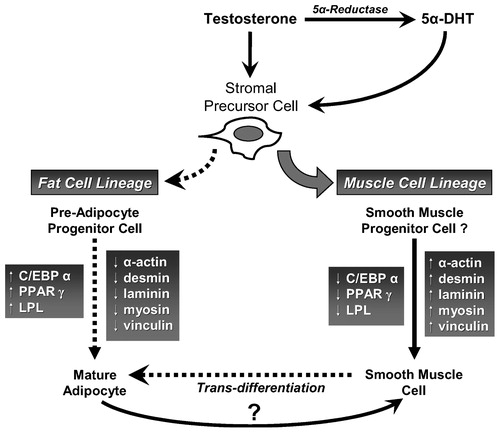
The mechanism by which androgens regulate growth and differentiation of vascular smooth muscle cells and adipocytes in the penis remains poorly understood. Bhasin et al. Citation[51] proposed that androgens promote the commitment of pluripotent stem cells into a muscle lineage and inhibit their differentiation into an adipocyte lineage. In a recent study, Singh et al. Citation[52] have shown that differentiation of pluripotent cells is androgen dependent. Both testosterone and dihydrotestosterone decreased the number of adipocytes and down-regulated the expression of the adipogenic markers PPAR-γ2 and C/EBPα. However, these mechanisms have yet to be investigated in tissue or cells from the corpus cavernosum. It is possible that pluripotent stem cells are present in the corpus cavernosum and that these cells respond to androgen deprivation by differentiation into an adipogenic lineage. Interestingly, hormone sensitive lipase (HSL) and lipoprotein lipase (LPL) have also been shown to be regulated by androgens Citation[53] and may contribute to lipid accumulation in castrated animals. Another possibility is the trans-differentiation of the cavernosal smooth muscle cells into other phenotypes. Corradi et al. Citation[54] have shown that inhibition of 5α-reductase activity induces stromal remodeling and smooth muscle de-differentiation in the prostate, suggesting that 5α-DHT deficiency promotes smooth muscle de-differentiation. While in several experimental systems vascular smooth muscle was shown to undergo trans-differentiation into other phenotypes Citation[55],Citation[56], there is no data in the literature on the trans-differentiation or de-differentiation of the trabecular smooth muscle in the corpus cavernosum.
The multifunctional properties of smooth muscle suggest heterogeneous sources of smooth muscle development via differentiation. The origin of smooth muscle in peripheral tissue of adult animals is thought to encompass differentiation of stem cells during embryogenesis and from precursor cells in adulthood. However, the exact origin of these cells remains unknown. A large body of literature exists with regard to the differentiation of progenitor cells into adipocytes Citation[57-61]. Regulation of differentiation of progenitor cells is dependent on a battery of hormones, growth factors and specific activation of a cascade of gene expression Citation[60-64]. A role for C/EBPα, PPARγ2 and LPL has been proposed in the differentiation of pre-adipocytes into adipocytes Citation[58-61],Citation[65-68]. Interestingly, increasing numbers of adipocytes in a given tissue may serve as a reservoir for biosynthesis of sex steroids that lead to more fat accumulation. Mechanisms of such pathways are under intensive investigation in many laboratories.
Summary and conclusions
Androgen deprivation by surgical or medical castration causes marked decrease in erectile function, as evidenced by the impaired response to cavernosal nerve stimulation. These functional changes were correlated with significant alterations in tissue structure that can adversely affect the material properties of the tissue and prevent adequate veno-occlusion. In addition to decreased smooth muscle, the increased number of adipocytes observed in the corpus cavernosum of orchiectomized animals may account, in part, for the observed venous leakage and erectile dysfunction. These androgen deficiency induced alterations in penile tissue suggest that androgens have a complex physiological role in erectile tissue. Androgens are believed to regulate: a) the expression and/or activity of nitric oxide synthase isoforms, phosphodiesterases and ion channels; b) the growth and state of differentiation of smooth muscle cells; c) connective tissue metabolism; and d) the growth, maintenance and/or differentiation of stromal cells into myogenic and adipogenic lineages. We suggest that androgen deprivation dynamically and reversibly alters the structure and function of the corpus cavernosum. Androgen deficiency is likely to affect multiple cellular components and molecular pathways to adversely change the structural and functional integrity of penile corpus cavernosum, contributing to erectile dysfunction. To this end, we postulate that, in the corpus cavernosum, androgens are critical for promoting and maintaining the myogenic lineage (). We further advance the hypothesis that, in erectile tissue, androgens promote differentiation of precursor stromal cells into smooth muscle cells, while androgen deprivation promotes differentiation of the precursor cells into adipocytes and/or facilitates trans-differentiation of smooth muscle into adipocytes. Based on this hypothesis, we expect that in cases of androgen deficiency the smooth muscle content will be reduced and adipogenesis increased. It is our view that androgen deficiency is a weapon of trabecular smooth muscle destruction due to inhibition of smooth muscle differentiation, de-differentiation of smooth muscle into other phenotypes, and promotion of programmed cell death and favoring adipogenic lineage, ultimately contributing to reduced smooth muscle content and erectile dysfunction.
Acknowledgements
This work was supported, in part, by grants R01-DK56846-01 and K01-DK02696-02 from NIDDK and by a non-restricted educational grant from Schering AG.
References
- Karadeniz T, Topsakal M, Aydogmus A, Gulgun C, Aytekin Y, Basak D. Correlation of ultrastructural alterations in cavernous tissue with the clinical diagnosis vasculogenic impotence. Urol Int 1996; 57: 58–61
- Mersdorf A, Goldsmith P C, Diederichs W, Padula C A, Lue T F, Fishman I J, Tanagho E A. Ultrastructural changes in impotent penile tissue: a comparison of 65 patients. J Urol 1991; 145: 749–758
- Ellis W J, Grayhack J T. Sexual function in aging males after orchiectomy and estrogen therapy. J Urol 1963; 89: 895–898
- Peters C A, Walsh P C. The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med 1987; 317: 599–604
- Rousseau L, Dupont A, Labrie F, Couture M. Sexuality changes in prostate cancer patients receiving antihormonal therapy combining the antiandrogen flutamide with medical (LHRH agonist) or surgical castration. Arch Sex Behav 1988; 17: 87–98
- Marumo K, Baba S, Murai M. Erectile function and nocturnal penile tumescence in patients with prostate cancer undergoing luteinizing hormone-releasing hormone agonist therapy. Int J Urol 1999; 6: 19–23
- Greenstein A, Plymate S R, Katz P G. Visually stimulated erection in castrated men. J Urol 1995; 153: 650–652
- Hirshkowitz M, Moore C A, O'Connor S, Bellamy M, Cunningham G R. Androgen and sleep-related erections. J Psychosom Res 1997; 42: 541–546
- Eri L M, Tveter K J. Safety, side effects and patient acceptance of the luteinizing hormone releasing hormone agonist leuprolide in treatment of benign prostatic hyperplasia. J Urol 1994a; 152: 448–452
- Eri L M, Tveter K J. Safety, side effects and patient acceptance of the antiandrogen Casodex in the treatment of benign prostatic hyperplasia. Eur Urol 1994b; 26: 219–226
- Aversa A, Isidori A M, De Martino M U, Caprio M, Fabbrini E, Rocchietti-March M, Frajese G, Fabbri A. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol 2000; 53: 517–522
- Aversa A, Isidori A M, Spera G, Lenzi A, Fabbri A. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. Clin Endocrinol 2003; 58: 632–638
- Aversa A, Isidori A M, Greco E A, Giannetta E, Gianfrilli D, Spera E, Fabbri A. Hormonal supplementation and erectile dysfunction. Eur Urol 2004; 45: 535–538
- Shabsigh R, Kaufman J M, Steidle C, Padma-Nathan H. Randomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil alone. J Urol 2004; 172: 658–663
- Seftel A D, Mack R J, Secrest A R, Smith T M. Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. J Androl 2004; 25: 963–972
- Foresta C, Caretta N, Rossato M, Garolla A, Ferlin A. Role of androgens in erectile function. J Urol 2004; 171: 2358–2362
- Gray P B, Singh A B, Woodhouse L J, Storer T W, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S. Dose-dependent effects of testosterone on sexual function, mood and visuospatial cognition in older men. J Clin Endocrinol Metab 2005; 90: 3838–3846
- Mills T M, Lewis R W, Stopper V S. Androgenic maintenance of inflow and veno-occlusion during erection in the rat. Biol Reprod 1998; 59: 1413–1418
- Mills T M, Stopper V S, Reilly C M. Sites of androgenic regulation of cavernosal blood pressure during penile erection in the rat. Int J Impot Res 1996; 8: 29–34
- Mills T M, Stopper V S, Wiedmeier V T. Effects of castration and androgen replacement on the hemodynamics of penile erection in the rat. Biol Reprod 1994; 51: 234–238
- Mills T M, Wiedmeier V T, Stopper V S. Androgen maintenance of erectile function in the rat penis. Biol Reprod 1992; 46: 342–348
- Baba K, Yajima M, Carrier S, Morgan D M, Nunes L, Lue T F, Iwamoto T. Delayed testosterone replacement restores nitric oxide synthase-containing nerve fibres and the erectile response in rat penis. BJU Int 2000; 85: 953–958
- Baba K, Yajima M, Carrier S, Akkus E, Reman J, Nunes L, Lue T F, Iwamoto T. Effect of testosterone on the number of NADPH diaphorase-stained nerve fibers in the rat corpus cavernosum and dorsal nerve. Urology 2000; 56: 533–538
- Nanasaki Y, Sakuma Y. Perineal musculature and its innervation by spinal motoneurons in the male rabbit: effects of testosterone. J Nippon Med Sch 2000; 67: 164–171
- Matsumoto A. Androgen stimulates neuronal plasticity in the perineal motoneurons of aged male rats. J Comp Neurol 2001; 430: 389–395
- Rand M N, Breedlove S M. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci 1995; 15: 4408–4416
- Yue P, Chatterjee K, Beale C, Poole-Wilson P A, Collins P. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 1995; 91: 1154–1160
- Deenadayalu V P, White R E, Stallone J N, Gao X, Garcia A J. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium-channel. Am J Physiol 2001; 281: H1720–H1727
- Scragg J L, Jones R D, Channer K S, Jones T H, Peers C. Testosterone is a potent inhibitor of L-type Ca2+ channels. Biochem Biophys Res Comm 2004; 318: 503–506
- Traish A M, Park K, Dhir V, Kim N N, Moreland R B, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology 1999; 140: 1861–1868
- Traish A M, Munarriz R, O'Connell L, Choi S, Kim S W, Kim N N, Huang Y H, Goldstein I. Effects of medical and surgical castration on erectile function in an animal model. J Androl 2003; 24: 381–387
- Luangkhot R, Rutchik S, Agarwal V, Puglia K, Bhargava G, Melman A. Collagen alterations in the corpus cavernosum of men with sexual dysfunction. J Urol 1992; 148: 467–471
- Persson C, Diederichs W, Lue T F, Yen T SB, Fishman I J, Mclin P, Tanagho E A. Correlation of altered penile ultrastructure with clinical arterial evaluation. J Urol 1989; 142: 1462–1468
- Mersdorf A, Goldsmith P C, Diederichs W, Padula C A, Lue T F, Fishman I J, Tanagho E A. Ultrastructural changes in impotent penile tissue: a comparison of 65 patients. J Urol 1991; 145: 749–758
- Wespes E, Goes P M, Schiffmann S, Deprierreux M, Vanderhaeghen J J, Schulman C C. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol 1991; 146: 1015–1017
- Jevtich M, Khawand N Y, Vidic B. Clinical significance of ultrastructural findings in the corpus cavernosa of normal and impotent men. J Urol 1990; 143: 289–293
- Nehra A, Goldstein I, Pabby A, Nugent M, Huang Y H, de las Morenas A, Krane R J, Udelson D, Saenz de Tejada I, Moreland R B. Mechanisms of venous leakage: a prospective clinicopathological correlation of corporeal function and structure. J Urol 1996; 156: 1320–1329
- Wespes E, Schiffmann S, Depierreux M, Vanderhaegan J J, Schulman C C. Is cavernovenous leakage related to a reduction of intracavernous smooth muscle fibers. Int J Impot Res 1990; 2: 30
- Rogers R S, Graziottin T M, Lin C M, Kan Y W, Lue T. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res 2003; 15: 26–37
- Traish A M, Kim N N. The physiological role of androgens in penile erection: regulation of corpus cavernosum structure and function. J Sex Med 2005; 2: 759–770
- Kimura K, Markowski M, Bowen C, Gelmann E P. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer Res 2001; 61: 5611–5618
- Sun M, Yang L, Feldman R I, Sun X M, Bhalla K N, Jove R, Nicosia S V, Cheng J Q. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem 2003; 278: 42992–43000
- Traish A M, Toselli P, Jeong S J, Kim N N. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl 2005; 26: 242–248
- Moon D G, Sung D J, Kim Y S, Cheon J, Kim J J. Bisphenol A inhibits penile erection via alteration of histology in the rabbit. Int J Impot Res 2001; 13: 309–316
- Moon D G, Lee K C, Kim Y W, Park H S, Cho H Y, Kim J J. Effect of TCDD on corpus cavernosum histology and smooth muscle physiology. Int J Impot Res 2004; 16: 224–230
- Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci 2005; 84: 319–327
- Goyal H O, Braden T D, Williams C S, Dalvi P, Williams J W, Srivastava K K. Exposure of neonatal male rats to estrogen induces abnormal morphology of the penis and loss of fertility. Reprod Toxicol 2004; 18: 265–274
- Goyal H O, Braden T D, Williams C S, Dalvi P, Mansour M M, Mansour M, Williams J W, Bartol F F, Wiley A A, Birch L, Prins G S. Abnormal morphology of the penis in male rats exposed neonatally to diethylstilbestrol is associated with altered profile of estrogen receptor-alpha protein, but not of androgen receptor protein: a developmental and immunocytochemical study. Biol Reprod 2004; 70: 1504–1517
- Goyal H O, Braden T D, Williams C S, Dalvi P, Mansour M M, Williams J W. Permanent induction of morphological abnormalities in the penis and penile skeletal muscles in adult rats treated neonatally with diethylstilbestrol or estradiol valerate: a dose-response study. J Androl 2005; 26(1)32–43
- Goyal H O, Braden T D, Williams C S, Dalvi P, Mansour M, Williams J W. Estrogen-induced abnormal accumulation of fat cells in the rat penis and associated loss of fertility depends upon estrogen exposure during critical period of penile development. Toxicol Sci 2005; 87(1)242–254
- Bhasin S, Taylor W E, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid N F. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol 2003; 58A: 1103–1110
- Singh R, Artaza J N, Taylor W E, Gonzalez-Cadavid N F, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003; 144: 5081–5088
- Anderson L A, McTernan P G, Harte A L, Barnett A H, Kumar S. The regulation of HSL and LPL expression by DHT and flutamide in human subcutaneous adipose tissue. Diabetes Obes Metab 2002; 4(3)209–213
- Corradi L S, Goes R M, Carvalho H F, Taboga S R. Inhibition of 5α-reductase activity induces stromal remodeling and smooth muscle de-differentiation in adult gerbil ventral prostate. Differentiation 2004; 72: 198–208
- Rucker-Martin C, Pecker F, Godreau D, Hatem S N. Dedifferentiation of atrial myocytes during atrial fibrillation: role of fibroblast proliferation in vitro. Cardiovasc Res 2002; 55: 38–52
- Johnson J L, van Eys G J, Angelini G D, George S J. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol 2001; 21: 1146–1151
- Hausman G J. Dexamethasone induced preadipocyte recruitment and expression of CCAAT/enhancing binding protein alpha and peroxisome proliferator activated receptor-gamma proteins in porcine stromal-vascular (S-V) cell cultures obtained before and after the onset of fetal adipogenesis. Gen Comp Endocrinol 2003; 133: 61–70
- Rosen E D. Molecular mechanisms of adipocyte differentiation. Ann Endocrinol (Paris) 2002; 63: 79–82
- Rosen E D, Hsu C, Wang X, Sakai S, Freeman M W, Gonzalez F J, Spiegelman B M. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 2002; 16: 22–26
- Rosen E D, Spiegelman B M. Molecular regulation of adipogenesis. Ann Rev Cell Dev Biol 2000; 16: 145–171
- Wright H M, Clish C B, Mikami T, Hauser S, Yanagi K, Hiramatsu R, Serhan C N, Spiegelman B M. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem 2000; 275: 1873–1877
- Dieudonne M N, Pecquery R, Boumediene A, Leneveu M C, Giudicelli Y. Androgen receptors in human preadipocytes and adipocytes: regional specificities and regulation by sex steroids. Am J Physiol 1998; 274: C1645–1652
- Garcia E, Lacasa M, Agli B, Giudicelli Y, Lacasa D. Modulation of rat preadipocyte adipose conversion by androgenic status: involvement of C/EBPs transcription factors. J Endocrinol 1999; 161: 89–97
- Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res 2002; 34: 737–745
- Wong Y C, Tam N N. Dedifferentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation 2002; 70: 633–645
- Chen W, Yang C C, Sheu H M, Seltmann H, Zouboulis C C. Expression of peroxisome proliferator-activated receptor and CCAAT/enhancer binding protein transcription factors in cultured human sebocytes. J Invest Dermatol 2003; 121: 441–447
- Bostrom K, Tintut Y, Kao S C, Stanford W P, Demer L L. HOXB7 overexpression promotes differentiation of C3H10T1/2 cells to smooth muscle cells. J Cell Biochem 2000; 78: 210–221
- Hu E, Tontonoz P, Spiegelman B M. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 1995; 92: 9856–9860