Abstract
Normally there is a gradual continual loss of cortical and trabecular bone in both men and women as they age. Osteopenia and osteoporosis are conditions in which the loss results in brittle bones that fracture easily. Males with low testosterone and hypogonadism are predisposed to osteoporosis and prevention tends to be overshadowed by the greater problem in postmenopausal women. The ability of the skeleton to resist external forces depends partly upon the amount of bone present and partly upon other factors including cancellous bone microarchitecture. This is examined in iliac crest bone biopsies from idiopathic osteoporotic men, mean age 60 ± 12 SD years [n = 16]. These were compared with a healthy control group (autopsy samples), mean age 30 ± 8.9 years [n = 28] with the aim of examining the pattern of cancellous atrophy in male idiopathic osteoporosis. Undecalcified specimens were embedded in methylmethacrylate and prepared for histomorphometry. Sections were analysed using an automated trabecular analysis system (TAS), whereby a binary image was created. Area measurements including the trabecular surface and distance measurements including the trabecular width were made. The binary image was thinned to its medial framework and the node and terminus number as indices of trabecular interconnection were recorded, together with the strut length. Results (median (range)) showed a lower percentage bone volume in the elderly osteoporotic male, 10.2% (5.4–23.1) compared to young normals 25.2% (14.6–43.9), p < 0.001. The trabeculae tended to be thinner, 95.7 µm (66.7–170.7) c.f. 120.8 µm (75.8–208.6) and considerably fewer in number, 11.1 (2.1–31.4) c.f. 48.3 (25.4–66.9), p < 0.001 per field and in particular the number of nodes, 2.1 (0.15–14) c.f. 40.6 (10.3–74.1) per field and the node: terminus ratio fell to 0.13 (0.01–1.19) c.f. controls 0.98 (0.24–6.69), p < 0.001. It was concluded that the pattern of cancellous atrophy in male idiopathic osteoporosis differs from normal aging and resembles that in postmenopausal women. Results using the automated TAS confirm previous observations made manually.
Introduction
Osteoporosis is a growing public health problem in men Citation[1] as well as women. The lifetime risk of osteoporotic fractures in men has been estimated to be 13% to 25%Citation[2], and the mortality from fractures in men is 30%, which is higher than in women Citation[3]. The cause of osteoporosis in an individual male is often incompletely defined; it may usefully be categorized as either secondary, or primary (i.e., idiopathic, involutional or senile) in nature and an overlap between these is regularly seen in clinical practice. Most commonly, osteoporosis in men is due to secondary causes, the three most frequent being hypogonadism (endogenous and iatrogenic, a common example of the latter being androgen-deprivation therapy), glucocorticoid excess (endogenous or more commonly exogenous), and alcohol overuse Citation[4],Citation[5]. These aetiologies account for 40% to 50% of all cases. Other disorders can contribute, however, including hyperthyroidism, hyperparathyroidism, multiple myeloma, idiopathic hypercalciuria, and intestinal malabsorption, among others Citation[6],Citation[7]. In total, one half of two thirds of men with osteoporosis have an identifiable medical condition or factor that results in bone loss and increased skeletal fragility Citation[8].
As well as affecting older men, primary or idiopathic osteoporosis may occasionally present in young men with symptomatic vertebral fractures Citation[9]. By definition, secondary causes of osteoporosis are absent in these patients. The pathogenesis is poorly understood, but may reflect disturbances in osteoblastic function Citation[10] or insulin-like growth factor Citation[11]. More recent reports have implicated the deficient acquisition of bone during skeletal maturation Citation[12], perhaps related to perturbations in sex steroid levels that occur in a familial-specific manner Citation[13]. Although the skeleton develops similarly in both sexes throughout childhood, it is likely that male-female differences in bone accumulation and geometric development during adolescence and puberty are a factor in the reduced lifetime fracture risk in men compared to women. The mechanism leading to these skeletal differences is poorly understood, but probably relates to differences in sex hormone action Citation[14]. Androgens stimulate greater formation of sub-periosteal bone, whereas estrogen apparently tends to inhibit this, leading to a mechanical advantage against fracture in men Citation[15]. There also may be differences in growth factors and mechanical loading via muscle that explains observed differences in bone mass between the sexes Citation[16]. The bone mass reaches a maximum about 10 years after linear growth stops, begins to decrease in the fourth decade, and declines to half its maximum value by the age of 80 Citation[5]. The peak bone mass, attained in early adult life is dependent primarily on genetic factors Citation[16],Citation[17] and recent studies measuring the bone mass at the spine and hip suggest that the peak value is achieved during the third and fourth decades Citation[18]. In another study the vertebral and femoral neck size and BMD (bone mineral density) rose more steeply with age in Caucasian boys than girls Citation[19]. It follows that the bone mass is lower in women than it is in men, and it is also lower in Caucasians than in African-Americans Citation[20].
Just as there are sex-specific differences in skeletal accrual described above, both women and men are subject to skeletal atrophy with aging and this appears to affect men less. The loss of cancellous bone, the main constituent of the vertebral bodies, has been described as similar in both men and women Citation[21], however the trabecular microarchitecture, as well as the bone mass (or relative trabecular bone volume in histological terms) is a factor for consideration in structural strength. In particular, a greater decline in the interconnection of the trabecular network in women than in men may contribute to a higher fracture risk in women Citation[22]. Additionally, androgen-dependent differences in sub-periosteal bone formation with age may help to reduce the effect of declining bone mass on fracture risk in men compared with women Citation[23]. Although cancellous bone comprises only 15% of the skeleton, the changes that occur in this type of bone after age 30 determines whether osteoporosis will develop. Cancellous bone decline with aging has been reported to begin in early adult life, occurring before the decline in cortical bone Citation[24], although others using a different technique disagree, suggesting that the fall in cancellous bone mass begins later Citation[25]. Whatever the relative time-scale, the microanatomical deterioration of cancellous bone loss may occur as a generalized thinning of the trabeculae, or as the perforation and fragmentation of the trabecular network Citation[26],Citation[27] with different biomechanical consequences. In recent years new noninvasive techniques have been widely applied to measuring bone mass or bone mineral density (BMD) with great precision in different skeletal regions Citation[22]. Nevertheless, there is now sufficient evidence to suggest that the complex microarchitecture of cancellous bone is a crucial factor in mechanical strength as well Citation[28],Citation[29] and this is not resolved adequately by these gross methods. On this basis the aim of the following article is to compare the microarchitecture of trabecular bone from the iliac crest (the standard bone biopsy site) of normal young men and elderly men with idiopathic osteoporosis using an automated image analysis procedure Citation[30], and to compare the results with those from previous studies using a manual method Citation[26],Citation[27].
Materials and methods
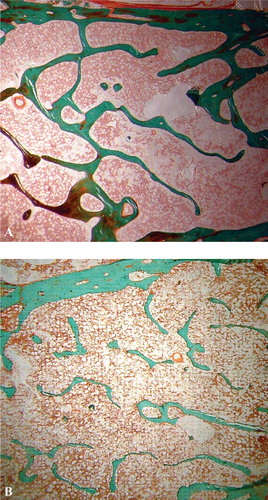
This was a retrospective investigation on archived material that was an unpublished adjunct to an earlier study Citation[31]. Patients who had undergone iliac crest biopsies and lumbar BMD measurements were identified from databases at the Royal Hallamshire Hospital, Sheffield. The records of these patients were reviewed and any who had received drugs for the treatment of osteoporosis (e.g., fluoride, biophosphonates and anabolic steroids) prior to biopsy or BMD measurements were excluded, as were those who were receiving or had received oral corticosteroids, or who had primary hyperparathyroidism or renal impairment (defined when serum creatinine exceeded the normal range). A total of 16 patients mean age 60 ± 12 years were eligible for study. Transiliac bone biopsies were taken from the elderly male subjects with primary osteoporosis after using local anaesthetic and an 8-mm diameter Bordier trephine. Bone mineral density was assessed using a Hologic 2000 densitometer within 3 months of the bone biopsy and generally during the same admission. At the same time, iliac crest autopsy specimens were assembled for comparison from 28 healthy young men (who had died suddenly with no history of bone disease and no treatment likely to affect the bone), mean age 30 ± 8.9 years: these samples were from an archive collection in the Hard Tissue Research Laboratory, University of Leeds, UK. All the material had received local ethical approval and was stored under the provisions of the Human Tissues Act. Specimens were fixed in 70% ethanol and embedded undecalcified in methylmethacrylate Citation[32],Citation[33]. Six sections, 8 µm thick were cut from each on a Jung K heavy duty microtome and stained by the Goldner method (). Since the sections were to be analysed using an automated image analysing system or trabecular analysis system (TAS) Citation[30] it was essential that the stains chosen provided good contrast between bony tissue and the marrow spaces, otherwise their separation when thresholding to create a binary image would not be reliable. Each section was placed upon the stage of a low power microscope fitted with a zoom lens. After calibrating the system the image of the whole section was captured by a closed circuit television camera attached to the microscope and to a VIP image analyser (Sight System, Newbury, UK). The captured image was thresholded to separate the bone trabeculae from any background ‘noise’ caused by the presence of stained marrow tissue. A binary image was created made up of 256 × 256 black and white pixels. Analysis consisted of the measurement of intact image when the area of interest was defined by an elastic window followed by the measurement of the thinned image when the selected part of the binary image was skeletonized or thinned to its medial axis. The comprehensive range of variables measured included the bone volume, trabecular thickness and trabecular separation from the intact image and the strut number, node number (junction between 2 or more trabeculae), terminus number (free apparently disconnected end of a trabecula) and the ratio of node:terminus (an index of connectivity) Citation[34] from the skeletonized image.
Figure 1. Photomicrographs showing the cancellous microanatomy in undecalcified transiliac histology sections from (A) young men where the network is well interconnected and (B) elderly men where the network is atrophied (×16).
Statistical analysis
Results were expressed as the median ± SD and the statistical significance of any differences between the two groups was determined using non-parametrical Mann-Whitney U test.
Results
A wide range of microarchitectural measurements are performed automatically by trabecular analysis system (TAS) and the most useful nine principal trabecular histomorphometrical variables are presented here (). As well as the relative trabecular bone volume (a measure of bone quantity), the node number (trabecular junctions), terminus number (trabecular ends), node/terminus ratio (index of connectivity), trabecular number and total trabecular length were determined. As expected, the relative trabecular bone volume [TBV] was significantly higher in young men than elderly men with primary osteoporosis (p < 0.001). The same distinction applied to all the other variables except the trabecular separation which was less in the young normal men. In particular there was a considerable decline in the number of trabeculae and associated surface, and in the number of nodes in the elderly osteoporotic men. Because the terminus number is subject to artifact it is of limited value alone but when combined with the node number as the node:terminus ratio it has been reported to be a useful index of trabecular interconnection Citation[34]. Also noteworthy is the trabecular width, which while reduced in mean value in the older group, seemed to be a less significant factor in their osteoporotic status and microarchitectural deterioration than the loss of trabeculae.
Table I. Comparison of trabecular architecture in iliac crest bone biopsy in young men and osteoporotic elderly men.
Discussion
It has previously been suggested that cancellous structural ‘quality’ as well as its quantity is a factor in the greater fracture predisposition found in aging women compared to men but that the structural distinction is largely lost in idiopathic osteoporosis Citation[26],Citation[27]. Using an automated image analysis procedure the evidence is consistent with a marked decrease in the trabecular number and interconnection in terms of wider separation and diminished nodes in elderly men with idiopathic osteoporosis. Unlike normal aging men where a significant decline in trabecular thickness is the main feature of atrophy (), the decrease in the trabecular thickness observed above is less than might be anticipated, possibly because there is some small consolidation response in the remaining disconnected trabeculae. The bone pattern in idiopathic osteoporotic men is similar to that described in postmenopausal women and also typical of hypogonadal men [J Aaron, personal communication]. The consequences of the precise pattern of trabecular bone atrophy are two-fold. First is the greater biomechanical disadvantage of a disconnected network compared with one that is merely thinned, where any disconnection can weaken the bone out of proportion to the amount that is lost Citation[26]. Second are the therapeutic implications where augmenting an attenuated network has a greater likelihood of success than trying to replace structural components that have been entirely lost.
Figure 2. Pattern of trabecular bone atrophy in men with age, when depressed formation causes widespread thinning of the network, and in idiopathic osteoporosis when increased resorption causes localized perforation and network disconnection.
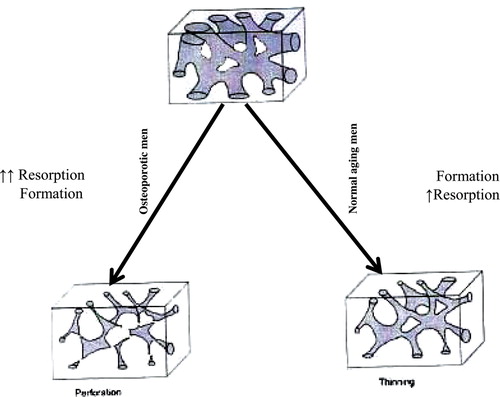
A principal limitation of the investigation is the relatively small number of subjects in each group due to the difficulties associated with invasive procedures and the acquisition of human material. The absence of a normal elderly cohort of men is also a limitation. However, in view of previous reports where the normal aging process has been described in detail Citation[26],Citation[27] it is unlikely that the pattern of extensive disconnection in these men is attributable to the normal skeletal aging process. In men this latter tends to be associated with a decline in bone formation, whereas in the present elderly subjects an increase in resorption is a more likely cause of network perforation, as is the case in postmenopausal women with vertebral crush fractures.
Bone development and growth are similar in boys and girls up to the start of puberty. Thereafter, skeletal dimorphism evolves with a greater bone mass in adult males than in adult females Citation[35]. Riggs and colleagues Citation[29] have suggested that excessive bone turnover with resorption activity marginally greater than formation is a cause of fragmentation and from the evidence above it seems that the trabecular nodes are especially vulnerable. The findings are consistent with those of Parfitt Citation[25], Malluche et al. Citation[36] and Chavassieus and Meunier [37] and a loss of trabecular connectivity in men with idiopathic osteoporosis, over and above the normal age-related thinning is apparently the pathological basis of their vertebral fractures.
Acknowledgements
I would like to thank Dr Jean Aaron and Mrs Pat Shore at the School of Biomedical Sciences, University of Leeds, UK, for support during my investigations in their Hard Tissue Research Laboratory, and also Professor John Kanis, University of Sheffield, UK.
References
- Orwoll E S, Klein R F. Osteoporosis in men. Endocr Rev 1995; 16: 87–116
- Nguyen T V, Eisman J A, Kelly P J, Sambrook P N. Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol 1996; 144: 255–263
- Myers A H, Robinson E G, Van Natta M I, Michelson J D, Collin K, Baker S P. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol 1991; 134: 1128–1137
- Hemandez-Avila M, Colditz G A, Stampfer M J, Rosner B, Speizer F E, Willet W C. Caffeine, moderate alcohol intake, and risk of fractures of the hip and forearm in middle-aged women. Am J Clin Nutr 1991; 54: 157–163
- Orwoll E S. Osteoporosis in men. Endocrinol Metab Clin North Am 1998; 27: 349–367
- Seeman E. Osteoporosis in men: epidemiology, pathophysiology, and treatment possibilities. Am J Med 1993; 95: 225–285
- Amin S, Felson D T. Osteoporosis in men. Rheum Dis Clin North Am 2001; 27: 19–47
- Khosla S, Lufkin E G, Hodgson S F, Fitzpatrik L A, Melton L J. Epidemiology and clinical features of osteoporosis in young individuals. Bone 1994; 15: 551–555
- Jackson J A, Kleerekoper M, Parffit M M, Rao D S, Villanueva A R, Frame B. Bone histomorphometry in hypogonadal and eugonadal men with spinal osteoporosis. J Clin Endocrinol Metab 1987; 65: 53–58
- Kuland E S, Rosen C, Casman F, Bilezikian J P. Insulin-like growth factor in men with idiopathic osteoporosis. J Clin Endocrinol Metab 1997; 82: 2799–2805
- Van Pottelbergh I, Goemaere S, Zmierczak H, De Bacquer D, Kaufman J M. Deficient acquisition of bone during maturation underlies idiopathic osteoporosis in men: evidence from a three-generation family study. J Bone Miner Res 2003; 18: 303–311
- Van Pottelbergh I, Goemaere S, Zmierczak H, De Bacquer D, Kaufman J M. Perturbed sex steroid status in men with idiopathic osteoporosis and their sons. J Clin Endocrinol Metab 2004; 89: 4949–4953
- Seeman E. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 2001; 86: 4576–4584
- Seeman E. The growth and age-related origins of bone fragility in men. Calcif Tissue Int 2004; 75: 100–109
- Orwall E. Osteoporosis in men. Primer on the metabolic bone diseases and disorders of mineral metabolism, M Favus. American Society for Bone and Mineral Research, Washington, DC 2003
- Smith D M, Khairi M RA, Johnston C C, Jr. The loss of bone mineral with aging and its relationship to risk of fracture. J Clin Invest 1975; 56: 311–318
- Mundy G R. Boning up on genes. Nature 1994; 367: 216–217
- Mundy G R. The genetics of osteoporosis. Endocrinologist 1995; 5: 176–179
- Lu P W, Cowell C T, Lloyds-Jones S A. Volumetric bone mineral density in normal subjects, aged 5–27 years. J Clin Endocrinol Metab 2000; 81: 1332–1339
- Henry Y M, Fatayerji D, Eastell R. Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteopor Int 2004; 15: 263–273
- Riggs B L, Wahner H W, Dunn W L. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest 1981; 67: 328–335
- Kalender W A, Felsenberg D, Louis O, Jonsson B, Thomson R G. Reference values for trabecular and cortical vertebral bone density in single and dual energy quantitative computed tomography. Eur J Radiol 1989; 9: 75–80
- Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002; 359: 1841–1850
- Parfitt A M, Mathews C HE, Villanueva A R. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. J Clin Invest 1983; 72: 1396–1409
- Aaron J E, Makins N B, Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orth 1987; 215: 26–71
- Aaron J E, Francis R M, Peacock M, Makins N B. Contrasting microanatomy of idiopathic and corticosteroid-induced osteoporosis. Clin Orth 1989; 243: 294–305
- Kleerekoper M, Villanueva A R. The role of three dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int 1985; 37: 594–597
- Riggs B L, Wahner H W, Melton L J. Rates of bone loss in the axial and appendicular skeletons of women: evidence of substantial vertebral bone loss prior to menopause. J Clin Invest 1986; 77: 1483–1491
- Aaron J E, Johnson D R, Kanis J A, Oakley B A, Higgins P O, Paxton S K. An automated method for the analysis of trabecular bone structure. Computers and Biomed Res 1992; 25: 1–16
- Hordon L D, Raisi M, Aaron J E, Paxton S K, Benton M, Kanis J A. Trabecular architecture in women and men of similar bone mass with and without vertebral fracture: 1- Two-dimentional histology. Bone 2000; 27: 271–276
- Shahtaheri S M, Aaron J E, Johnson D R. The impact of mammalian reproduction on cancellous bone architecture. J Anat 1999; 188: 407–412
- Saino H, Carter D H, Natali A J, Shahtaheri S M, Aaron J E. Evidence of an extensive exercise collagen type III proximal domain in rat femur. Bone 2003; 32: 660–668
- Garrahan N J, Mellish W E, Compston J E. A new method for the two-dimensional analysis of bone structure in human iliac crest biopsies. J Microsc 1986; 142: 341–349
- Vanderschueren D, Vandenput S B, Boonen S, Kaufman J M, Zmierczak H. Androgen and bone. Endo Rev 2004; 25: 389–425
- Malluche H H, Meyer W, Sheman D, Massery S C. Quantitative bone histology in 84 normal American subjects. Calcif Tissue Int 1982; 34: 449–455
- Chavassieux P, Meunier P J. Histomorphometric approach to bone loss in men. Calcif Tissue Int 2001; 69: 209–213