Aging phenomena are generally felt to be related to the interaction between both genetic and environmental factors, and one of the most prominent alterations that occurs with aging is the reduced ability to appropriately respond or adapt to stress. Although the entire body is involved, many prominent aging theories concentrate on the endocrine system, because hormones play such an essential role in integrating, modulating and counter-regulating responses to both internal and environmental stresses. Among the hormonal changes that might be related to aging, a primary role is likely exerted by the aging-related deficit of anabolic hormones, promoting a milieu that favours catabolism. This aging-related deficiency in anabolic hormone milieu takes several different forms, being relatively sudden and dramatic in the case of estrogen (E2) in women, although being more gradual and steady for testosterone (Te) in men, and dihydroepiandosterone (DHEA) and growth hormone in both genders. This editorial will focus on age-related changes in overall anabolic status in the aging male and how it may be connected to complex and interacting abnormalities in the overall hormonal milieu.
The anabolic deficit associated with aging is potentially related to several clinical conditions typically observed in the ‘frail elderly’: sarcopenia; poor muscle quality, muscle weakness hypertrophy of adipose tissue, especially in central depots; loss of skeletal mass and integrity; and impaired neurotransmission and neurotrophism. Most research in this field has focused on finding a simple and direct relationship between the plasma concentration of a single hormone, and one or more specific clinical aging outcomes. As an example, there are numerous studies that have attempted to find a simple correlation between either DHEA or Te and muscle mass and/or strength. Although occasionally a direct link is detectable, when this ‘holy grail’ relationship is not detected, a lack of important association is inferred. However, this interpretation is naïve and overly simplistic, because it does not take into account: (1) the many complex steps involved in determining the physiological effect(s) of any single hormone; (2) the numerous possible interaction and compensatory activities among multiple hormone working in a similar (anabolic) direction; and (3) the crucial importance of opposing catabolic hormones on the overall anabolic/catabolic balance that may be paramount in determining a given clinical outcome such as muscle or bone mass.
The complexity within a single hormone system
Synthesis/secretion
Age-related impairment in synthesis/secretion of anabolic hormones (e.g. Te and DHEA/S) can develop because of primary abnormalities at the levels of the central nervous system, hypothalamus, pituitary and end-organ. Indeed, often, as with Te, there is evidence of impairment at more than one level. This is especially true within aging systems. ‘Aging’per se, genetic predisposition, environmental exposures, chronic diseases and long-term drugs administration can all produce significant impairments in steroid hormone secretion. The potential pathogenic role at each of these different steps (hypothalamus, pituitary, cortico-adrenal and testicular glands) can be directly detected by the presence of morphological and structural abnormalities (cell loss and pathology). Abnormalities can also be revealed by aberrations in well-known functional indexes such as the circadian rhythmicity, and the quantitative and qualitative response of end organs to the appropriate prior releasing or stimulating hormone.
Binding
The binding of released hormone is another potential step for age-related changes within a single anabolic system. Most of Te and DHEA circulating in the plasma after secretion are bound to SHBG and/or albumin, with only a small fraction being free. Although DHEA is bound only to albumin, Te is tightly bound to SHBG and only loosely bound to albumin. This binding characteristic allows the concentration of SHBG to exert a pivotal role in regulating the anabolic effects of Te by firmly sequestering it in the bound (inactive) state. This dual binding system for Te can also complicate the relationship between the measured total plasma concentration and an expected clinical outcome. Therefore, it is important to understand the multitude of factors that can influence serum SHBG levels. Hepatic synthesis of SHBG increases with aging (possibly related to insulin resistance) and thus, for any given serum concentration, less bound hormone is free and active. Certain other conditions can also enhance the levels of SHBG: increased thyroxine and/or cortisol concentrations; liver pathology like cirrhosis; a vegetarian or high FFA diet; long-term treatments with certain drugs like anticonvulsants; and exposure to environmental xenoestrogens.
Metabolism
Anabolic hormones like Te and DHEA also undergo metabolic bioconversions that can dramatically modulate their biological effects. Te can be bioconverted to estrogens by aromatase, which is abundant in many tissues, including adipose tissue. Because aromatase is preferentially expressed in preadipocytes compared with mature adipocytes, its activity can be significantly modulated both by the total amount of adipose tissue in the body and by factors that interfere with the PPARy-mediated maturation of preadipocytes. A decline in aromatase activity can be induced by environmental toxins found in plasticisers, by drugs like fenofibrate and cyclooxigenase inhibitors, by flavonoids contained in red wine and grape seeds and finally by insufficient concentrations of vitamin D. These conditions may therefore limit normal bioconversion which could be important in men and in postmenopausal women. On the contrary, aromatase efficiency increases physiologically with aging, and can be further activated in some pathological situations like hepatocellular carcinoma, adrenocortical and testicular tumors and liver cirrhosis.
Peripherally, Te can also be bioconverted to DHT by 5-α-reductase enzymatic activity. An age-related decline of this enzyme has been well described. Drugs can further affect this enzyme with increased activity from indomethacin, and reductions associated with both non-steroidal inhibitors like benzoquinolinones and carboxylic acid derivatives, and steroidal inhibitors like azasteroids and progesterone derivatives.
The two major forms of DHEA (free and sulfo-conjugated) are both primarily produced by the adrenal gland, but are metabolically inter-convertible by sulfo-transferase and sulfatase enzymatic activity found in many peripheral tissues. Because DHEA is known to be the active form of this steroid, the availability of ubiquitous tissue sulfatases can significantly modulate the overall biological effect. Finally, because at least part of anabolic activity of DHEA is mediated by its bioconversion to Te, in an intracrine fashion within cells, any condition which interacts with this metabolic step might profoundly alter the anabolic milieu of the whole body. This intracrine function is regulated in a compensatory fashion by the ambient concentration of Te.
Receptors
Acquired androgen receptor abnormalities such as anti-receptor antibodies or androgen receptor gene CAG polymorphism can produce ‘androgen resistance’ and mitigate any anabolic clinical outcome. Furthermore, a number of transcription factors can bind to the promoter/enhancer or repressor sites for steroid hormone receptors and affect their ability to activate RNA polymerase. The stability and the availability of these transcription modifying factors can change with aging and diminish the efficacy of post-receptor steps.
Interactions and compensatory activities of multiple hormones that act in the same anabolic or in the catabolic direction
The intricacies described above for a single hormone system are further complicated when we consider that the anabolic milieu is modulated simultaneously and in parallel by several hormone systems (Te, DHEA, E2, GH-IGF), all of which are in some way diminished with aging. This creates the potential for a ‘multiple dysregulation syndrome’. Therefore, a reasonable clinical research approach should truly simultaneously account for all of the anabolic hormone systems and the potentially elaborate manner in which they may interact. Considering this, it becomes clear that a partial failure of a single hormone system might be partially or fully compensated by one or more parallel systems without producing a significant detrimental downstream clinical outcome. The effective compensation, which mitigates the appearance of a significant downstream clinical outcome, also lessens the likelihood of finding a statistically significant relationship between the diminished hormone concentration and the final clinical outcome of interest. It would also seem reasonable that in the absence of a significant clinical problem there is no obvious need for any treatment. However, the risk of finding an important clinical outcome becomes statistically more likely, when partial failure of two or more hormone systems are present simultaneously.
Although a modest deficit in any one anabolic system can be potentially mitigated by counter-regulatory increases in hormones, a more complete failure of any single system might not be compensated by enhancement of parallel systems. In this condition of near-complete failure, one would be more likely to detect a simple direct correlation with an associated clinical outcome. Furthermore, this situation would best be treated by replacement of the specific deficient hormone. Unfortunately, this condition is more likely to occur in younger individuals, while multiple more modest deficiencies are probably more common in the older patient.
Complexity increases further, when we consider that a decline of each single anabolic hormone might interact with a parallel hormone systems at each of the different steps previously described (Sections Synthesis/secretion, Binding, Metabolism and Receptors): synthesis/secretion, transport, peripheral metabolism, receptor and post-receptor mechanisms.
A final layer of complexity is added by considering how age-related alterations in catabolic hormone systems might affect the overall anabolic:catabolic balance of the hormonal milieu. With aging, a small but discernable increase of cortisol (and possibly thyroid hormone) secretion has been documented. Excessive activation of one or more catabolic systems could produce a ‘relative anabolic deficit’, in the face of normal or only slightly reduced levels of anabolic hormones. Therefore, the ratio between blood concentrations of anabolic hormones (Te or DHEA) and catabolic hormones (cortisol and thyroid hormones) might reveal statistically significant and clinically important correlations with downstream outcome measures that could not be detected by analysis of any one hormone or set of parallel hormones.
In addition to a direct effect of catabolic hormone systems on a target outcome, we must consider the possibility that cortisol and thyroid hormones may also influence anabolic systems at any of the multiple steps from hormone synthesis to the post-receptor message.
Summary and conclusions
In the search for a relationship between the age-associated decline in an anabolic endocrine system and impairment of clinical parameter (e.g. muscle mass or strength), a prudent clinical investigator must be careful to fully consider: (1) the complexity of each single anabolic hormone system from synthesis to post-receptor activity; (2) the multiple ways in which parallel anabolic systems can interact with and counter-regulate each other; and (3) how opposing catabolic systems can modify the overall anabolic:catabolic balance ().
Figure 1. The complexity of a single anabolic hormone system involves the moments of synthesis, transport, peripheral metabolism and finally the activation of the receptor and postreceptor mechanism. Furthermore, interactions and counter-regulatory activities are involved of hormones working in the same and in the opposite directions.
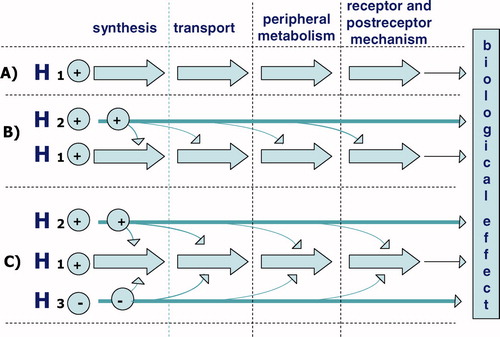
A scenario, in which there is a profound deficit in a single hormone system, and a clear correlation to a clinical outcome, may be common in younger individuals but relatively rare with aging. Evidence-based medical treatment in this case would consist of replacement of the specific deficient hormone. We postulate that with aging, partial deficiencies in multiple anabolic systems, along with possible augmentation of opposing catabolic systems, would be the more common scenario leading to a clinically important outcome. Given the multiple layers of complexity described above, it should not be surprising, therefore, when researchers are unable to find statistically significant relationships between a single hormone concentration and its hypothesised associated clinical outcome. This may limit the degree to which we can rely only on evidenced based research to guide our therapeutic decisions in older individuals. In light of the discussion above, we feel that it is necessary to add a new term to the nomenclature that presently defines the action of hormones at various distances from their point of secretion (endocrinology, paracrinology, autocrinology and intracrinology). This new term syncrinology comes from the Greek roots ‘to secrete together’. Syncrinology can be used to signify a mechanism of action, by which multiple hormones of both similar and opposite effects converge simultaneously on a single target. As an example, for the target effect of anabolic protein synthesis, homeostasis requires the harmonised action of several anabolic and catabolic hormone systems. The relative balance between these anabolic and catabolic systems varies during the lifespan (and with significant external stresses), according to the requisite metabolic requirements. In the first decades of life, the balance would decidedly favour anabolism in order to meet the requirements of growth. With aging there is a shift in syncrinology that reverses the balance to catabolic, in a body that teleologically was not intended to be immortal.