Abstract
Background: Opioids are an effective treatment for chronic non-malignant pain (CNP). Long-term use risks and side effects such as opioid-induced androgen deficiency (OPIAD) exist. This could be measured by saliva testosterone (Sal-T).
Objectives: To evaluate OPIAD in long-term opioid use in CNP patients.
Methods: A cross-sectional study included CNP male outpatients under opioid treatment. Total-Testosterone (Total-T), Free-Testosterone (Free-T), Bio-Testosterone (Bio-T) and Sal-T were measured. Correlations were calculated by Spearman’s rho (SPSS 20).
Results: From 2012 to 2014, 134 from 249 (54%) consecutive male outpatients reported erectile dysfunction (ED), 37% of them related to opioids and 19% evidenced OPIAD. A total of 120 subjects (94 cases and 26 matched-controls) were included. A significantly lower luteinizing hormone, Total-T and Free-T were found, as well as, a significant correlation between Sal-T and Total-T (r = 0.234, p = 0.039), Bio-T (r = 0.241, p = 0.039), IIEF (r = 0.363, p = 0.003) and HAD-anxiety (r = −0.414, p = 0.012) in OPIAD patients. Sal-T levels were significantly lower in patients with severe–moderate ED versus mild ED (p = 0.045) and in patients with severe ED versus moderate–mild ED (p = 0.036).
Conclusions: These data demonstrate the high prevalence of ED in long-term use of opioids, part of this is associated to OPIAD, which can be tested by Sal-T as a non-invasive approach.
Introduction
Opioid therapy is one of the most effective forms of analgesia currently. Chronic pain adult males exposed to long-use prescription opioids, increase the risk of opioid adverse events such as androgen deficiency (OPIAD) [Citation1–4]. This syndrome is characterized by an inadequate production of sex hormones, particularly testosterone. Symptoms that may manifest include reduced libido, erectile dysfunction (ED), fatigue, hot flashes, anxiety and depression [Citation5,Citation6]. None of these signs and symptoms are specific of OPIAD but may raise suspicion. In fact, opioid-induced endocrinopathy should be considered in any patient receiving daily opioid treatment in an amount equivalent to 100 mg of morphine or more [Citation7]. Patients should be asked routinely about symptoms suggestive of sex hormone deficiency before treatment and at regularly scheduled follow-up medical visits. However, despite its high frequency, OPIAD is rarely considered for treatment, that usually consist in androgen replacement for males and dehydroepiandrosterone (DHEA) supplementation for females [Citation8], and, prevalence of abnormally low serum testosterone levels has historically been scarcely reported, roughly 6% in urology clinics [Citation9–11]. Furthermore, it is well known, that low levels of testosterone in men are related to a higher risk of cardiovascular disease and a decreased survival [Citation12–15].
Serum testosterone concentration is the principal laboratory test used to diagnose male OPIAD. There is no consensus as to when laboratory tests should be ordered, but it is reasonable to do so when patients report one or more suggestive symptoms [Citation16]. Recently, salivary testosterone (Sal-T) has been suggested as a biomarker for androgen deficiency diagnosis [Citation17]. Saliva is a widely accepted sample source for steroid analysis and offers a non-invasive and stress-free, alternative to plasma and serum [Citation18]. In healthy adult men, testosterone circulates in plasma either free (Free-T, 2.2%) or bound to serum proteins (albumin, sex hormone binding globulin (SHBG); Total-T, 44%) [Citation19,Citation20], and a highly significant correlation between Sal-T, Total-T and Free-T has been described in eugonadal and hypogonadal men with normal renal function [Citation21,Citation22]. Unfortunately, the usefulness of Sal-T was not further investigated in male sexual dysfunction with androgen deficiency.
The aim of this study was to investigate if Sal-T could become a reliable non-invasive tool to screen androgen deficiency. For this purpose, the following steps were performed: (i) to establish the correlation of Sal-T with circulating Total-T; (ii) to determine the correlation of Sal-T with clinical parameters such as International Index of Erectile Function questionnaire scores (IIEF) and (iii) to detect androgen deficiency through Sal-T analysis.
Methods
This protocol was approved by the Ethics Committee for Clinical Research of the University General Hospital of Alicante (Spain). All participants signed informed consents before enrolment in study protocols, and the study were performed according to the Declaration of Helsinki.
Chronic pain patients and controls
A cross-sectional study was developed for two years including consecutive 249 chronic non-cancer pain (CNP) male outpatients with long-use of opioids that spontaneously reported ED; together with 26 controls with the same characteristics and geographical areas but without spontaneous ED report. All subjects came from the Clinic Pain Unit of University General Hospital of Alicante (Spain). Salivary and blood samples from subjects' anterocubital vein were drawn in the morning.
Inclusion criteria were: adult CNP male patient’s diagnosed according to International Association for the Study of Pain (IASP)’s criteria [Citation23], receiving oral and/or transdermal opioid treatment for at least one year, and with spontaneous ED in heterosexual patient’s reports. Patients with apparent infections, other inflammatory diseases, malignancies or use of testosterone, glucocorticoids, bisphosphonates, calcitonin or parathyroid hormone were excluded from the study. None of the patients were taking hormone medication (supplementation or deprivation) or phosphodiesterase type 5 (PDE5) inhibitors.
Data collection
All the data provided were collected as part of the routine clinical procedure and in line with current guidelines. ED was recorded based on patient’s spontaneous notification in routine clinical controls. A physical exam was performed including body mass index (BMI, kg/m2), blood pressure (mmHg), heart rate (bpm) and the most common comorbidities (hypertension, diabetes, dyslipidaemia or obesity). Baseline socio-demographic and lifestyle characteristics were recorded together with familiar medical history and current intake of abuse drugs including alcohol and nicotine ( and Supplementary Table 1).
Table 1. Summary of clinical and socio-demographical characteristics of the participants.
Pain severity was determined using a commonly used self-reported Visual Analogue Scale (VAS), with 0 indicating “no pain”, and 10 indicating “the worst possible pain”. Based on this, the pain severity in individuals was classified as mild (VAS ≤ 3 cm), moderate (VAS 4–6 cm) or severe (VAS ≥ 7 cm). Quality of life standardized measures developed by the EuroQol Group (EQ-VAS) were used in this study. Scores ranged from 0 (the worst health status) to 100 (the best health status). A pain expert psychologist evaluated psychological health by Hospital Anxiety and Depression Scale (HAD). The HAD is comprised of two subscales, Depression and Anxiety. Each subscale has a score ranging from 0 to 21. Items are rated on a four-point Likert-type scale ranging from 0 to 3, generating a scale range of 0–42 points, with higher scores representing greater symptom severity. Scores of 0–7 indicate normal levels; 8–10 indicate borderline abnormal anxiety and depression levels and 11–21 suggest abnormal levels of anxiety and depression.
Patients who reported ED were routinely derived to Andrology Unit for correct physical examination to confirm ED according to the Standard Operating Procedures in Sexual Medicine [Citation24] and International Classification of Diseases (ICD) [Citation25]. Only heterosexual patients were included in this study, to make the results more comparable and to prevent possible bias using the IIEF questionnaire (range 5–75 scores). This questionnaire has 15 items that evaluate different sexual aspects, the most important being Erectile Function domain (IIEF-EF), with a range from 0 to 30 scores which classifies EF as 0–10 severe, 11–16 moderate, 17–25 mild and ≥26 normal [Citation26].
Sample collection
Salivette® system was used. It was used for the saliva sample with each swab placed beneath the tongue for at least 3 min. All subjects were instructed not to brush their teeth 2 h prior to saliva collection. Then, salivettes were centrifuged at 570×g for 2 min and supernatants were frozen at −20 °C until processing. Salivary testosterone levels were analysed.
Blood samples were drawn in the morning, after overnight fast, for determination of blood glucose, urea, creatinine, uric acid, total cholesterol, high and low density lipoprotein cholesterol (HDL-cholesterol and LDL-cholesterol), triglycerides, total bilirubin, alanine amino transferase (ALT), aspartate amino transferase (AST), gamma glutamyltranspeptidase (GGT), albumin, total proteins, prostate specific antigen (PSA), thyroid stimulating hormone (TSH), follicular stimulating hormone (FSH), luteinizing hormone (LH), prolactin, total testosterone, SHBG and total testosterone.
To minimize pre-analytical errors, saliva was discarded if contaminated with blood.
Hormone variables
Total serum testosterone can be divided into three components; roughly half of testosterone is bound to the carrier molecule sex hormone-binding globulin (SHBG), almost all of the remainder is bound to albumin, and 1–2% is unbound or free. Testosterone binds so tightly to SHBG that it is functionally unavailable to cells. In contrast, albumin-bound testosterone dissociates readily, meaning that this component and the free component are available for cells. The term “bioavailable testosterone” (Bio-T) refers to a combination of the albumin-bound and free portions [Citation27].
Total-T was measured by chemiluminescent immunoassay (CLIA, UniCel DXI 800, Access testosterone Beckman Coulter). Free-T was calculated by determining Total-T, SHBG (nmol/L) and albumin (kinetic nephelometry, immunochemical systems IMMAGE Beckman Coulter), from the equation FT = ([T] − (N× [FT]))/(Kt{SHBG-[T]+N[FT]}), where Kt (109 L/mol) is the association constant of SHBG for T, and N= (Ka[3.6 × 104 L/mol, association constant of albumin for T]×Ca [albumin concentration, g/L]) + 1, as described by Vermeulen et al. [Citation28].
Bio-T was calculated by adding to the Free-T the testosterone bound to albumin, obtained by the product of KaCa and Free-T. Sal-T was measured by a radioimmunoassay (RIA) for the quantification of Total-T in serum (Coat-A-Count, Siemens) with some in-house modifications in order to adapt it to saliva. In order to increase the sensitivity, the overnight incubation time was prolonged, the reaction volume increased and the calibration curve adapted after successive dilutions of the lower concentration calibrator, achieving an analytical sensitivity of 34 pg/ml and an intraserial CV of 7.9%.
Statistics
Data were expressed as mean ± standard deviation when normally distributed and as median (nonparametric distribution) for parameters with non-normal distribution, unless otherwise specified [Citation29]. Differences were evaluated with one-way analysis of variance or Kruskal–Wallis test, according to normality. Correlations were assessed using Spearman's or Pearson's method when not normally or normally distributed, respectively. Unpaired two-sided Student's t-tests were used for comparison of means of normally distributed parameters. In all other cases, Mann–Whitney U test was used for comparisons between groups. Stepwise multiple linear or logistic regressions were applied for multivariate analysis, for continuous or categorical dependent variable, respectively. All statistical analyses were performed on SPSS (Statistical Package for the Social Sciences; Chicago, IL) for Windows v20. The level of statistical significance was p <0.05.
Results
From 1 May 2012 to 27 July 2014, a total of 747 (249 males, 33%) consecutive patients with long-use of opioids were nursed at the Clinic Pain Unit of University General Hospital of Alicante. In global, 54% (134/249) reported spontaneous ED. Forty of them (30%) were excluded by previously diagnosed hypogonadism or renal insufficiency, hepatic impairment, major psychiatric disorders, and other reasons for ED or chronic debilitating diseases. Patients were also excluded if they were not engaged in steady heterosexual activity.
Finally, a total of 94 patients with ED (37%) were included in the present study (cases, 59.5 ± 11.8 years, 19% OPIAD). Also, 26 non-ED patients (controls, 55.9 ± 14.7 years, p = 0.402) were retrieved, according to routine pain screenings. The baseline socio-demographic and clinical characteristics of the participants are reported in .
All subjects (n = 120) had documented history of chronic pain with a median duration of 5,5 years and were under long-use of opioid treatment. The most common comorbidity was dyslipidemia (56%, total cholesterol 180 ± 40 mg/dl), followed by hypertension (40%, blood pressure 132/76 mmHg), diabetes (22.3%, fasting glucose 132 ± 36 mg/dl) and obesity (46%, BMI > 30). Patients use: 74% major opioids, 34% tramadol, 40% analgesic, 84% adjuvant drug (most prevalent, 39% antidepressants and 70% anticonvulsants, Supplementary Table 1).
Analysis of pain intensity (VAS), mean quality of life-VAS, HAD-anxiety, HAD-depression, IIEF and IIEF-EF for ED cases and controls are reported in . In general, pain intensity was mostly severe–moderate with a mean of 5.8 ± 2.5 cm (22% mild, 37% moderate, 41% severe). Most subjects present a normal or borderline result in the HAD-anxiety questionnaire (41% normal, 21% borderline case, 38% case) and depression-HAD (46% normal, 25% borderline case, 29% case). IIEF and IIEF-EF were significantly lower in patients with ED, and especially in patients presenting OPIAD (p < 0.05). Most of the patients with ED presented an IIEF-EF score that corresponds to severe conditions (68% severe, 10% moderate, 22% mild).
Table 2. VAS, HAD and IIEF scores for controls and ED patients.
Blood analysis and Sal-T levels are summarized in . Total-T, Free-T, SHBG and Sal-T levels were lower in patients presenting OPIAD (). Furthermore, Total-T and Free-T in these patients were not only significantly lower (p < 0.01), but also it was up to two-fold lower than in controls or No-OPIAD patients.
Table 3. Blood analysis and salivary testosterone levels.
ED and hormone level correlations
Mean hormone values analyzed by ED severity (severe, moderate, mild and normal) are summarized in . Total-T, Free-T, Bio-T and Sal-T levels were significantly lower in patients with severe ED ( and ).
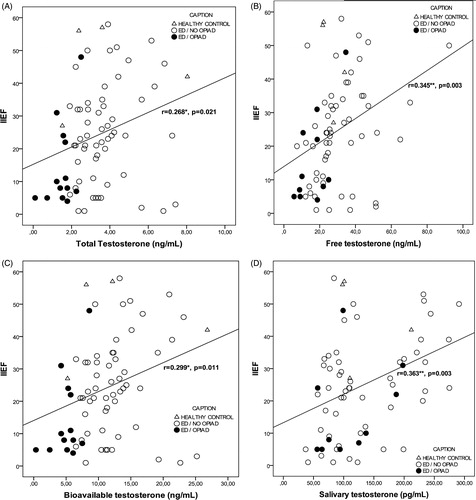
Figure 1. Correlation analysis of salivary testosterone with total (A), free (B) and bioavailable testosterone (C). Each dot corresponds to a different participant. *Correlation is significant (p ≤ 0.05).
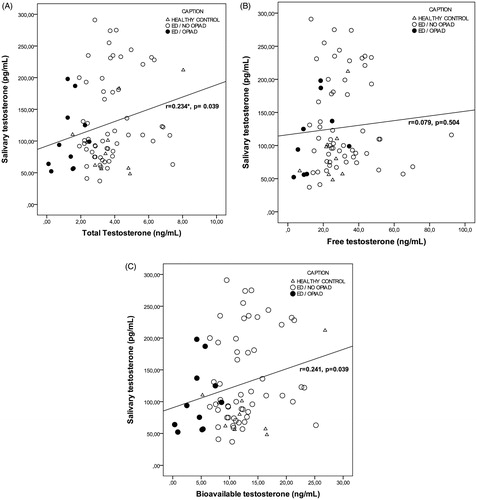
Table 4. Testosterone levels in ED patients according to ED severity.
In addition, we analyzed correlations between testosterone levels and HAD-anxiety and IIEF/IIEF-EF results in the samples and in ED patients (Supplementary Table 2 and 3, respectively). In our sample, we observed a significant correlation (p < 0.05) between Sal-T and Total-T (r = 0.234, p = 0.039), Bio-T (r = 0.241, p = 0.039), IIEF (r = 0.363, p = 0.003), and HAD-anxiety (r = −0.414, p = 0.012) ( and , and data not shown). Also, a positive correlation was found between Total-T and IIEF (r = 0.268, p = 0.021) (), HAD-anxiety (r = 0.370, p = 0.017), and HAD-depression (r = 0.337, p = 0.033); and between Bio-T and Free-T (r = 0.501, p = 0.000), IIEF (r = 0.299, p = 0.011) (), and HAD-anxiety (r = 0.357, p = 0.026).
Discussion
This study shows a high prevalence of ED spontaneously reported in 37% of males with chronic pain under long-use of opioids, 19% of them with OPIAD. Some studies demonstrated a direct correlation between testosterone deficiency and chronic opioid use but this is the first study that demonstrates the value of Sal-T in the diagnosis of androgen deficiency [Citation30]. In fact, Sal-T levels were significantly lower in patients with severe–moderate ED compared to patients with mild IIEF-EF score.
Opioid-induced endocrinopathy is one of the most common, yet least often, diagnosed consequences of prolonged opioid therapy. More or less, all guidelines suggest chronic opioid use as a common cause of hypogonadism [Citation5]. It has been shown that prolonged opioid therapy affects testosterone plasma levels. Although wide interindividual variations exist, mean testosterone levels decline with age, and differences between populations have been observed [Citation31–36]. At age 75, the mean Total-T level in the morning is about two-thirds of the mean level in men aged 20–30 years, whereas the mean Free-T and Bio-T levels are only 40% of the mean levels in younger men. Furthermore, the circadian rhythm of serum testosterone levels is generally lost or attenuated in elderly men [Citation37,Citation38]. In fact, some groups in developing countries exhibit significantly lower Sal-T levels compared to industrialized western populations as well as more attenuated age related declines [Citation39–41].
While there are no current standards for monitoring these patients, the available evidence suggests that we should routinely screen patients on opioid treatment for manifestations of hypogonadism and determine testosterone levels [Citation5,Citation42,Citation43]. This study supports the usefulness of morning Sal-T testing as a non-invasive approach to screen androgen status in men with ED and CNP.
Campbell et al. [Citation44,Citation45] reported some differences in aging patterns in association with different life styles, cultures and ethnic groups. Also, patterns of age related decline of male testosterone are variable depending on the measurements used (serum Total-T, Free-T), and on the age ranges in question [Citation46–49]. Inter- and intraindividual testosterone variations are also associated with illness severity and body composition such as amount of skeletal muscle [Citation50–53], as well as adipose tissue deposition and distribution [Citation54–57]. These results suggest that the commonly reported decline of testosterone values after the third decade of life observed in western countries may not be caused by aging per se. Instead, any association between testosterone and age may be a result of various factors, including energy availability and utilization during maturation, body composition, as well as age-related life style or illness [Citation58,Citation59]. Even more, males with anxiety mood also tend to show lower testosterone levels indicating that hormones and pain perception are changed by anxiety [Citation60]. In our study, a significant correlation was found between Sal-T, IIEF and anxiety symptoms.
Chronic administration of painkillers, such as opioids, requires the physician to be aware of both the consequences that can develop due to long-term testosterone impairment and the available means to restore and maintain physiological testosterone levels. It has been shown that even a single administration of morphine in men was able to reduce enormous testosterone levels in few hours inducing a dramatic hypogonadism that persists throughout the treatment [Citation61,Citation62]. Unfortunately, OPIAD remains known just as the effect of many medications on sexual function, since patients and professionals are often uncomfortable discussing about sexuality, thus, it remains under-recognized and under-treated [Citation63]. Testosterone has an appreciable role where adequate serum levels are required in males and females for libido and sexuality; cellular growth; maintenance of muscle mass and bone; healing; blood–brain barrier; and for central nervous system maintenance [Citation64]. Also, it has been suggested that androgen insufficiency disrupts cellular signalling pathways and produces pathological alterations in penile tissues leading to ED. Even more, OPIAD persistence is important not only because of the endocrine aspect of the illness, but also because it cannot be excluded that OPIAD in chronic pain patients could determine increasing pain sensitivity [Citation65]. Due to the pivotal role of testosterone, adequate serum levels are required for multiple functions such as muscle mass maintenance, cellular growth or sexuality. In order to maintain adequate serum levels, testosterone replacement therapy should be considered, however, there is no rule regarding when to start treatment. It is important to establish when to start testosterone replacement treatment therapy and which candidates can benefit from the treatment [Citation64]. Testosterone replacement therapy has been shown to improve body composition, pain sensitivity, sexual desire and aspects of quality of life [Citation66], however, men on long-term testosterone treatment should be monitored periodically for secondary effects.
Also, testosterone deficiency appears to be even more underdiagnosed in women [Citation67,Citation68]. Women receiving chronic opioid therapy may manifest a similar constellation of symptoms related to testosterone deficiency as men; however, these symptoms may be undiagnosed due to underappreciation of this phenomenon by both patient and practitioner. Pending problems to use Sal-T are related to the certainty of reference ranges (potential influence of gender, age, ethnicity, genotype and pathologic deviations), duration of effects, dose–response correlations and potential induction of binding proteins after long-term opioid use. Potential interferences from blood leakage into the buccal mucosa should be considered, e.g. by determination of blood related proteins.
The initial assessment of men with ED and/or diminished libido should include determination of testosterone level. These symptoms, with or without a testosterone deficiency, might be related to co-morbidities [Citation5]. Thus, patients with long use of opioids should be routinely screened for ED due to its high prevalence. The findings of our study suggest the usefulness of morning Sal-T analysis as a non-invasive approach to screen androgen status in men with ED and CNP. Further research is needed to determine if treatment is beneficial in improving sexual dysfunction symptoms and OPIAD. Also, studies of longer duration could demonstrate continued and further improvement in long-term ED symptoms.
Declaration of interest
The authors report no declarations of interest. The financial support of this project by Spanish Pain Society (2013) is gratefully acknowledged. This study was supported by an unrestricted Research Grant from Bayer Hispania.
Supplementary material available online
Supplementary_material_testo.doc
Download MS Word (144.5 KB)Acknowledgements
We wish to acknowledge the helpful clinical assistance we received from Mrs. Raquel Martín (Pain Health care assistant), Piedad Ortega and Marta Puerto (Pharmacy Research Fellowships), Dr. Olga Alda (Pain Researcher) and Mrs. Guillermina Ferrández (Andrology Nurse).
References
- Jovey RD, Ennis J, Gardner-Nix J, et al. Use of opioid analgesics for the treatment of chronic noncancer pain – a consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manag 2003;8:3A–28A
- Brown RT, Zuelsdorff M, Fleming M. Adverse effects and cognitive function among primary care patients taking opioids for chronic nonmalignant pain. J Opioid Manag 2006;2:137–46
- Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab 2000;85:2215–22
- Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage 1994;9:126–31
- Lunenfeld B, Mskhalaya G, Zitzmann M, et al. Recommendations on the diagnosis, treatment and monitoring of hypogonadism in men. Aging Male 2015;18:5–15
- Wang C, Nieschlag E, Swerdloff RS, et al. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male 2009;12:5–12
- Morales AJ, Haubrich RH, Hwang JY, et al. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf) 1998;49:421–32
- Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Phys 2012;15:ES145–56
- Niv D, Devor M, European Federation of IC. Position paper of the European Federation of IASP Chapters (EFIC) on the subject of pain management. Eur J Pain 2007;11:487–9
- Finch PM, Roberts LJ, Price L, et al. Hypogonadism in patients treated with intrathecal morphine. Clin J Pain 2000;16:251–4
- Kohler TS, McVary KT. The relationship between erectile dysfunction and lower urinary tract symptoms and the role of phosphodiesterase type 5 inhibitors. Eur Urol 2009;55:38–48
- Gheorghiu I, Moshyk A, Lepage R, et al. When is bioavailable testosterone a redundant test in the diagnosis of hypogonadism in men? Clin Biochem 2005;38:813–18
- Anantharaman P, Schmidt RJ. Sexual function in chronic kidney disease. Adv Chronic Kidney Dis 2007;14:119–25
- Kaplan AL, Hu JC, Morgentaler A, et al. Testosterone therapy in men with prostate cancer. Eur Urol 2016;69:894–903
- Morgentaler A. Controversies and advances with testosterone therapy: a 40-year perspective. Urology 2016;89:27–32
- Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain 2009;25:170–5
- Cardoso EM, Contreras LN, Tumilasci EG, et al. Salivary testosterone for the diagnosis of androgen deficiency in end-stage renal disease. Nephrol Dial Transplant 2011;26:677–83
- Groschl M. Current status of salivary hormone analysis. Clin Chem 2008;54:1759–69
- Snyder PJ. Might testosterone actually reduce mortality? J Clin Endocrinol Metab 2008;93:32–3
- Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981;53:58–68
- Morley JE, Perry HM, 3rd, Patrick P, et al. Validation of salivary testosterone as a screening test for male hypogonadism. Aging Male 2006;9:165–9
- Arregger AL, Contreras LN, Tumilasci OR, et al. Salivary testosterone: a reliable approach to the diagnosis of male hypogonadism. Clin Endocrinol (Oxf) 2007;67:656–62
- Cardenas DD, Turner JA, Warms CA, et al. Classification of chronic pain associated with spinal cord injuries. Arch Phys Med Rehabil 2002;83:1708–14
- Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med 2010;7:337–48
- Ghanem HM, Salonia A, Martin-Morales A. SOP: physical examination and laboratory testing for men with erectile dysfunction. J Sex Med 2013;10:108–10
- Kobori Y, Koh E, Sugimoto K, et al. The relationship of serum and salivary cortisol levels to male sexual dysfunction as measured by the International Index of Erectile Function. Int J Impot Res 2009;21:207–12
- Moisey R, Swinburne J, Orme S. Serum testosterone and bioavailable testosterone correlate with age and body size in hypogonadal men treated with testosterone undecanoate (1000 mg IM-Nebido). Clin Endocrinol (Oxf) 2008;69:642–7
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84:3666–72
- Yeap BB, Alfonso H, Chubb SA, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography–tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab 2012;97:4030–9
- Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, et al. Hypogonadism and sexual dysfunction in male cancer survivors receiving chronic opioid therapy. J Pain Symptom Manage 2003;26:1055–61
- Ellison PT, Bribiescas RG, Bentley GR, et al. Population variation in age-related decline in male salivary testosterone. Hum Reprod 2002;17:3251–3
- Nahoul K, Roger M. Age-related decline of plasma bioavailable testosterone in adult men. J Steroid Biochem 1990;35:293–9
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 2002;57:M76–99
- Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 2001;86:724–31
- Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab 1991;73:1016–25
- Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nat Rev Urol 2015;12:641–50
- Corona G, Mannucci E, Ricca V, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl 2009;32:720–8
- Rabijewski M, Papierska L, Kozakowski J, et al. The high prevalence of testosterone deficiency in population of Polish men over 65 years with erectile dysfunctions. Aging Male 2012;15:258–62
- Ellison PT, Panter-Brick C. Salivary testosterone levels among Tamang and Kami males of central Nepal. Hum Biol 1996;68:955–65
- Bribiescas RG. Testosterone levels among Aché hunter-gatherer men: a functional interpretation of population variation among adult males. Hum Nat 1996;7:163–88
- Bribiescas RG. An evolutionary and life history perspective on human male reproductive senescence. Ann N Y Acad Sci 2010;1204:54–64
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007;52:54–70
- Martinez-Jabaloyas JM, Queipo-Zaragoza A, Pastor-Hernandez F, et al. Testosterone levels in men with erectile dysfunction. BJU Int 2006;97:1278–83
- Campbell B, Leslie P, Campbell K. Age-related changes in testosterone and SHBG among Turkana males. Am J Hum Biol 2006;18:71–82
- Campbell B, O'Rourke MT, Lipson SF. Salivary testosterone and body composition among Ariaal males. Am J Hum Biol 2003;15:697–708
- Ongphiphadhanakul B, Rajatanavin R, Chailurkit L, et al. Serum testosterone and its relation to bone mineral density and body composition in normal males. Clin Endocrinol (Oxf) 1995;43:727–33
- Jinrui H, Itoh N, Nitta T, et al. Changes in the salivary testosterone level in aged. Hinyokika Kiyo 1994;40:807–11
- Li JY, Li XY, Li M, et al. Decline of serum levels of free testosterone in aging healthy Chinese men. Aging Male 2005;8:203–6
- Kang YG, Bae CY, Kim S, et al. Age-related change in serum concentrations of testosterone in middle-aged Korean men. Aging Male 2003;6:8–12
- Spratt DI, Cox P, Orav J, et al. Reproductive axis suppression in acute illness is related to disease severity. J Clin Endocrinol Metab 1993;76:1548–54
- Snyder PJ, Peachey H, Hannoush P, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab 1999;84:2647–53
- Bhasin S, Woodhouse L, Casaburi R, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 2001;281:E1172–81
- Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab 2005;90:678–88
- Dicker A, Ryden M, Naslund E, et al. Effect of testosterone on lipolysis in human pre-adipocytes from different fat depots. Diabetologia 2004;47:420–8
- Svartberg J, von Muhlen D, Schirmer H, et al. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromsø Study. Eur J Endocrinol 2004;150:65–71
- Derby CA, Zilber S, Brambilla D, et al. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 2006;65:125–31
- Tsai EC, Boyko EJ, Leonetti DL, et al. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 2000;24:485–91
- Nieschlag E, Lammers U, Freischem CW, et al. Reproductive functions in young fathers and grandfathers. J Clin Endocrinol Metab 1982;55:676–81
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002;87:589–98
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab 1999;84:3681–5
- Aloisi AM, Aurilio C, Bachiocco V, et al. Endocrine consequences of opioid therapy. Psychoneuroendocrinology 2009;34:S162–8
- Aloisi AM, Pari G, Ceccarelli I, et al. Gender-related effects of chronic non-malignant pain and opioid therapy on plasma levels of macrophage migration inhibitory factor (MIF). Pain 2005;115:142–51
- Aurilio C, Ceccarelli I, Pota V, et al. Endocrine and behavioural effects of transdermal buprenorphine in pain-suffering women of different reproductive ages. Endocr J 2011;58:1071–8
- De Maddalena C, Bellini M, Berra M, et al. Opioid-induced hypogonadism: why and how to treat it. Pain Phys 2012;15:ES111–8
- Fraser LA, Morrison D, Morley-Forster P, et al. Oral opioids for chronic non-cancer pain: higher prevalence of hypogonadism in men than in women. Exp Clin Endocrinol Diabetes 2009;117:38–43
- Basaria S, Travison TG, Alford D, et al. Effects of testosterone replacement in men with opioid-induced androgen deficiency: a randomized controlled trial. Pain 2015;156:280–8
- Elliott JA, Horton E, Fibuch EE. The endocrine effects of long-term oral opioid therapy: a case report and review of the literature. J Opioid Manag 2011;7:145–54
- Davis S. Testosterone deficiency in women. J Reprod Med 2001;46:291–6