Abstract
We present a case report of an atypical giant pituitary adenoma secreting follicle-stimulating hormone (FSH). A 55-year-old patient presented for erectile dysfunction, loss of libido and fatigue. The biochemical evaluation showed very high FSH serum levels in the presence of central hypogonadism. Neither testicular enlargement nor increased sperm count was observed, thus a secretion of FSH with reduced biological activity was supposed. The histological examination after neuro-surgery showed an atypical pituitary adenoma with FSH-positive cells. Hypogonadism persisted and semen analyses impaired until azoospermia in conjunction with the reduction in FSH levels suggesting that, at least in part, this gonadotropin should be biologically active. Thus, we hypothesized a concomitant primary testicular insufficiency. The patient underwent short-term treatment trials with low doses of either recombinant luteinizing hormone (LH) or human chorionic gonadotropin (hCG) in three consecutive treatment schemes, showing an equal efficacy in stimulating testosterone (T) increase. This is the first case of atypical, giant FSH-secreting pituitary adenoma with high FSH serum levels without signs of testicular hyperstimulation, in presence of hypogonadism with plausible combined primary and secondary etiology. Hypophysectomized patients may represent a good model to assess both pharmacodynamics and effective dose of LH and hCG in the male.
Introduction
Pituitary adenomas are benign neoplasms, mainly represented by prolactin-secreting adenomas (25–30% of cases) and by nonfunctioning pituitary adenoma (NFPA) (25–30% of cases) [Citation1]. Epidemiologic data indicate that gonadotropin-secreting pituitary adenomas (GSPA) represent about 10% of benign pituitary tumors [Citation2]. However, advances in immunocytochemistry, electron microscopy, cell culture and molecular techniques demonstrated that 80–90% of clinically NFPA are gonadotrope-derived and either express intact gonadotropins or their subunits [Citation2]. Thus, GSPA could be under-diagnosed and an incidence of around 25–35% of whole pituitary adenomas is estimated [Citation1–3].
In 1976, Snyder and Sterling reported the first case of GSPA in a 51-year-old man with a pituitary adenoma causing bitemporal hemianopsia, hypopituitarism and macroorchidism [Citation4]. Since this first clinical description, the biological characteristics of GSPA were investigated by in vitro studies. On the contrary, clinical features of GSPA derive only from a limited number of case reports available in the literature. GSPA may produce and secrete either low levels of intact follicle-stimulating hormone (FSH), luteinizing hormone (LH), or only their biologically inert α- or β-subunits. Generally, GSPA becomes clinically evident when the tumor growth leads to neurological symptoms and visual field defects [Citation5]. Rarely, the clinical diagnosis of GSPA is possible when the functional hormone is secreted by the tumor and signs and symptoms are present. Ovarian hyperstimulation in women [Citation2,Citation6] and testicular enlargement in men [Citation7–9] represent peculiar clinical manifestations occurring mainly in case of FSH-secreting GSPA rather than LH [Citation6]. Moreover, nuanced clinical pictures have been shown in male patients, such as clinical or subclinical hypogonadism with serum testosterone (T) levels either slightly below or within the normal range. In particular, according to the literature, the underlying mechanism of FSH-secreting GSPA-related hypogonadism would consist in a destruction of normal pituitary LH-secreting cells and alteration of normal LH secretion [Citation6]. However, only few GSPA cases are associated with a gonadotropin-related T increase, resulting in testicular enlargement, increased sperm count and precocious puberty [Citation6].
A large analysis of randomly selected patients with GSPA revealed that these tumors are mostly classified as typical adenomas [Citation10]. Thus, the follow-up approach to GSPA without clinical manifestations is usually considered similar to NFPA. Here, we present the first case of an atypical FSH-secreting pituitary adenoma detected for the presence of symptomatic hypogonadotropic hypogonadism, in which the Leydig cell activity was studied during the follow-up by short-term treatment trials with low doses of either recombinant LH or human chorionic gonadotropin (hCG).
Case presentation
A 55-year-old man was referred to Unit of Endocrinology of Modena for the appearance of fatigue, depression, decreased libido and erectile dysfunction (ED) in the last 6 months. In anamnesis, no alcohol or smoke consumption was referred. Moreover, the patient was not married and did not have children.
The past medical history included reduced growth of the left forearm, as a consequence of radio-therapeutic treatment of hemangioma at the age of 6 months. He underwent two-stage thyroidectomy for follicular variant of papillary thyroid carcinoma in 1974, complicated with permanent post-surgical hypoparathyroidism. In addition, the patient was followed up at the Hematology Unit of Modena for Jak-2 myeloproliferative syndrome in stable conditions since 7 years. Finally, the patient was followed for hypertension. The patient’s chronic therapy consisted in levothyroxine, carvedilol, quinapril, calcium carbonate and calcitriol. The written informed consent has been obtained by the patient for the publication of this case report.
Investigations
The physical examination revealed overweight (body mass index: 29.94 kg/m2) and a mild decline of virilization and beard growth. Physical examination was otherwise unremarkable, with a blood pressure well controlled (140/75 mmHg, heart rate 72 pulses). The andrological examination revealed small testicles. An ultrasound scan (Esaote® My Lab25 Gold) of the scrotum confirmed the testicular volume reduction (right: 4.15 ml; left: 2.75 ml), with homogeneous parenchyma, ordinary perfusion and no signs of varicocele (). The claimed ED was evaluated performing the validated international index of erectile function (IIEF)-15, resulting in a severe ED [erectile function (EF) domain score = 6].
Table 1. Biochemical and ultrasound evaluations performed at baseline (presurgery) and three months after neuro-surgery.
Biochemical testing () showed low serum total T level with LH inappropriately in the normal range, suggesting a central hypogonadism (). Basal serum FSH was significantly higher than normal range (72.5 mIU/ml), whereas serum inhibin B and anti-Mullerian hormone (AMH) were below the normal range (). Other biochemical examinations showed appropriateness of levothyroxine, calcium and calcitriol therapy, no recurrence of thyroid carcinoma and stable Jak-2 myeloproliferative syndrome (). Morning serum cortisol levels were low (2.5 μg/dl), with a normal increase after adrenocorticotroph stimulating hormone (ACTH)-stimulation (Synacthen 0.25 mg i.v.). Insulin growth factor-1 (IGF-1) serum levels were below the first centile of normality for age and sex (). Finally, sperm count showed a severe oligoastenoteratozoospermia (). Genetic tests confirmed 46, XY karyotype and the absence of Y-chromosome microdeletions.
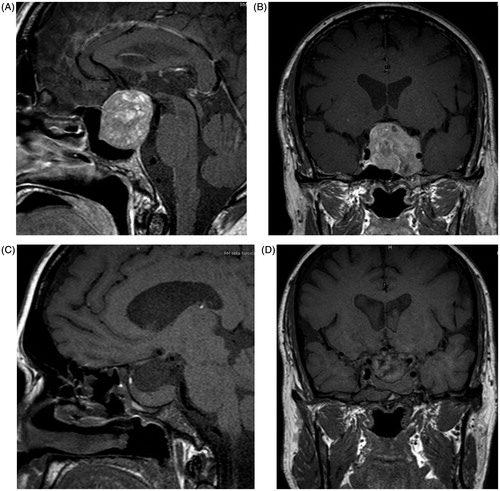
Magnetic resonance imaging (MRI) of the pituitary gland revealed a macroadenoma (38 mm × 46 mm × 42 mm), occupying the suprasellar cistern, raising the optic chiasm and the second ventricle and invading the cavernous sinus on the left side, involving the cerebellar interpeduncular cistern (). Computerized visual field analysis (Humphrey Field Analyzer®) showed a marked reduction of visual acuity in the inferior fields of the left eye, without any impairment in the opposite eye.
Treatment
The patient underwent trans-sphenoidal surgical removal of the pituitary macroadenoma, complicated with a complete deficit of the left third cranial nerve, resulting in diplopia and eyelid ptosis. Histology revealed a neoplasm consisting of monomorphic epithelial cells with eosinophilic clear cytoplasm, arranged in nests and trabeculae. Immunohistochemical staining of the pituitary adenoma specimen was positive for FSH in about 90% of cells and negative for all other pituitary hormones. The Ki-67 proliferative index was about 8% and 5% of the cells showed an overexpression of p53.
Outcome and follow-up
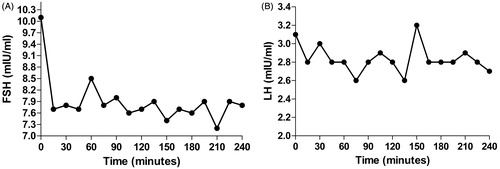
During the follow-up visit after 3 months, ED and loss of libido persisted, without changes in the EF score at IIEF-15 questionnaire (EF = 6). However, an improvement of diplopia and left eyelid ptosis was recorded. Biochemical testing showed a significant reduction of FSH serum levels (8.6 mIU/ml) together with the persistence of central hypogonadism (). Prolactin serum levels remained in the normal range. The assessment of serum FSH and LH levels every 15 min for 4 h showed apulsatile secretion pattern according to the Santen and Bardin method [Citation11] (). In particular gonadotropins, peak did not exceed the 20% of the nadir (2.9 versus 2.5 mIU/ml for LH and 8.5 versus 7.6 mIU/ml for FSH) (). Sperm count showed further impairment of both sperm quality and quality, with azoospermia (). Other biochemical tests are summarized in .
Figure 2. FSH (A) and LH (B) pulsatility evaluated with consecutive blood samples performed each 15 min for 4 h.
A detailed ultrasound scan was repeated confirming the persistence of small bilateral testes (). MRI 3 months after surgery confirmed the complete removal of pituitary tumor with healthy pituitary tissue located in the extreme right side of the pituitary stalk ().
Symptoms of hypogonadism and ED persisted 12 months after pituitary surgery. Gonadotropins remained in normal range (LH 3.3 mIU/ml and FSH 9.7 mIU/ml) with deficiency of total T levels (0.98 ng/ml) and no recurrence of pituitary adenoma was confirmed at MRI.
Hormonal treatment
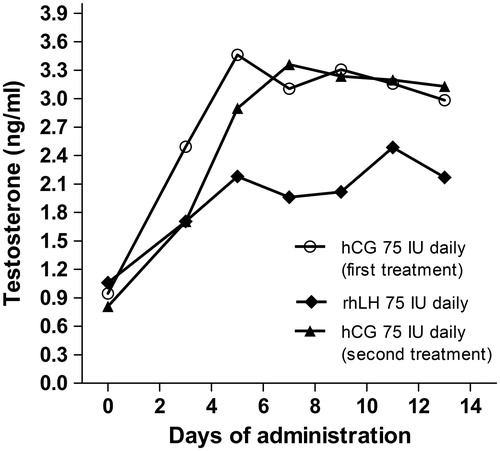
The persistence of hypogonadism after pituitary surgery in presence of normal serum LH remained unclear. To better understand the hypothalamic–pituitary–gonadal axis function in our patient, we tested Leydig cell function by a brief gonadotropin treatment. In particular, we asked the question whether LH was able to stimulate testosterone production [Citation12,Citation13]. Currently, hCG administration is the mostly used therapeutic option to increase intratesticular testosterone [Citation14]. However, although LH and hCG act on the same receptor, they show different in vitro activities [Citation15], with different kinetics and potency. Recombinant LH is used in controlled ovarian stimulation (COS) in combination with FSH when a central hypogonadism is caused by the concomitant use of a GnRH analog [Citation16]. Thus, we designed a brief stimulation test with low doses of either LH or hCG. Assuming that the long-standing hypogonadism could have determined a reduction in the Leydig cells responsiveness, three different schemes were consecutively administered: (i) hCG (Gonasi HP, IBSA Pharmaceutics) 75 IU daily i.m. for 2 weeks; (ii) recombinant LH (rh-LH) (Luveris, Merck) 75 IU daily i.m. for 2 weeks; (iii) hCG again (Gonasi HP, IBSA Pharmaceutics) 75 IU daily i.m. for further 2 weeks. Each stimulation scheme was followed by 2 weeks of wash-out from the drug. The first hCG administration was undertaken to sensitize (if necessary) the Leydig cells to further gonadotropin stimulations. The following rh-LH and hCG administrations were used to compare in vivo the response between the two gonadotropins. In the absence of clinical experience on administration of rh-LH in male hypogonadic patients to restore eugonadism, we selected a mild stimulation, comparable to that used in women undergoing COS. Serum total T levels were measured by isotopic dilution-liquid chromatography-tandem mass spectrometry at the Centre for Applied Biomedical Research of the University of Bologna – Sant’Orsola-Malpighi Hospital (Bologna) [Citation17] in serum samples taken before and every others day after the start of the treatment. All three treatments led to a significant increase in serum total T levels compared to baseline (Kruskal–Wallis, p = 0.004, p = 0.010 and p = 0.004, respectively). Although the maximal T increase reached after hCG administration seemed higher compared to after rh-LH (3.46 versus 2.49 ng/ml), no significant differences were found (Mann–Whitney, p = 0.245) (). Interestingly, T increased immediately after the first injection and it maintained a steady state by both approaches (). LH serum levels increased after rH-LH administration from 2.7 to 5.7 mIU/ml, suggesting that a minimal, apulsatile LH increase was able to stimulate Leydig cells.
Following this test with hCG and LH, the patient opted for androgen replacement therapy, which was initiated with intramuscular injections of testosterone undecanoate 1000 mg, every 12 weeks. Six months after the androgen therapy, serum T raised to the lower limit of the normal range (2.83 ng/ml), with a slight improvement of hypogonadal symptoms and ED (IIEF-15 EF domain = 17).
Discussion
This is an interesting case of giant GSPA discovered for the onset of biochemical and clinical hypogonadism. Alongside the detection of low serum T levels and inappropriately normal LH levels, high serum FSH levels were detected. These findings, together with the presence of a large pituitary adenoma, confirmed the diagnosis of FSH-secreting GSPA. However, despite the very high FSH serum levels, the patient did not show typical signs and symptoms previously described in men with FSH-secreting GSPA [Citation1,Citation2,Citation18]. In particular, in this case very small testes, severe oligoastenoteratospermia and low levels of both inhibin B and AMH were initially found, suggesting the concomitance of primary hypogonadism.
At baseline, despite the low T serum levels, LH was in the normal range. This central hypogonadism could be explained by the tumor mass effect, although the pathogenesis remains unclear so far. Moreover, after surgery T serum levels did not increase, notwithstanding the resolution of the tumor mass effect. The lack of LH pulsatility after surgery, in the presence of normal LH basal levels, could explain the maintenance of hypogonadism [Citation19]. However, the short-term treatment with apulsatile rh-LH increased T serum levels in our patient, together with a significant increase of LH serum levels (from 2.7 to 5.7 mIU/ml). The T increase, although LH was administered only once daily, suggests that the human Leydig cell T production does not require pulsatile LH administration mimicking the physiological secretion. As a confirmation, in vitro studies showed that the continuous, apulsatile exposure of steroidogenic cells to both hCG and LH, activates the intracellular cyclic adenosine monophosphate (cAMP) production and consequently the steroids synthesis [Citation20,Citation21]. Thus, the pathogenesis of central hypogonadism in our patient remains unclear.
In the literature, one case described a FSH-secreting GSPA with increased spermatogenesis [Citation22] and one case with testicular enlargement [Citation18], confirming the known importance of FSH on initiation and maintenance of spermatogenesis [Citation23]. Moreover, activating mutations of the FSH receptor with undetectable FSH levels are described to support normal spermatogenesis both in the presence [Citation24] and in the absence [Citation25] of T. However, the high serum FSH levels found in our patient were associated with impaired spermatogenesis and low testicular volume, suggesting either a possible lack of FSH bioactivity, or a concomitant primary damage of the tubular testicular compartment. The reduced T serum levels could be associated with reduced intratesticular T levels, limiting the positive effect of FSH on the spermatogenetic activity of the testis. Indeed, there is wide evidence in favor of the needed interaction among FSH, LH and T in the testis to maintain normal spermatogenesis [Citation26,Citation27]. However, after removal of the pituitary neoplasm, serum FSH decreased and sperm quality progressively worsened, until azoospermia, suggesting that FSH was at least in part bioactive in our case. Thus, a primary damage of tubular testicular could not be ruled out, as suggested by the reduced level of tubular testicular function markers, such as AMH and inhibin B. To this purpose, the exclusion of Y-chromosome microdeletions and karyotype abnormalities supports the hypothesis of an idiopathic spermatogenetic failure.
Our case demonstrates, for the first time, that a daily intramuscular injection of the same low dosage (75 IU) of either rh-LH or hCG increases serum T levels, quickly reaching a steady state. Although hCG is expected to be more potent than rh-LH, both treatments significantly increased serum T levels. Thus, a similar action of LH and hCG on the Leydig cell T production is supposed. In vitro models of human granulosa cells suggested that hCG and LH activate differently signal transduction by the same receptor [Citation20,Citation21], although they show an equal activity on T-production by murine Leydig cells [Citation28]. A similar in vivo comparison between rh-LH and hCG was not performed systematically so far. Considering the long-lasting central hypogonadism in our patient, we first sensitized Leydig cells with hCG. Our findings suggest that both hCG and rh-LH could be considered as therapeutic options for hypogonadism in men. Clinical trials based on rh-LH could be useful to find new therapeutic approaches to restore fertility in hypogonadotropic hypogonadic patients.
Finally, this FSH-secreting GSPA could be classified as atypical pituitary adenoma, according to the following World Health Organization (WHO) diagnostic criteria: (i) excessive p53 immunoreactivity, (ii) ki-67 proliferative index greater than 3% and (iii) increased mitotic activity. Histologic analysis showed a ki-67 of about 8% and a 5% of p53 immunoreactivity. Zada et al. reviewed 121 surgically removed pituitary tumors, in which atypical adenoma represented the 15% of cases [Citation29]. Among these, only one tumor expressed immunoreactivity for gonadotropin markers, in the absence of detectable gonadotropin levels [Citation29]. Thus, here, we described the first clinical case of FSH-secreting GSPA, with elevated FSH serum levels together with central hypogonadism.
In conclusion, we report the first case of atypical giant FSH-secreting pituitary adenoma with high serum FSH levels without clinical signs of testicular hyperstimulation, in presence of hypogonadism with plausible primary and secondary etiology. Two previous studies reported the cases of giant GSPA, although no information about the histology was reported [Citation30,Citation31]. Moreover, the majority of patients reported in the literature showed LH and T normal or elevated [Citation9]. Only in one case, T serum levels were below the normal range, even if gonadotropins were at the upper limit of normality [Citation31]. Moreover, we describe a new therapeutic option able to restore serum and probably intratesticular T in case of central hypogonadism. Considering the different activity of LH and hCG in vitro and that Leydig cells do not produce only steroids, but are also important for production of INSL3 and activation of vitamin D [Citation32] the use of rLH in hypogonadal men should be further and more comprehensively explored. Hypophysectomized patients may represent a good model to assess both pharmacodynamics and effective dose of LH and hCG in the male.
Declaration of interest
All authors declare no conflict of interest.
Acknowledgements
D.S. is a PhD fellow of the Doctorate School in Clinical and Experimental Medicine of the University of Modena and Reggio Emilia, Italy.
References
- Mehta GU, Jane JA. Jr Pituitary tumors. Curr Opin Neurol 2012;25:751–5
- Ho DM, Hsu CY, Ting LT, Chiang H. The clinicopathological characteristics of gonadotroph cell adenoma: a study of 118 cases. Hum Pathol 1997;28:905–11
- Saeger W, Ludecke DK, Buchfelder M, et al. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 2007;156:203–16
- Snyder PJ, Sterling FH. Hypersecretion of LH and FSH by a pituitary adenoma. J Clin Endocrinol Metab 1976;42:544–50
- Jaffe CA. Clinically non-functioning pituitary adenoma. Pituitary 2006;9:317–21
- Snyder PJ. Gonadotroph and other clinically nonfunctioning pituitary adenomas. Cancer Treat Res 1997;89:57–72
- Clemente M, Caracseghi F, Gussinyer M, et al. Macroorchidism and panhypopituitarism: two different forms of presentation of FSH-secreting pituitary adenomas in adolescence. Horm Res Paediatr 2011;75:225–30
- Heseltine D, White MC, Kendall-Taylor P, et al. Testicular enlargement and elevated serum inhibin concentrations occur in patients with pituitary macroadenomas secreting follicle stimulating hormone. Clin Endocrinol 1989;31:411–23
- Ntali G, Capatina C, Grossman A, Karavitaki N. Clinical review: functioning gonadotroph adenomas. J Clin Endocrinol Metab 2014;99:4423–33
- Young WF Jr, Scheithauer BW, Kovacs KT, et al. Gonadotroph adenoma of the pituitary gland: a clinicopathologic analysis of 100 cases. Mayo Clinic Proc 1996;71:649–56
- Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 1973;52:2617–28
- Coviello AD, Bremner WJ, Matsumoto AM, et al. Intratesticular testosterone concentrations comparable with serum levels are not sufficient to maintain normal sperm production in men receiving a hormonal contraceptive regimen. J Androl 2004;25:931–8
- Page ST, Kalhorn TF, Bremner WJ, et al. Intratesticular androgens and spermatogenesis during severe gonadotropin suppression induced by male hormonal contraceptive treatment. J Androl 2007;28:734–41
- Crosnoe LE, Grober E, Ohl D, Kim ED. Exogenous testosterone: a preventable cause of male infertility. Transl Androl Urol 2013;2:106–13
- Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol 2014;383:203–13
- Maia MC, Approbato MS, da Silva TM, et al. Use of recombinant luteinizing hormone for controlled ovarian hyperstimulation in infertile patients. JBRA Assist Reprod 2016;20:78–81
- Fanelli F, Belluomo I, Di Lallo VD, et al. Serum steroid profiling by isotopic dilution-liquid chromatography–mass spectrometry: comparison with current immunoassays and reference intervals in healthy adults. Steroids 2011;76:244–53
- Lahlou N, Le Nestour E, Chanson P, et al. Inhibin and follicle-stimulating hormone levels in gonadotroph adenomas: evidence of a positive correlation with tumour volume in men. Clin Endocrinol 1993;38:301–9
- Nachtigall LB, Boepple PA, Pralong FP, Crowley WF Jr. Adult-onset idiopathic hypogonadotropic hypogonadism – a treatable form of male infertility. N Engl J Med 1997;336:410–15
- Casarini L, Lispi M, Longobardi S, et al. LH and hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PloS One 2012;7:e46682
- Casarini L, Riccetti L, De Pascali F, et al. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol 2016;422:103–14
- Zarate A, Fonseca ME, Mason M, et al. Gonadotropin-secreting pituitary adenoma with concomitant hypersecretion of testosterone and elevated sperm count. Treatment with LRH agonist. Acta Endocrinol 1986;113:29–34
- van Alphen MM, van de Kant HJ, de Rooij DG. Follicle-stimulating hormone stimulates spermatogenesis in the adult monkey. Endocrinology 1988;123:1449–55
- Casas-Gonzalez P, Scaglia HE, Perez-Solis MA, et al. Normal testicular function without detectable follicle-stimulating hormone. A novel mutation in the follicle-stimulating hormone receptor gene leading to apparent constitutive activity and impaired agonist-induced desensitization and internalization. Mol Cell Endocrinol 2012;364:71–82
- Gromoll J, Simoni M, Nieschlag E. An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hypophysectomized man. J Clin Endocrinol Metab 1996;81:1367–70
- Weinbauer GF, Nieschlag E. Endocrine control of germ cell proliferation in the primate testis. What do we really know? Adv Exp Med Biol 1997;424:51–8
- Johnston H, Baker PJ, Abel M, et al. Regulation of Sertoli cell number and activity by follicle-stimulating hormone and androgen during postnatal development in the mouse. Endocrinology 2004;145:318–29
- Riccetti LD, Pascali F, Gilioli L, et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse Leydig cells in vitro. Reprod Biol Endocrinol
- Zada G, Woodmansee WW, Ramkissoon S, et al. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg 2011;114:336–44
- Usui T, Aoyama T, Hatano T, et al. A giant FSH-producing pituitary adenoma protruding into the lateral ventricles. Intern Med (Tokyo, Japan) 2008;47:1753–4
- Wang X, Ge L, Cui Y, et al. A FSH-secreting pituitary macroadenoma causing a testosterone deficiency syndrome. Int J Fertil Steril 2014;8:99–104
- Ferlin A, Selice R, Carraro U, Foresta C. Testicular function and bone metabolism – beyond testosterone. Nat Rev Endocrinol 2013;9:548–54