Abstract
We conducted a systematic review to evaluate the efficacy and adverse effects of newer drugs used to treat lower urinary tract symptoms (LUTS). The drugs were either Food and Drug Administration (FDA) approved for benign prostatic hyperplasia (BPH) or not FDA approved for BPH but have been evaluated for treatment of BPH since 2008. We searched bibliographic databases through September 2017. We included randomized controlled trials (RCTs) lasting one month or longer published in English. Outcomes of interest were LUTS assessed by validated measures. Efficacy was interpreted using established thresholds indicating clinical significance that identified the minimal detectable difference. Twenty-three unique, generally short-term, RCTs evaluating over 9000 participants were identified. Alpha-blocker silodosin and phosphodiesterase type 5 inhibitor tadalafil were more effective than placebo in improving LUTS (moderate strength evidence) but these drugs had more adverse effects, including abnormal ejaculation (silodosin). Anticholinergics were only effective versus placebo when combined with an alpha-blocker. Evidence was generally low strength or insufficient for other drugs. Evidence was insufficient to assess long-term efficacy, prevention of symptom progression, need for surgical intervention, or long-term adverse effects. Longer trials are needed to assess the effect of these therapies on response rates using established minimal detectable difference thresholds, disease progression, and harms.
Introduction
Benign prostatic hyperplasia (BPH), a common condition in elderly men, refers to the noncancerous unregulated proliferation of smooth muscle, connective tissue, and glandular epithelium within the prostate [Citation1]. Causation of BPH is not widely understood. Risk factors include aging and family history but BPH may also be associated with changes in hormonal levels, including elevated estradiol and low testosterone [Citation2,Citation3], diabetes [Citation4], obesity [Citation3,Citation5], and elevated markers of inflammation [Citation6].
Lower urinary tract symptoms due to benign prostatic hyperplasia (BPH) include storage symptoms (daytime urinary urgency and nocturia) and/or voiding disturbances (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding) [Citation7,Citation8]. LUTS may be bothersome and can negatively impact quality of life (QoL) [Citation8,Citation9].
Pharmacological management of LUTS has evolved over the last 25 years. Alpha-blockers (AB) and 5-alpha reductase inhibitors (5-ARIs) have been used to treat LUTS attributed to BPH for decades. Recently, newer drugs in these classes and drugs in other classes approved for other indications, have shown promise. Silodosin, a newer AB, was approved by the Food and Drug Administration (FDA) for treatment of BPH in 2008 [Citation10]. Anticholinergic drugs approved for overactive bladder (OAB) symptoms have the potential to alleviate LUTS attributed to BPH [Citation11]. These agents work directly on the bladder smooth muscle as opposed to ΑBs and 5-ARIs, which target the prostate. Tadalafil, a phosphodiesterase type 5 inhibitor (PDE-5) used for the treatment of erectile dysfunction (ED), was approved for the treatment of BPH in 2011. Other PDE-5 inhibitors have been used off-label for LUTS, both alone and in combination with ABs. A new class of drugs, beta-3 adrenoceptor agonists, was recently developed to treat OAB. Their proposed advantages over anticholinergics include potentially lower rates of adverse effects and potentially smaller risk of urinary retention [Citation11].
The aim of our review was to determine the efficacy and adverse effects of newer medications alone or in combination with older medications for LUTS attributed to BPH. This systematic review summarizes and updates findings from a report conducted by the Agency for Healthcare Research and Quality (AHRQ)/Minnesota Evidence-based Practice Center [Citation12].
Materials and methods
Literature search and study selection
We developed an a priori written protocol, which together with a technical report that incorporated input from key stakeholders, a multidisciplinary Technical Expert Panel, and public comment, are available at the AHRQ website (http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviewsand reports/?productid=2067&pageaction=displayproduct). Bibliographic databases, including MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), were searched to identify relevant RCTs published through September 2017 [Citation12]. We also searched relevant systematic reviews and the Clinical Trials (www.clinicaltrials.gov) and the FDA (www.fda.gov/Drugs) websites to identify additional completed and ongoing studies.
Two independent investigators screened titles and abstracts to identify studies meeting potentially eligibility criteria. Two independent investigators reviewed the full text of those references to determine eligibility. We included randomized controlled trials (RCTs) that evaluated efficacy of treatments involving newer drugs in men aged at least 45 years with LUTS attributed to BPH. Newer drugs were defined as those either FDA approved for BPH since 2008 or not FDA approved for BPH but have been evaluated for BPH treatment since 2008. Included RCTs were at least one month in duration with no minimum sample size and limited to English language.
Data extraction and quality assessment
Study, participant, intervention, and outcomes data were extracted by one investigator and reviewed and verified for accuracy by a second investigator. Risk of bias (low, medium, or high) of eligible studies was assessed by one investigator and reviewed by a second using methods recommended by AHRQ [Citation13]. The primary predefined outcomes of interest were LUTS measured by validated questionnaires: the International Prostate Symptom Score (I-PSS)/American Urological Association Symptom Index scores (AUA-SI), BPH Impact Index (BII), and the I-PSS QoL question. I-PSS/AUA scores range from 0 to 35 with higher scores indicating more severe symptoms. In addition to changes in I-PSS/AUA scores, we extracted the percentage of responders to treatment based on changes in I-PSS/AUA scores as defined by the study investigators (e.g. ≥3 point reduction from baseline; > 25% reduction) if reported. We also assessed common and serious medication adverse effects (AE) as well as overall and treatment related study withdrawal.
Data synthesis and analysis
The mean efficacy between groups was interpreted using established thresholds indicating clinical significance that identified the minimal detectable difference (MDD) in I-PSS (i.e. a reduction of three points indicates slight improvement) and BII (i.e. a reduction of 0.5 points) scales () [Citation14]. Johnston et al. [Citation15] suggest an interpretation of the differences between groups in relation to the established minimal important difference. This approach suggests that when the weighed mean difference (WMD) is equal to or larger than the MDD, many patients may have gained detectable benefits from treatment; when the WMD is at least half of the MDD but less than the MDD, an appreciable number of participants have likely achieved a clinically meaningful improvement; and when the WMD is less than one-half of the MDD, it is unlikely that an appreciable number of participants achieve detectable benefits. Following this guidance, we concluded that statistically significant differences were clinically meaningful when the WMD was at least 50% of the MDD. Therefore, the statistically significant WMD between treatment groups for post-treatment or change in I-PSS must be equal to or greater than −1.5 and the WMD between treatment groups for post-treatment or change in BII must be equal to or greater than −0.25. No threshold was established for the I-PSS QoL question. Responses to this question are ordinal and range from 0–6. We used an MDD of 1 to assess efficacy and comparative effectiveness. Therefore, if this question was analyzed as a continuous variable, we required statistical significance and a WMD of at least 0.5 to conclude a clinically meaningful difference. Additional primary outcomes included disease progression and/or treatment failure (prevention/delay of need for surgical intervention; acute urinary retention (AUR), or an increase [worsening] of at least 4 I-PSS points from baseline).
Table 1. Symptom and quality of life scales measuring LUTS attributed to BPH.
We pooled data when at least three trials reported similar comparisons and outcomes. Proportion of I-PSS responders or mean changes in I-PSS scores were pooled using a Hartung, Knapp, Sidik, and Jonkman (HKSJ) method [Citation16] for random effects model in Stata [Citation17]. The HKSJ method is more conservative than the DerSimonian-Laird method commonly used in meta-analyses and lowers the possibility of overly narrow confidence intervals common with DerSimonian-Laird. Other outcomes were pooled in RevMan [Citation18] and the DerSimonian-Laird random effects confidence intervals were converted to HKSJ confidence intervals [Citation16]. Study variance was assessed with τ2 and the magnitude of heterogeneity was measured with the I 2 statistic. If substantial heterogeneity was present (i.e. I2 ≥70%), sensitivity analyses were undertaken or results were stratified to assess treatment effects based on patient or study characteristics [Citation19,Citation20]. We pooled across different AB’s unless there were at least three trials for a given agent.
We rated strength of evidence (SOE), our confidence in the estimates of effect, for the primary outcomes as high, moderate, low or insufficient based on the four domains of risk of bias, directness, consistency, and precision [Citation21]. High SOE indicates high confidence that further research is very unlikely to change the confidence in the estimate of effect, meaning that the evidence reflects the true effect. Insufficient SOE indicates that evidence either is unavailable or does not permit a conclusion
Results
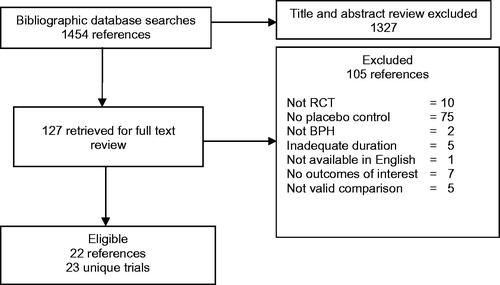
We identified 1454 citations, of which 127 required full text review after title and abstract screening, and 22 studies reporting results from 23 unique RCTs met eligibility criteria for inclusion in this review (). Over 9000 participants were enrolled. The alpha-blocker silodosin was studied in four trials (reported in three articles) [Citation22–24]; anticholinergics tolterodine and solifenacin were studied in five trials [Citation25–29]; PDE-5 inhibitors tadalafil, sildenafil, and vardenafil were studied in 12 trials [Citation30–42]; and a beta-3 agonist mirabegron was studied in a trial [Citation43]. Baseline characteristics of the included RCTs are shown in . Across trials, the mean age of men was 56–67 y and mean IPSS ranged from 16–21. These characteristics did not vary widely across intervention classes.
Table 2. Baseline characteristics of the eligible placebo-controlled trials.
Alpha-blocker
Silodosin
Three reports of four eligible 12-week trials randomized males with BPH (n = 1759) to silodosin 8 mg daily versus placebo [Citation22–24]. Three trials reported industry sponsorship [Citation22,Citation23]. Risk of bias was low in three trials [Citation22,Citation23] and moderate in one trial [Citation23].
Silodosin improved LUTS more than placebo (). The proportion of responders, defined as men with a > 25% reduction in baseline I-PSS score, was reported in two trials [Citation22,Citation24]. Based on this definition most men had a response to both Silodosin and placebo. However, the proportion responding was higher with silodosin (66 and 76%) than placebo (51% in both trials) with corresponding risk ratios of 1.3 (95%CI 1.1–1.5) [Citation22] and 1.5 (95%CI 1.2–1.9) [Citation24] (high SoE). Mean change from baseline in I-PSS scores was larger with silodosin than placebo, −6.9 vs. −4.0 points. While the WMD of −2.7 points (95%CI −3.9 to −1.4; I 2 and τ2 = 0%) did not reach the MDD of three points it was greater than 1.5 indicating that “many” silodosin recipients may have had detectable benefits from treatment versus placebo (moderate SoE). Improvement in the I-PSS QoL, reported in two trials, favored silodosin, with 32 and 43% reporting being “delighted, pleased, or mostly satisfied” compared with 23 and 33% with placebo (p = 0.001 and 0.03, respectively) (high SoE) [Citation22,Citation23].
Table 3. Evidence overview of comparisons of interventions (with ≥3 trials).
Withdrawals due to adverse effects (5 vs. 2%; p = 0.001) and participants reporting one or more adverse effects (53 vs. 38%; p < 0.0001) were higher with silodosin than placebo (high SoE). The most common adverse effect with silodosin was abnormal ejaculation (22 vs. 1% for placebo; p < 0.001). We found limited information on serious adverse effects. One trial reported that serious adverse effects were infrequent and similar with silodosin and placebo, approximately 1 and 2%, respectively [Citation23].
Anticholinergics
Tolterodine monotherapy
Two trials compared tolterodine 4 mg daily with placebo [Citation25,Citation26]. One trial (n = 439), 12 weeks in duration, enrolled men with OAB symptoms [Citation25]. The second trial (n = 76) was a 24-week study that enrolled men with prostates at least 25 ml in size [Citation26]. Both trials excluded individuals with a baseline postvoid residual volume of ≥200 ml, reported industry sponsorship and had low risk of bias. Mean changes from baseline in I-PSS and I-PSS QoL scores were similar with tolterodine and placebo (low SoE). Estimated reductions in I-PSS scores from baseline were between six and seven points in both groups. AUR was reported in five participants with tolterodine and four participants with placebo [Citation25,Citation26]. Withdrawals due to adverse effects were not reported. Dry mouth was reported more frequently with tolterodine than placebo (9 vs. 2%; p = 0.002).
Tolterodine in combination with alpha-blocker
The 24-week trial described previously (n = 76) also compared tolterodine 4 mg combined with tamsulosin 0.2 mg to placebo in men with LUTS and OAB symptoms [Citation26]. Unlike the monotherapy results, combination therapy improved LUTS and QoL more than in placebo (low SoE). Reduction in I-PSS scores from baseline was −14.7 points for the combination group compared to −7.2 points for the placebo group. The mean difference between groups (−7.5 points) was greater than the MDD of three points. Mean improvement in QoL scores from baseline was −3.2 and −1.4 points for combined and placebo groups, respectively (p < 0.001; low SoE). Dry mouth was reported more frequently with combination therapy than placebo (26 vs. 3%; p = 0.02).
Solifenacin monotherapy
One 12-week trial (n = 222) compared solifenacin with daily doses of 3, 6, or 9 mg to placebo in men with LUTS and OAB symptoms [Citation27]. Individuals with a baseline postvoid residual of >200 ml were excluded. Risk of bias was moderate. We assessed efficacy outcomes based on the commonly used 6 mg dose. Change in I-PSS scores was similar between groups, −6 points for solifenacin 6 mg and −6.3 points for placebo with a mean difference of −0.3 points (95%CI −1.74 to 2.34; low SoE). AUR requiring catheterization was reported in one participant allocated to solifenacin 9 mg. Evidence was insufficient to draw conclusions about comparative withdrawals and withdrawal due to adverse effects.
Solifenacin in combination with alpha-blocker
Three 12-week trials (n = 1857) compared combination solifenacin 3, 6, or 9 mg and AB tamsulosin 0.4 mg with placebo in men with LUTS attributable to BPH and OAB [Citation27–29]. Two studies excluded patients with baseline postvoid residuals >150 [Citation28] or >200 ml [Citation27], respectively. All were industry-sponsored and had low risk of bias. We based efficacy outcomes on the commonly used 6 mg dose.
In contrast to monotherapy, solifenacin 6 mg with the addition of tamsulosin 0.4 mg improved LUTS more than in placebo (moderate SoE) (). Mean reduction in I-PSS scores with combination was 7.3 points compared with 5.7 points with placebo. The WMD of −1.5 points (95%CI −1.8 to −1.2; I2 and τ2 = 0%) did not meet the MDD of three points but indicated probable detectable benefits from treatment for many combination therapy recipients versus placebo. The magnitude of effect of combination therapy with 9 mg solifenacin appeared lower than with 6 mg. Improvement in I-PSS QoL scores was similar with combination therapy compared to placebo (low SoE).
Among the three trials, 11 cases ( <1% of participants) of AUR were reported with combination therapy and none with placebo. Withdrawal due to adverse effects and the proportion of participants reporting ≥1 adverse effect were similar with combination therapy and placebo (insufficient/low SoE). Compared with placebo, combination therapy was more likely to cause dry mouth (9 vs. 1%; p < 0.001) and constipation (3 vs. <1%; p = 0.05) [Citation27,Citation28].
Phosphodiesterase type 5 inhibitors (PDE-5s) Tadalafil
Ten eligible 12-week trials randomized men with LUTS attributed to BPH (n = 4407) to tadalafil versus placebo [Citation30–39]. Approximately 75% of participants had a history of ED. The dose of tadalafil used most frequently was 5 mg daily, which is the FDA-approved dose for LUTS. All trials reported industry sponsorship and had low to moderate risk of bias.
Tadalafil 5 mg improved LUTS more than placebo (). A responder analysis from one trial (n = 281), defined as a ≥ 3 point reduction from baseline I-PSS score, reported 49% responded with tadalafil compared with 36% with placebo (low SoE) [Citation39]. Tadalafil 5 mg improved mean I-PSS scores from baseline more than placebo, −5.5 vs. −3.4 points (moderate SoE). The WMD of −1.8 points (95%CI −2.3 to −1.3; I2 and τ2 = 0%) indicated that many tadalafil recipients may have had detectable benefits from treatment versus placebo. Tadalafil daily doses of 10 [Citation38] and 20 mg [Citation37,Citation38] showed larger effect sizes suggesting a dose-response relationship (test for subgroup differences I2 = 76%, p = 0.006). Tadalafil 5 mg improved BII scores more than placebo and the WMD of −0.5 (95%CI −0.8 to −0.3) achieved established MDD (moderate SoE). Changes in I-PSS QoL were similar (high SoE). Incidence of AUR was rare, reported in two participants with placebo in two trials [Citation31,Citation38]. No other indicators of disease progression/treatment failure were reported.
Participants allocated to tadalafil 5 mg were more likely to withdraw due to an adverse effect but the absolute difference was small, approximately two percentage points (high SoE). The proportion of withdrawals due to adverse effects increased at higher doses but the differences between doses were not significant. The proportion reporting at least one adverse effect was higher with tadalafil 5 mg than placebo, 29 versus 22% (p = 0.0003; (high SoE). Four trials reported dyspepsia, which was uncommon but more frequent with tadalafil use (3 vs. 0% for placebo; p = 0.001) [Citation32,Citation33,Citation37,Citation38]. Short-term, serious adverse effects were rare and reported in similar proportions with tadalafil and placebo (approximately 1% each). Three myocardial infarction deaths were reported in three trials, two with tadalafil [Citation34,Citation35] and one with placebo [Citation37].
Sildenafil
Two trials compared sildenafil with placebo in men with LUTS [Citation40,Citation41]. One three-month trial (n = 369) enrolled men with ED in addition to LUTS and compared sildenafil 50 mg daily or before sexual activity (increasing to 100 mg at 2 weeks and returning to 50 mg if 100 mg was not tolerated) with placebo [Citation40]. The trial reported industry sponsorship and had low overall risk of bias. In men with LUTS and ED, sildenafil improved mean I-PSS scores more than placebo, −6.3 vs. −1.9 points (low SoE). The mean difference of 4.4 points exceeded the MDD. Evidence was insufficient for the BII and I-PSS QoL. Evidence was insufficient for withdrawals due to adverse effects and proportion reporting one or more adverse effects. Headache and dyspepsia were reported more frequently with sildenafil than placebo (11 vs. 3% and 6 vs. 1%, respectively p < 0.05 for both). Two serious adverse effects were reported with sildenafil, including one severe acute cerebrovascular stroke.
The second study (n = 176) was an eight-week, four-arm trial that randomized men to sildenafil 25 mg daily, 25 mg twice a day, 50 mg daily, or placebo [Citation41]. ED was reported in 12% of the participants. The trial reported industry sponsorship and had moderate overall risk of bias. Improvements from baseline were −7.2, −5.8 and −4.6 points for the sildenafil 25 mg daily, sildenafil 25 mg twice daily, and placebo groups, respectively. All sildenafil treatment groups improved IPSS QoL scores more effectively than placebo (p < 0.01 for all groups). Evidence was insufficient for the IPSS and I-PSS QoL. Evidence was insufficient for withdrawals due to adverse effects. The incidences of treatment-emergent adverse events differed significantly between groups (p = 0.012) and ranged from 23 (50 mg daily) to 47% (25 mg twice daily) compared to 16% in the placebo group (low SoE). Specific events were not described. Two serious adverse effects were reported with sildenafil, including one acute myocardial infarction in a participant in the sildenafil 50 mg group.
Vardenafil
One eight-week trial (n = 222) compared vardenafil 10 mg twice daily to placebo [Citation42]. Approximately 60% of participants reported ED or ejaculatory problems at baseline. The trial was industry sponsored and had low risk of bias. Vardenafil improved mean I-PSS scores more than placebo, −5.9 vs. −3.6 points (low SoE). The mean difference between groups of −2.3 points (95%CI −3.64 to −0.90) indicated that many vardenafil recipients may have benefited from treatment versus placebo. Study withdrawals due to adverse effects and the proportion reporting one or more adverse effect were higher with vardenafil than placebo (8 vs. 2%; p = 0.05 and 30 vs. 16%; p = 0.02, respectively;low SoE). Common adverse effects included headaches, flushing, and dyspepsia. Myocardial infarction and hypertensive crisis were reported in two participants with vardenafil.
Beta 3 agonist
Mirabegron
One 12-week trial (n = 200) assessed the efficacy of daily mirabegron doses of 50 and 100 mg daily doses compared with placebo [Citation43]. The study had low risk of bias and was industry sponsored. Mean I-PSS score changes from baseline were −6.2, −4.8, and −5.0 points in the 50 mg, 100 mg, and placebo groups and the differences between groups were not statistically significant. The information provided was insufficient for effect size calculation or pooling across dose levels for any outcome or adverse effect (insufficient SoE).
Discussion
We found that some newly used drugs improved LUTS attributed to BPH when compared with placebo, even given the strong placebo response observed in many of the included trials. Some drugs increased harms, although the adverse effects were generally not severe and the event rate was low. The AB silodosin was more effective than placebo but was associated with an increased rate of adverse effects, mainly abnormal ejaculation. Tadalafil, the only FDA-approved PDE-5 for BPH, also was more effective than placebo but the rates of adverse effects were also higher. Anticholinergics, developed to treat OAB, were not superior to placebo in reducing LUTS. Improvements greater than placebo were only observed when these drugs were combined with an established AB. In addition, anticholinergics were more likely to cause dry mouth and constipation. For other newer drugs such as mirabegron, sildenafil, and vardenafil, the strength of evidence was low or insufficient to assess efficacy and adverse effects. Our results are generally consistent with those of previous systematic reviews of silodosin, tadalafil, and anticholinergics [Citation44–46]. However, these older reviews did not interpret efficacy based on thresholds indicating clinically meaningful differences, did not formally grade strength of evidence, and did not include some newer studies.
This critical analysis on the body of evidence on the efficacy of newer drugs and drug combinations sheds an important light on the methodological quality of research studies in this important field of urology. Ideally, studies should identify the proportion of patients (“responders”) in both the active drug(s) and placebo groups that achieved a MDD (e.g. the three point reduction from baseline for the I-PSS/AUA symptom score) in addition to the average change in symptom scores. This information is more readily interpreted by clinicians and patients alike and allows the calculation of absolute effect size measures such as numbers-needed-to-treat and numbers-needed-to-harm. Responders to treatment based on the I-PSS/AUA symptom score was only available for silodosin and tadalafil and in some situations was based on percentage improvement from baseline rather than the established three point change. No responder analyses were reported for any other treatments and outcomes and for some outcomes, an MDD has yet to be established.
A further limitation of the existing evidence is the short duration RCTs (only one trial was longer than 12 weeks) and lack of data on longer-term outcomes or adverse effects. Similarly, there were no high quality observational studies that could have filled this gap. Give the chronic nature of LUTS and that medical therapy for LUTS is typically instituted for longer than 12 weeks, often for many years, this shortcoming limits the applicability of this body of evidence to clinical practice. Available data are further limited by the paucity of information on medication adherence, clinical progression (as measured by increases in I-PSS scores and rates of surgical intervention), and complication rates (such as the development of acute urinary retention). Applicability is limited by the inclusion and exclusion criteria of trials for certain agents. For example, studies evaluating anticholinergics commonly excluded men with higher postvoid residual urine volumes, thereby potentially reducing the rates if treatment-related adverse effects. Lastly, while there is great interest in personalizing treatment for male LUTS based on patients primary symptoms (such as nocturia), medical comorbidities (such as concomitant ED), pretreatment with other agents, and patient age, we found no studies that were preplanned to address such potential subgroup effects.
It is unlikely that our review was affected by language bias given that all trials for drugs subject to FDA approval require registration at clinicaltrials.gov and that we found no additional publications for trials within two years of completion. Publication bias is always a potential concern, especially in a research area in which most trials are industry sponsored. However, given the paucity of trials (less than 10 per comparison), we were unable to apply formal statistical testing for publication bias. Strengths of this review include its rigorous methods that followed a written, a priori protocol that was developed with stakeholder input. We focused on pre-specified outcomes of direct patient importance and gave equal weights to potential treatment benefits and harms. Our study further distinguishes itself from most systematic reviews on agents to treat male LUTS by qualifying all analyses by a transparent and methodologically rigorous strength of evidence rating that included such domains such as inconsistency and imprecision.
Newer therapies silodosin and tadalafil improved short-term LUTS more than placebo but led to higher adverse effects. Data were not available to assess long-term efficacy or prevention of disease progression. Trials with longer duration of treatment and follow-up are needed to assess the effect of these therapies on response rates using established MDD thresholds, disease progression, and long-term outcomes.
Notes on contributors
Roderick MacDonald is a research specialist affiliated with the Minneapolis Veterans Affairs Healthcare System Center for Chronic Disease Outcomes Research.
Michelle Brasure is a project manager and librarian affiliated with the Division of Health Policy and Management, University of Minnesota, School of Public Health.
Philipp Dahm is a urologist affiliated with the Minneapolis Veterans Affairs Healthcare.
Carin M. Olson is a research specialist affiliated with the Division of Health Policy and Management, University of Minnesota, School of Public Health.
Victoria A. Nelson is a research specialist affiliated with the Division of Health Policy and Management, University of Minnesota, School of Public Health.
Howard A. Fink is a physician affiliated with the Geriatric Research Education and Clinical Center, Minneapolis Veterans Affairs Healthcare System.
Michael C. Risk is a urologist affiliated with the Minneapolis Veterans Affairs Healthcare.
Bruce Rwabasonga is a research specialist affiliated with the Division of Health Policy and Management, University of Minnesota, School of Public Health.
Timothy J. Wilt is a Professor of Medicine and staff physician at the Minneapolis VA Health Care System and a core investigator within the Minneapolis Veterans Affairs Healthcare System Center for Chronic Disease Outcomes Research.
Disclosure statement
The authors report no conflicts of interest
Additional information
Funding
References
- Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S11–S18.
- Asiedu B, Anang Y, Nyarko A, et al. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male. 2017;20:17–22.
- Kaplan SA, Lee JY, O'Neill EA, et al. Prevalence of low testosterone and its relationship to body mass index in older men with lower urinary tract symptoms associated with benign prostatic hyperplasia. Aging Male. 2013;16:169–172.
- Ferreira FT, Daltoé L, Succi G, et al. Relation between glycemic levels and low tract urinary symptoms in elderly. Aging Male. 2015;18:34–37.
- Zucchetto A, Tavani A, Dal Maso L, et al. History of weight and obesity through life and risk of benign prostatic hyperplasia. Int J Obes (Lond). 2005;29:798–803.
- Arivazhagan J, Nandeesha H, Dorairajan LN, et al. Association of elevated interleukin-17 and angiopoietin-2 with prostate size in benign prostatic hyperplasia. Aging Male. 2017;20:115–118.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803.
- Jarvis TR, Chughtai B, Kaplan SA. Bladder outlet obstruction and BPH. Curr Bladder Dysfunct Rep. 2014;9:372–378.
- Hollingsworth JM, Wilt TJ. Lower urinary tract symptoms in men. BMJ. 2014;349:g4474.
- McVary K, Roehrborn C, Avins A. American Urological Association Guideline: management of Benign Prostatic Hyperplasia (BPH). Revised, 2010. American Urological Association Education and Research: Inc; 2010.
- Suarez O, Osborn D, Kaufman M, et al. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep. 2013;14:580–584.
- Brasure M, MacDonald R, Dahm P, et al. Newer medications for lower urinary tract symptoms attributed to benign prostatic hyperplasia: a review. Comparative Effectiveness Review No. 178. (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-2012- 00161-I.) AHRQ Publication No. 16-EHC024-EF. Rockville, MD: Agency for Healthcare Research and Quality; [Internet] May 2016. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- Viswanathan M, Ansari M, Berkman N, et al. Assessing the risk of bias of individual studies in systematic reviews of health care interventions. Agency for Healthcare Research and Quality Methods Guide for Comparative Effectiveness Reviews. [Internet] March 2012. AHRQ Publication No. 12-EHC047-EF. Available from: www.effectivehealthcare.ahrq.gov/
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770–1774.
- Johnston BC, Patrick DL, Busse JW, et al. Patient-reported outcomes in meta-analyses-Part 1: assessing risk of bias and combining outcomes. Health Qual Life Outcomes. 2013;11:109.
- IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25.
- StataCorp. Stata statistical software: release 14. College Station, TX; 2015. Available from: https://www.stata.com
- Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen:The Nordic Cochrane Centre, The Cochrane Collaboration; 2012.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011;64:1187–1197.
- Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.1.0. [Updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org
- Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. Methods guide for comparative effectiveness reviews (Prepared by the RTI-UNC Evidence-based Practice Center under Contract No. 290-2007-10056-I). AHRQ Publication No. 13(14)-EHC130-EF. Rockville (MD) Agency for Healthcare Research and Quality; [Internet] 2013. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- Chapple CR, Montorsi F, Tammela TL, et al. Silodosin therapy for lower urinary tract symptoms in men with suspected benign prostatic hyperplasia: results of an international, randomized, double-blind, placebo- and active-controlled clinical trial performed in Europe. Eur Urol. 2011;59:342–352.
- Marks LS, Gittelman MC, Hill LA, et al. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol. 2009;181:2634–2640.
- Kawabe K, Yoshida M, Homma Y, et al. Silodosin, a new alpha1A-adrenoceptor-selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019–1024.
- Kaplan SA, Roehrborn CG, Rovner ES, et al. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296:2319–2328.
- Cai JL, Zhou Z, Yang Y, et al. Efficacy and safety of medium-to-long-term use of tolterodine extended release with or without tamsulosin in patients with benign prostate hyperplasia and larger prostate size: a double-blind, placebo-controlled, randomized clinical trial. Chin Med J. 2016;129:2899–2906.
- Van Kerrebroeck P, Haab F, Angulo JC, et al. Efficacy and safety of solifenacin plus tamsulosin OCAS in men with voiding and storage lower urinary tract symptoms: results from a phase 2, dose-finding study (SATURN). Eur Urol. 2013;64:398–407.
- Van Kerrebroeck P, Chapple C, Drogendijk T, et al. Combination therapy with solifenacin and tamsulosin oral controlled absorption system in a single tablet for lower urinary tract symptoms in men: efficacy and safety results from the randomised controlled NEPTUNE trial. Eur Urol. 2013;64:003–1012.
- Kaplan SA, He W, Koltun WD, et al. Solifenacin plus tamsulosin combination treatment in men with lower urinary tract symptoms and bladder outlet obstruction: a randomized controlled trial. Eur Urol. 2013;63:158–165.
- Takeda M, Yokoyama O, Lee SW, et al. Tadalafil 5 mg once-daily therapy for men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results from a randomized, double-blind, placebo-controlled trial carried out in Japan and Korea. Int J Urol. 2014;21:670–675.
- Yokoyama O, Yoshida M, Kim SC, et al. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a randomized placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201.
- Takeda M, Nishizawa O, Imaoka T, et al. Tadalafil for the treatment of lower urinary tract symptoms in Japanese men with benign prostatic hyperplasia: results from a 12-week placebo-controlled dose-finding study with a 42-week open-label extension. Low Urin Tract Symptoms. 2012;4:110–119.
- Oelke M, Giuliano F, Mirone V, et al. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol. 2012;61:917–925.
- Egerdie RB, Auerbach S, Roehrborn CG, et al. Tadalafil 2.5 or 5 mg administered once daily for 12 weeks in men with both erectile dysfunction and signs and symptoms of benign prostatic hyperplasia: results of a randomized, placebo-controlled, double-blind study. J Sex Med. 2012;9:271–281.
- Porst H, Kim ED, Casabe AR, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:1105–1113.
- Kim SC, Park JK, Kim SW, et al. Tadalafil administered once daily for treatment of lower urinary tract symptoms in Korean men with benign prostatic hyperplasia: results from a placebo-controlled pilot study using tamsulosin as an active control. Low Urin Tract Symptoms. 2011;3:86–93.
- Dmochowski R, Roehrborn C, Klise S, et al. Urodynamic effects of once daily tadalafil in men with lower urinary tract symptoms secondary to clinical benign prostatic hyperplasia: a randomized, placebo controlled 12-week clinical trial. J Urol. 2010;183:1092–1097.
- Roehrborn CG, McVary KT, Elion-Mboussa A, et al. Tadalafil administered once daily for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a dose finding study. J Urol. 2008;180:1228–1234.
- McVary KT, Roehrborn CG, Kaminetsky JC, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–1407.
- McVary KT, Monnig W, Camps JL Jr, et al. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177:1071–1077.
- Ko WJ, Han HH, Ham WS, et al. Daily use of sildenafil 50mg at night effectively ameliorates nocturia in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia: an exploratory multicenter, double-blind, randomized, placebo-controlled study. Aging Male. 2017;20:81–88.
- Stief CG, Porst H, Neuser D, et al. A randomised, placebo-controlled study to assess the efficacy of twice-daily vardenafil in the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Eur Urol. 2008;53:1236–1244.
- Nitti VW, Rosenberg S, Mitcheson DH, et al. Urodynamics and safety of the β3-adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol. 2013;190:1320–1327.
- Novara G, Tubaro A, Sanseverino R, et al. Systematic review and meta-analysis of randomized controlled trials evaluating silodosin in the treatment of non-neurogenic male lower urinary tract symptoms suggestive of benign prostatic enlargement. World J Urol. 2013;31:997–1008.
- Dong Y, Hao L, Shi Z, et al. Efficacy and safety of tadalafil monotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a meta-analysis. Urol Int. 2013;91:10–18.
- Kaplan SA, Roehrborn CG, Abrams P, et al. Antimuscarinics for treatment of storage lower urinary tract symptoms in men: a systematic review. Int J Clin Pract. 2011;65:487–507.