Abstract
The male pelvic floor muscles comprise a pair of compound muscle layers referred to as the levator ani. Studies have shown that pelvic floor muscle strength is affected by physical activity. This study aimed to assess male pelvic floor muscle strength through manometry of the external anal sphincter and evaluation of its association with physical activity level, BMI, and rectal pressure in healthy men. To assess physical activity level over the previous week, we used the International Physical Activity Questionnaire 8 (IPAQ). Pelvic floor muscle strength was assessed via anorectal manometry. The results shows a negative correlation between resting pressure and MET, a positive correlation between rectal pressure and resting and maximum pressure. The novel finding of this study is a negative correlation between walking and pelvic floor strength. This study raises new questions about the understanding of the interaction among physical activity, intraabdominal pressure, and pelvic floor strength in the physiology of continence in men.
Introduction
The pelvic floor muscles comprise a pair of compound muscle layers referred to as the levator ani, which consists of the iliococcygeus, pubococcygeus, puborectalis and ischiococcygeus muscles. Muscles fibres from the puborectalis muscle are associated with the external anal sphincter [Citation1]. Interestingly, the pelvic floor muscles contract or relax en masse [Citation2]. Some studies have shown that pelvic floor muscle strength is affected by physical activity; however, consensus of this effect is lacking [Citation3–8].
Pelvic floor muscle dysfunction is common after radical prostatectomy. Urinary incontinence after radical prostatectomy occurs in 6–87% of cases [Citation9,Citation10]; nevertheless, the role of the pelvic floor muscles in post-prostatectomy is not yet fully understood. Physiotherapy for men undergoing radical prostatectomy involves pre- and post-operative pelvic floor assessments and strength training [Citation4,Citation6,Citation11]. Risk factors for developing post-prostatectomy incontinence include age, prior resection of the prostate, high body mass index (BMI) and comorbidities [Citation12–14]. However, studies are in disagreement about the effect of physical activity level on pelvic floor muscle strength. Evidence indicates that pelvic floor function and pre-surgical morphology are associated with recovery from urinary incontinence in men [Citation15,Citation16].
A direct association was observed between pelvic floor muscle thickness and time to urinary incontinence recovery after radical prostatectomy. Patients with thicker muscles regained continence more quickly after surgery [Citation15], and the use of pre-operative pelvic floor muscle training shortened the duration of post-operative urinary incontinence [Citation16].
Physiological changes like decline, with ageing, in serum concentrations of testosterone is a fact and some men will eventually develop symptoms with clinical consequences such as frailty, changes in body composition, muscle weakness and osteoporosis. On the other hand, testosterone supplementation in specific cases will increase muscle mass strength, function and enhance quality of life [Citation17–20]. However, to achieve the best lifestyle, the men must to optimise dietary habits, as well as to exercise and to abstain from smoking life-long [Citation17]. These studies show that the decline of the ageing testosterone concentration associated with lifestyle may affect the function and strength of skeletal muscles, such as the muscles of the male pelvic floor.
Thus, it is essential to study the pelvic floor muscles in healthy men to characterise the differences among individuals and identify possible factors contributing to them. Studies of the normal function or dysfunction of the male pelvic floor as well as a rapid and inexpensive methods for assessing the pelvic floor muscles are lacking.
This study aimed to assess pelvic floor muscle strength through manometry of the external anal sphincter and evaluation of its association with physical activity level, BMI and rectal pressure in healthy men.
Methods
We evaluated 31 men with a mean age of 51.8 ± 4.9 years (range, 45–61 years). Individuals with a history of pelvic floor diseases, pelvic surgeries, anorectal diseases or surgeries, neurological disorders, orthopaedic or rheumatic diseases, diabetes mellitus, urinary or faecal incontinence, or cancer were excluded. This study was approved by the ethics committee of the Federal University of Juiz de Fora, and all the patients provided written informed consent in accordance with the tenets of the Declaration of Helsinki.
Age, BMI, physical activity level, and pelvic floor muscle assessment were documented for all patients. BMI is defined via the following calculation: body mass in kilograms divided by the square of the body height in metres (kg/m2) [Citation21]. To assess physical activity level over the previous week, we used the International Physical Activity Questionnaire (IPAQ), version 8 long form validated for the Portuguese language. Time spent performing high-, moderate- and low-intensity activities within the domains of work, transportation and domestic, gardening and leisure activities is used to calculate the metabolic equivalent of task (MET) minutes (MET.min/week). One MET corresponds to the oxygen consumption of 3.5 ml/kg/min or 1 kcal/kg/hour.
Pelvic floor muscle strength was assessed via anorectal manometry as described by Morais et al. [Citation22]. The patients were positioned in the left lateral decubitus position and a probe 0.6 mm in diameter connected to a balloon that was 1.5 cm in diameter and 2.5-cm-long (inflated with saline) was connected to a single channel to measure pressure (mmHg). With the balloon already inflated and placed at the external level of the anus, the pressure was zeroed. The balloon was then introduced into the rectum 7 cm from the anal edge to record the rectal pressure. The balloon was then gradually pulled 1 cm increments to the external anal sphincter, the location of greatest pressure in the anal canal. After pressure stabilisation, the resting pressure of the external anal sphincter was recorded. Finally, the patient was instructed to contract the anal sphincter at full force three times while squeezing the balloon. The mean pressure value of three contractions was considered the maximum contraction pressure [Citation22].
The parametric distribution of the data was evaluated using the Kolmogorov–Smirnov test. Pearson correlation coefficients or Spearman tests were used for correlation analysis. Normally distributed data were analysed using analysis of variance (two-way ANOVA) test. Nonparametric data were analysed using the Kruskal–Wallis test. Values of p < .05 were considered statistically significant. All statistical analyses were performed with the aid of Prism 5.0 software.
Results
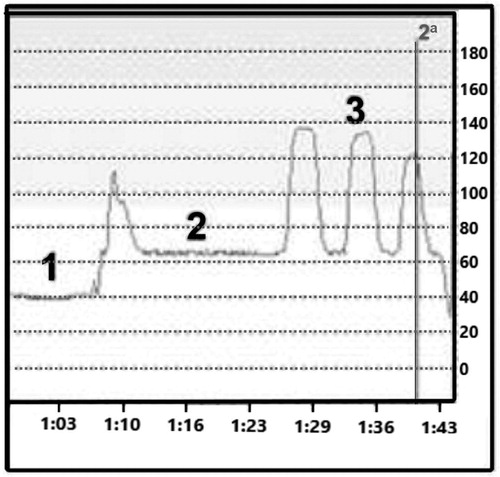
Pelvic floor strength was assessed by a manometry test. shows a representative test of rectal pressure, resting pressure and maximum contraction.
Figure 1. Representative manometric test. 1, rectal pressure; 2, resting pressure (in the anal canal); 3, maximum pressure (three voluntary contractions). The X axis shows time in minutes, while the Y axis shows pressure in mmHg.
The results show the mean values of age, BMI (mean, 26 ± 3.6 kg/m2; range, 18–36.3 kg/m2), rectal pressure (44.4 ± 13.8 cm/H2O; range, 21–87 cm/H2O), resting pressure (103.3 ± 28.4 cm/H2O; 64–171 cm/H2O) and maximum contraction (164.9 ± 32.4 cm/H2O; 130–238 cm/H2O). Rectal pressure and maximum contraction pressure values were normally distributed.
The results of the IPAQ questionnaire were as follows: six (19%) had a low level, 10 (32.3%) had a moderate level, and 15 (48.4%) had a high level of physical activity.
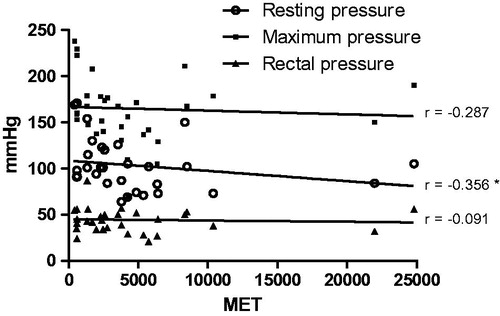
When evaluating the correlation between physical activity level and manometry values, we found that the resting pressure and maximum contraction decreased as physical activity increased. In fact, patients with a low physical activity level had a mean maximum contraction pressure of 193.7 ± 40.2mm/H2O, while individuals with high physical activity level had a mean value of 155.4 ± 26.6 mm/H2O (p < .05). We observed a negative correlation between resting pressure and MET but no significant difference between MET and rectal or maximum pressures ().
Figure 2. Correlation between physical activity level in multiples of resting metabolic rate (MET) and anorectal manometry values (mmHg). Resting pressure, maximum pressure, and rectal pressure was assessment. Spearman test correlation. *p < .05.
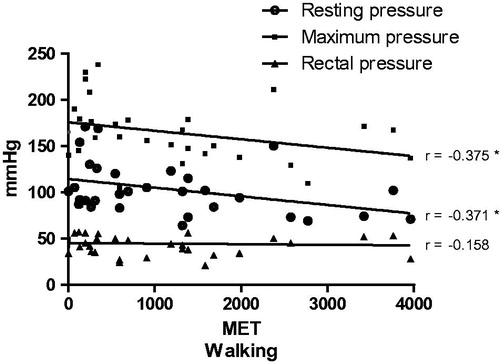
The analysis of manometry and the MET spent walking (treadmill) revealed a negative correlation (p < .05) between resting pressure and maximum pressure ().
Figure 3. Correlation between multiples of the resting metabolic rate (MET) corresponding to walking and the anorectal manometry values (mmHg). Resting pressure, maximum pressure, and rectal pressure were assessed. Spearman test correlation. *p < .05.
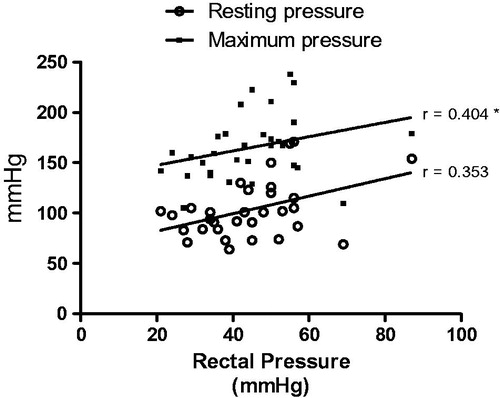
In assessing the association between the studied variables (), we observed a positive association between maximum pressure and rectal pressure (p < .05).
Figure 4. Correlation between rectal pressure (mmHg) and maximum and resting pressure (mmHg). Spearman test correlation. *p < .05.
There were no statistically significant differences in BMI or manometry values regarding physical activity level measured by the IPAQ.
Discussion
Pelvic floor dysfunction occurs owing to a complex interaction among surgical procedures, pathological condition, physical activity type, age, body composition and others. Urinary incontinence is the significant medical complication of pelvic floor dysfunction. Some studies have shown that pelvic floor strength can influence the treatment of urinary incontinence [Citation6,Citation23]. However, there are limited data regarding the relationship between pelvic floor strength and urinary incontinence in men. More data are clearly required to understand pelvic floor function in healthy men.
In our study, all patients had maximum contraction pressure of the pelvic floor within the normal range, which would be expected for a healthy population. Our values were similar to previously reported values for maximum contraction (range, 130–296 cm/H2O) [Citation24,Citation25]. Meanwhile, the maximum pressure value was normally distributed, characterising what happens to biological variables [Citation26]. Similarly, maximum contraction pressure of the pelvic floor was normally distributed in 19 healthy men a mean 40 years of age (mean value, 154 ± 21 cm/H2O), as was resting pressure (mean value, 72 ± 24 cm/H2O) [Citation27]. The variability of pelvic floor strength in healthy patients may have relevance as a risk factor for the development of urinary incontinence after radical prostatectomy. The association between muscle thickness and urinary incontinence was verified [Citation15].
Interestingly, the present study provides new data about pelvic floor strength in healthy men. We observed a negative correlation between pelvic floor strength and physical activity level. This negative correlation was stronger when we analysed pelvic floor strength and walking. This apparently controversial result can be partially explained by studying the pelvic floor strength of athletes since such data on non-athletes are scarce.
The resting and maximum contraction pressures were negatively associated with physical activity level. The negative correlation was stronger between walking and pressure (resting and maximum). Pelvic floor muscle weakening can be related to intense physical activity. Increase intra-abdominal pressure during intense physical activity overloads the pelvic floor, which can induce fatigue and weakness [Citation5,Citation7,Citation28–30]. The chronic increase in intra-abdominal pressure seems associated with pelvic floor dysfunction in female athletes, especially in activities that encourage increased intra-abdominal pressure such as sports involving impact, jumping, running, balls, and weight lifting [Citation5,Citation28–30]. In fact, people with high physical activity levels have a greater incidence of urinary incontinence. Some high-impact exercises increase pelvic floor overload, which can be a medical complication in individuals with a weak pelvic floor [Citation5,Citation29]. A study showed decreased force of vaginal or pelvic floor contractions after vigorous exercise in women, suggesting fatigue of the pelvic floor after vigorous activity [Citation7]. Thus, one can infer that the practice of specific physical activity and muscle strengthening does not generally cause a corresponding increase in pelvic floor strength, contributing to muscular imbalance between the abdominal muscles and the pelvic floor [Citation7,Citation28,Citation31,Citation32].
The pre-prostatectomy pelvic floor strength is crucial to continence recovery. The probability of incontinence at 4 weeks after prostatectomy is higher in men with moderate or weak pelvic floor muscles, whereas men with a strong pelvic floor muscle were less likely to develop urinary incontinence [Citation6]. However, another study reported no relationship between pre-operative physical activity levels and post-prostatectomy urinary incontinence, although patients had high overall preoperative physical levels and low overall incontinence rates [Citation3].
In this study, the rectal pressure values were also normally distributed (44.4 ± 13.8 cm/H2O; range, 21–87 cm/H2O). There was a positive association between rectal pressure and maximum pressure and a trend towards a positive association with resting pressure of the pelvic floor. In fact, studies have indicated a positive association between rectal pressure and anal sphincter pressure, suggesting a mechanism that prevents rectal escape. In individuals without pelvic floor dysfunction, increased abdominal pressure is accompanied by increased tone in both types of muscle fibres (fast and slow contraction) of the pelvic floor to retain faecal and urinary continence [Citation11].
Although our study did not show a significant association between BMI and pelvic floor strength, other studies showed that patients with higher BMI, obesity, an increased sagittal diameter of the abdomen, and the accumulation of visceral fat have higher rectal pressure [Citation31,Citation33,Citation34]. A systematic review showed that patients with an above-normal BMI are more likely to develop pelvic floor disorders, urinary or faecal incontinence, and prolapse of the pelvic organs [Citation34].
Injury or dysfunction of the pelvic floor such as radical prostatectomy, vigorous sports, or pregnancy can causes imbalances of the pelvic floor muscles and abdominal pressure that result in urinary incontinence [Citation5,Citation13]. Patients with a higher rectal pressure are at risk of developing incontinence after injuries to the pelvic floor as observed in patients undergoing radical prostatectomy [Citation35]. In addition, obese patients require more time to recover urinary functions. Similarly, obese subjects with urinary incontinence notice improvements after weight loss since it reduces rectal pressure and regains the balance between rectal pressure and pelvic floor strength [Citation34]. Studies should assess not only the pelvic floor strength as a risk factor for incontinence after prostatectomy but the rectal pressure as well. The concept that the balance between rectal pressure and pelvic floor strength is important in the pathophysiology of stress urinary incontinence can be inferred through our findings.
This study raises new questions about the understanding of the interaction among physical activity, intraabdominal pressure, and pelvic floor strength in the physiology of continence in men. New data are needed to elucidate some unanswered questions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Vodusek DB. Anatomy and neurocontrol of the pelvic floor. Digestion. 2004;69:87–92.
- Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation: mass contraction of the pelvic floor muscles. Int Urogynecol J. 1998;9:28–32.
- Mungovan SF, Huijbers BP, Hirschhorn AD, et al. Relationships between perioperative physical activity and urinary incontinence after radical prostatectomy: an observational study. BMC Urol. 2013;13:67.
- Parekh AR, Feng MI, Kirages D, et al. The role of pelvic floor exercises on post-prostatectomy incontinence. J Urol. 2003;170:130–133.
- Bø K. Urinary incontinence, pelvic floor dysfunction, exercise and sport. Sports Med. 2004;34:451–464.
- Manley L, Gibson L, Papa N, et al. Evaluation of pelvic floor muscle strength before and after robotic-assisted radical prostatectomy and early outcomes on urinary continence. J Robotic Surg. 2016;10:331–335.
- Lindland Ree M, Nygaard I, Bø K. Muscular fatigue in the pelvic floor muscles after strenuous physical activity. Acta Obstet Gynecol Scand. 2007;86:870–876.
- Martinho NM, Silva VR, Marques J, et al. The effects of training by virtual reality or gym ball on pelvic floor muscle strength in postmenopausal women: a randomized controlled trial. Braz J Phys Ther. 2016;20:248–257.
- Braslis K, Santa-Cruz C, Brickman AL, et al. Quality of life 12 months after radical prostatectomy. BJU International. 1995;75:48–53.
- Van Kampen M, De Weerdt W, Van Poppel H, et al. Effect of pelvic-floor re-education on duration and degree of incontinence after radical prostatectomy: a randomised controlled trial. Lancet. 2000;355:98–102.
- Dorey G. Restoring pelvic floor function in men: review of RCTs. Br J Nurs. 2005;14:1014–1018. 1020-1.
- Leliefeld HH, Stoevelaar HJ, McDonnell J. Sexual function before and after various treatments for symptomatic benign prostatic hyperplasia. BJU Int. 2002;89:208–213.
- Cornel EB, de Wit R, Witjes JA. Evaluation of early pelvic floor physiotherapy on the duration and degree of urinary incontinence after radical retropubic prostatectomy in a non-teaching hospital. World J Urol. 2005;23:353–355.
- Kumar A, Samavedi S, Bates AS, et al. Continence outcomes of robot-assisted radical prostatectomy in patients with adverse urinary continence risk factors. BJU Int. 2015;116:764–770.
- Song C, Doo CK, Hong JH, et al. Relationship between the integrity of the pelvic floor muscles and early recovery of continence after radical prostatectomy. J Urol. 2007;178:208–211.
- Pacik D, Fedorko M. Literature review of factors affecting continence after radical prostatectomy. SMJ. 2017;38:9–17.
- Lunenfeld B, Nieschlag E. Testosterone therapy in the aging male. Aging Male. 2007;10:139–153.
- Strollo F, Strollo G, Morè M, et al. Low-intermediate dose testosterone replacement therapy by different pharmaceutical preparations improves frailty score in elderly hypogonadal hyperglycaemic patients. Aging Male. 2013;16:33–37.
- Judge LW, Bellar DM, Hoover DL, et al. Effects of acute androstenedione supplementation on testosterone levels in older men. Aging Male. 2016;19:161–167.
- Krause Neto W, de Assis Silva W, Polican Ciena A, et al. Total training load may explain similar strength gains and muscle hypertrophy seen in aged rats submitted to resistance training and anabolic steroids. Aging Male 2017. DOI:10.1080/13685538.2017.1365832
- Sampaio LR, Figueiredo VdC. Correlation between body mass index and body fat distribution anthropometric indices in adults and the elderly. Revista De Nutrição. 2005;18:53–61.
- Morais MBd, Sdepanian VL, Tahan S, et al. A manometria anorretal (método do balão) no diagnóstico diferencial da doença de Hirschsprung. Rev Assoc Med Bras. 2005;51:313–317.
- Celiker Tosun O, Kaya Mutlu E, Ergenoglu AM, et al. Does pelvic floor muscle training abolish symptoms of urinary incontinence? A randomized controlled trial. Clin Rehabil. 2015;29:525–537.
- Castro L. Tópicos em gastroenterologia. 2001: Medsi.
- Saad LH, Coy CS, Fagundes JJ, et al. Sphincteric function quantification by measuring the capacity to sustain the squeeze pressure of the anal canal. Arq Gastroenterol. 2002;39:233–239.
- Imbelloni LE, Beato L, Tolentino AP, et al. Automatic blood pressure monitors. Evaluation of three models in volunteers. Rev Bras Anestesiol. 2004;54:43–52.
- Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterology. 1999;94:773–783.
- Silva LH, Serezuella KC, Bordini A, et al. Relação da incontinência urinária de esforço com a prática de atividade física em mulheres nulíparas. Salusvita. 2005;24:195–218.
- Caetano AS, Tavares MdCGC, Lopes MHBdM. Urinary incontinence and physical activity practice. Rev Bras Med Esporte. 2007;13:270–274.
- Kikuchi A, Niu K, Ikeda Y, et al. Association between physical activity and urinary incontinence in a community-based elderly population aged 70 years and over. Eur Urol. 2007;52:868–874.
- Higa R, Lopes MHBdM. Factors associated with urinary incontinence in women. Rev Bras Enferm. 2005;58:422–428.
- Virtuoso JF, Mazo GZ, Menezes EC. Urinary incontinence and perineal muscle function in physically active and sedentary elderly women. Rev Bras Fisioter. 2011;15:310–317.
- Fang CB, Klug WA, Aguida HAC, et al. Rectal and anal pressure variation during defecation in patients with acquired megacolon. Rev Bras Colo-Proctol. 2007;27:264–268.
- Greer WJ, Richter HE, Bartolucci AA, et al. Obesity and pelvic floor disorders: a systematic review. Obstet Gynecol. 2008;112:341–349.
- Ahlering TE, Eichel L, Edwards R, et al. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology. 2005;65:740–744.
