Abstract
Background: There is growing evidence showing a putative association between high-risk human papillomavirus (HR-HPV) infection and an increased risk of PCa.
Objective: The aim of the current meta-analysis was to evaluate the association between HPV infection and PCa risk.
Methods: This analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. We included all studies on HPV DNA or antibodies detected in biopsy tissues or sera. Available data were extracted from the article, including means and standard deviations in all case-control groups.
Results: Thirty studies that investigated the link between HPV-16 and -18 were identified as eligible for this systematic review and meta-analysis, including a total of 6321 participants. The pooled OR showed increased risk of PCa (OR =1.37; p < .01) in men positive for HPV-16. There were seven studies with 2391 PCa cases and 4059 controls investigating the association between HPV-18 infection and PCa risk. Significant heterogeneity between study was found in the pooled analyzes. The pooled OR did not show increased risk of PCa (OR =0.80; p = .49) in men positive for HPV-18.
Conclusions: This meta-analysis suggests that HPV-16 infection could represent a risk factor for PCa, whereas we found no such association for HPV-18. Further well-conducted studies could be useful to confirm this conclusion.
Introduction
Prostate cancer (PCa) continues to pose significant health care challenges worldwide. It remains the commonest non-dermatologic cancer in men with 233,000 estimated new cases per year in USA and it is the second most common reason for death due to cancer [Citation1].
Unfortunately, there are few knowledge about the underlying mechanisms this disease. In this regard, both environmental and hereditary components are supposed to play crucial roles in prostate carcinogenesis [Citation2–6]. Moreover, there is growing evidence showing a putative association between high-risk human papillomavirus (HR-HPV) infection and an increased risk of PCa [Citation2,Citation7,Citation8].
Sutcliffe et al. proposed that the sexually transmitted diseases (STDs) may serve an important role in the initiation of PCa [Citation9]. Viral infection may induce chronic and recurrent in ammation of the prostate, and we hypothesize that in ammation of the prostate induced by STDs may increase the risk of PCa [Citation10].
To this regard, it should be point out that inflammation at the tissue level is present in approximately 17% of all cancer cases [Citation11]. Biological studies have provided evidence that active oxygen and nitrogen radicals produced by the inflamed tissue increase the risk of cancer by suppressing antitumor activity and stimulating carcinogenesis [Citation12,Citation13].
It is plausible that in men with pre-existent prostatic inflammation, HR-HPV infection, often localized at the prostate gland level, may contribute to the development of chronic inflammation and regenerative “risk factor” lesions [Citation14]. The immune system plays a key role in preventing tumor formation by recognizing and destroying malignant cells. On the contrary, the selective pressure exerted by the immune system secondary to HPV infection causes tumors to develop multiple mechanisms to escape immune detection, including the induction of immune tolerance, local suppression of the immune response, and systematic disruption of T-cell signaling [Citation15]. Based on these considerations, the aim of the current meta-analysis is to evaluate the association between HR-HPV infection of 16 and 18 genotype and PCa risk.
Methods
Systematic literature search
This analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines [Citation16]. We performed a systematic literature search of PubMed, EMBASE, Cochrane, and Academic One File databases using Medical Subject Headings (MeSH) indexes, keyword searches, and publication types until December 2016. The search terms combined text words and MeSH terms. For example, the search terms for “cross-sectional”, “prostate cancer”, “human papillomavirus”, “HR-HPV”, “HPV16”, “HPV18”, “risk factors”, and “prostate sample”.
We included all studies on HPV DNA or antibodies detected in biopsy tissues or sera. With respect to HPV detection methods in prostate cancer tissues were used the PCR primers, type-specific PCR primers, and both combined, non-PCR methods as in situ hybridization (ISH), immunohistochemistry and HC2. Exclusion criteria were as follows: studies on immunosuppression patients, case reports, publications not in English, studies without extractable data from the original article, and studies lacking HPV infection. Available data were extracted from the article, including means and standard deviations in all case-control groups.
Study selection
Citation lists of retrieved articles were screened manually to ensure sensitivity of the search strategy. References of the included papers were hand searched to identify other potential relevant studies. Studies were reviewed by two independent reviewers (GIR and AEC); differences in opinion were discussed in consultation with the last author (SLV).
Data were extracted independently by two reviewers. Discrepancies for inclusion between the investigators were resolved by discussion or further consultation with a third author.
Statistical analysis
Meta-analysis was conducted to determine the pooled odds ratio (OR) for HR-HPV and risk of prostate cancer (PCa), based on histological biopsy. We constructed 2 × 2 table including number of events/total number of patients with PCa and number of events/total number of patients with HR-HPV-16 or -18 infection.
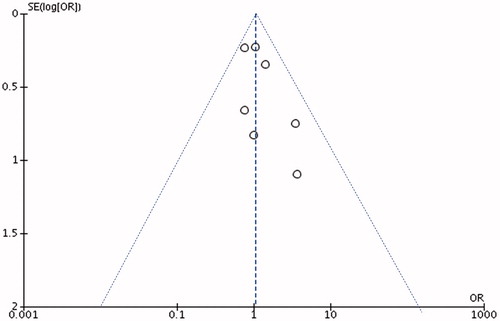
Natural logarithm (ln) of the OR [ln(OR)] were calculated to estimate the risk of PCa in patients with HR-HPV. Standard error (SE) of the lnOR was calculated through a first-order Taylor series conversion, where SE[ln(OR)] = (1/OR) * SE[OR]. Begg’s and Egger’s methods were used to assess publication bias, using log transformation of the effect size. The shape of the funnel plots is used to show symmetry and Egger’s methods are applied to indicate significant publication bias for the analyzes exploring association between PC risk and HPV-16 or HPV-18 infection.
Statistical heterogeneity was assessed using the Cochran-Q and I2 statistics. Specifically, statistical heterogeneity was tested using the chi-square test. I2 ≤ 50%, the variation of the studies was considered to be homogenous, the fixed effect model was adopted. If I2 > 50%, there was significant heterogeneity between studies, the random effects model was used. For estimating the association between HPV methods of detection and risk of PCa, a multivariate logistic regression analysis, adjusted for year of study and age has been performed. All p values are two-tailed, α ≤ 0.05 was considered statistically significant (p ≤ .05). The analyzes were performed using RevMan software v.5.1 (Cochrane Collaboration, Oxford, UK) and using SPSS v. 19 software (SPSS Inc, IBM Corp, Somers, NY).
Results
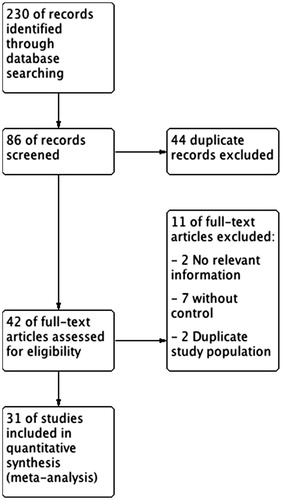
In total, 230 references were identified from the initial online databases and relevant references. After evaluating the title and abstract of each study, 137 studies were excluded as they did not meet the inclusion criteria. Subsequently, we carefully read the full texts of the remaining 96 studies. Thirty studies were identified as eligible for this systematic review, with a total of 6321 participants ( and ).
Table 1. Characteristics of included studies.
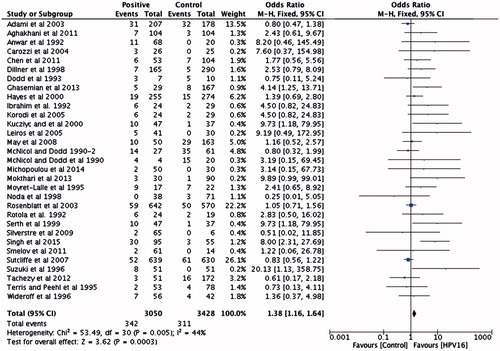
There were 31 studies with 3050 PCa cases and 3428 controls investigating the association between HPV-16 infection and PCa risk. No significant heterogeneity between studies was found in the pooled analyzes. The pooled OR showed increased risk of PCa (OR =1.38 [95% CI: 1.16–1.64]; p < .01) in men positive for HPV-16 ().
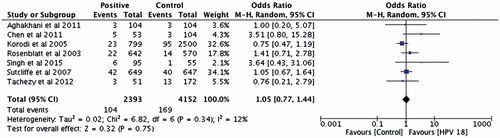
There were seven studies with 2393 PCa cases and 4152 controls investigating the association between HPV-18 infection and PCa risk. Significant heterogeneity between study was found in the pooled analyzes. The pooled OR did not show increased risk of PCa (OR =1.05 [95% CI: 0.77–1.44]; p = .75) in men positive for HPV-18 ().
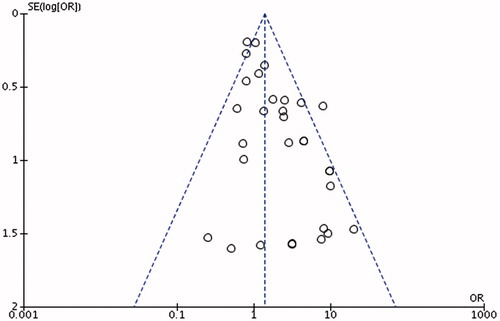
Funnel plot was generated to evaluate publication bias using the Egger’s funnel plots ( and ).
For study investigating HPV-16, we showed significant heterogeneity (bias =1.26 [95% CI: 0.67–1.85]; p < .0) while not for HPV-18 (bias =1.09 [95% CI: −0.67 to 2.85]; p = .17).
The association between HPV methods of detection and risk of PCa has been evaluated by the use of a multivariate logistic regression analysis, adjusted for year of study and age has been performed. We did not find any association between method of detection and risk of PCa (OR =1.12 [95% CI: 0.91–1.39]; p = .21).
Discussion
Prostate cancer is a topic of great interest also for endocrinologists; in particular, the management of androgen therapy [Citation17] and the reflections of the major metabolic diseases on the risk of developing it [Citation18,Citation19]. HPV infections concern the field of STDs and represent another element of cultural linkage.
The HR-HPV infection is the most common infection among young, sexually active individuals. Indeed, it is estimated that about 75–80% of sexually active individuals will become infected in their lifetime [Citation20]. Another important aspect of the clinical management of HR-HPV infection in men compared with women concerns the possible differences in the following parameters: viral load, duration of natural infection, absence of symptoms, lower antibody response, anatomical site differences, and lower incidence [Citation21].
Understanding the factors that influence the progression from latent to clinical cancer is therefore essential to identify patients at high risk of progression at the time of diagnosis and understand the target to prevent and control the passage between infection and clinical disease [Citation22].
Three meta-analyses have been published on the correlation between HR-HPV infections and the risk of prostate cancer [Citation2,Citation7,Citation8]. However, their results are discordant. In the present article, we showed that HPV-16 infection was significantly associated with an increased risk of PCa (OR =1.38), whereas HPV-18 was not (OR =1.05).
In parallel, some studies showed no significant association between HR-HPV and PCa [Citation9,Citation23–25].
In a previous study, we showed that the prevalence of HR-HPV infection in patients with microbial male accessory gland infection was ≈30%, whereas the prevalence decreased to ≈20% among patients with male accessory gland inflammation only [Citation20]. In this regard, it has been shown that HR-HPV could be identified in the prostatic secretions of patients with type III prostatitis and might be associated with the degree of intraprostatic inflammation. This strengthens the evidences about the relationship between HR-HPV and prostatic inflammation [Citation26].
High-risk HPV infections usually last 1–2 years [Citation27], which means that HR-HPV can replicate in host tissues for months in the face of constant cellular turnover and immune surveillance. Its ability to do so depends largely on its potential to regulate both viral and host gene expression [Citation28]. In addition to its differentiation-dependent life cycle organization, HR-HPV actively suppresses both innate and adaptive immune responses [Citation27]. The HPV oncoproteins E6 and E7 are essential for the normal virus life cycle and throughout the cancer development pathway from benign precursor lesions to invasive carcinomas [Citation29,Citation30].
Unfortunately, although HR-HPV has been recognized as an important risk factor of oral, cervical, anal cancer, its role in prostate cancer is far to be understood. However, HR-HPV infection and incidence rate remains high in developing countries due to lack of awareness, followed by mass screening programs, various socioeconomic issues, and low usage of preventive vaccines [Citation31].
Furthermore, a high incidence of HR-HPV DNA has been reported in semen analysis from sexually active men, with and without risk factors for HR-HPV, and from infertile patients [Citation32]. Foresta and colleagues [Citation32] investigated the presence of HPV DNA sequences in semen samples of asymptomatic young adult men who had unprotected intercourse, and reported the presence of HPV semen infection in 10% of these men. Moreover, they reported that a high incidence of HR-HPV infection in sperm was found not only in individuals with risk factors but also in asymptomatic infertile men [Citation32].
Based on these premises, sexual intercourse should be considered an important pathway to the exposure of microorganisms such as HR-HPV infection.
Articles and publications about expressed prostate secretions virus infections have been limited, with only some isolated case reports. CMV, HSV-2 and HR-HPV are the most common virus, all of which can be found in healthy populations, where they will cause latent infection or mild symptoms [Citation33].
Xiao et al. reported that WBC count and lecithin levels were significantly correlated with HR-HPV infection illustrating that there was some relationship between HR-HPV and prostatic inflammation [Citation26].
Before concluding, this meta-analysis exhibited some limitations. In particular, heterogeneity of included studies, as concerning definition of HR-HPV infection and methodology of such studies may represent some drawbacks of the present work.
Conclusion
Results of this meta-analysis of cross-sectional studies showed that HPV-16 infection is associated with PCa. Although the heterogeneity of current literature data concerning techniques of diagnosis of HPV in patients with PCa, HPV infection should be considered as a potential risk factor of PCa.
Disclosure statement
The authors have no conflicting interests to declare.
Additional information
Notes on contributors
Giorgio I. Russo
Giorgio I. Russo is with Department of Urology, University of Catania, Italy.
Aldo E. Calogero
Aldo E. Calogero is a Professor at Department of Andrology and Endocrinology, University of Catania, Italy.
Rosita A. Condorelli
Rosita A. Condorelli is with Department of Andrology and Endocrinology, University of Catania, Italy.
Guido Scalia
Guido Scalia is a Professor at Department of Virology, University of Catania, Italy.
Giuseppe Morgia
Giuseppe Morgia is a Professor at Department of Urology, University of Catania, Italy.
Sandro La Vignera
Sandro La Vignera is a Professor at Department of Andrology and Endocrinology, University of Catania, Italy.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA: Cancer J Clin. 2014;64:9–29.
- Lin Y, Mao Q, Zheng X, et al. Human papillomavirus 16 or 18 infection and prostate cancer risk: a meta-analysis. Iran J Med Sci. 2011;180:497–503.
- Dicitore A, Grassi ES, Borghi MO, et al. Antitumor activity of interferon-β1a in hormone refractory prostate cancer with neuroendocrine differentiation. J Endocrinol Invest. 2017;40:761–770.
- Sideris S, Aoun F, Martinez CN, et al. Role of corticosteroids in prostate cancer progression: implications for treatment strategy in metastatic castration-resistant patients. J Endocrinol Invest. 2016;39:729–738.
- Urquiza-Salvat N, Pascual-Geler M, Lopez-Guarnido O, et al. Adherence to Mediterranean diet and risk of prostate cancer. Aging Male. 2018 [cited Mar 15]; [7 p.]. DOI:10.1080/13685538.2018.1450854
- Pascual-Geler M, Urquiza-Salvat N, Cozar JM, et al. The influence of nutritional factors on prostate cancer incidence and aggressiveness. Aging Male. 2018 [cited 2017 Sep 20]; [9 p.]. DOI:10.1080/13685538.2017.1379491
- Bae JM. Human papillomavirus 16 infection as a potential risk factor for prostate cancer: an adaptive meta-analysis. Epidemiol Health. 2015;37:e2015005.
- Yang L, Xie S, Feng X, et al. Worldwide prevalence of human papillomavirus and relative risk of prostate cancer: a meta-analysis. Sci Rep. 2015;5:14667.
- Sutcliffe S, Giovannucci E, Gaydos CA, et al. Plasma antibodies against Chlamydia trachomatis, human papillomavirus, and human herpesvirus type 8 in relation to prostate cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16:1573–1580.
- Yin B, Liu W, Yu P, et al. Association between human papillomavirus and prostate cancer: a meta-analysis. Oncol Lett. 2017;14:1855–1865.
- Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264.
- Lonkar P, Dedon PC. Reactive species and DNA damage in chronic inflammation: reconciling chemical mechanisms and biological fates. Int J Cancer. 2011;128:1999–2009.
- Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49.
- De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269.
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100.
- Isidori AM, Balercia G, Calogero AE, et al. Outcomes of androgen replacement therapy in adult male hypogonadism: recommendations from the Italian society of endocrinology. J Endocrinol Invest. 2015;38:103–112.
- Esposito K, Chiodini P, Capuano A, et al. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest. 2013;36:132–139.
- Gacci M, Russo GI, De Nunzio C, et al. Meta-analysis of metabolic syndrome and prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:146–155.
- La Vignera S, Vicari E, Condorelli RA, et al. Prevalence of human papilloma virus infection in patients with male accessory gland infection. Reprod Biomed Online. 2015;30:385–391.
- Partridge JM, Koutsky LA. Genital human papillomavirus infection in men. Lancet Infect Dis. 2006;6:21–31.
- Carozzi F, Tamburrino L, Bisanzi S, et al. Are biomarkers evaluated in biopsy specimens predictive of prostate cancer aggressiveness? J Cancer Res Clin Oncol. 2016;142:201–212.
- Tachezy R, Hrbacek J, Heracek J, et al. HPV persistence and its oncogenic role in prostate tumors. J Med Virol. 2012;84:1636–1645.
- Korodi Z, Dillner J, Jellum E, et al. Human papillomavirus 16, 18, and 33 infections and risk of prostate cancer: a Nordic nested case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:2952–2955.
- Adami HO, Kuper H, Andersson SO, et al. Prostate cancer risk and serologic evidence of human papilloma virus infection: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2003;12:872–875.
- Xiao J, Ren L, Lv H, et al. Atypical microorganisms in expressed prostatic secretion from patients with chronic prostatitis/chronic pelvic pain syndrome: microbiological results from a case-control study. Urol Int. 2013;91:410–416.
- Songock WK, Kim SM, Bodily JM. The human papillomavirus E7 oncoprotein as a regulator of transcription. Virus Res. 2017;231:56–75.
- Frazer IH. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384:410–414.
- Thomas JT, Hubert WG, Ruesch MN, et al. Human papillomavirus type 31 oncoproteins E6 and E7 are required for the maintenance of episomes during the viral life cycle in normal human keratinocytes. Proc Natl Acad Sci USA. 1999;96:8449–8454.
- Flores ER, Allen-Hoffmann BL, Lee D, et al. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J Virol. 2000;74:6622–6631.
- Husain RS, Ramakrishnan V. Global variation of human papillomavirus genotypes and selected genes involved in cervical malignancies. Ann Glob Health. 2015;81:675–683.
- Garolla A, Pizzol D, Foresta C. The role of human papillomavirus on sperm function. Curr Opin Obstet Gynecol. 2011;23:232–237.
- Foresta C, Garolla A, Zuccarello D, et al. Human papillomavirus found in sperm head of young adult males affects the progressive motility. Fertil Steril. 2010;93:802–806.
- Leskinen MJ, Vainionp R, Syrjnen S, et al. Herpes simplex virus, cytomegalovirus, and papillomavirus DNA are not found in patients with chronic pelvic pain syndrome undergoing radical prostatectomy for localized prostate cancer. Urology. 2003;61:397–401.