Abstract
Testosterone is the predominant gonadal androgen in men. Low testosterone levels are found to be associated with an increased in metabolic risk and systematic inflammation. Since adipose tissue is a source of inflammatory cytokines, testosterone may regulate inflammation by acting on adipose tissue. This review aimed to explore the role of testosterone in inflammation and its mechanism of action. Both animal studies and human studies showed that (1) testosterone deficiency was associated with an increase in pro-inflammatory cytokines; (2) testosterone substitution reduced pro-inflammatory cytokines. The suppression of inflammation by testosterone were observed in patients with coronary artery disease, prostate cancer and diabetes mellitus through the increase in anti-inflammatory cytokines (IL-10) and the decrease in pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α). Despite these, some studies also reported a non-significant relationship. In conclusion, testosterone may possess anti-inflammatory properties but its magnitude is debatable. More evidence is needed to validate the use of testosterone as a marker and in the management of chronic inflammatory diseases.
Introduction
Inflammation is an important classic host defense mechanism against infection and injury. It is also intimately linked with a broad range of noninfectious diseases. Increasing evidence showed that chronic inflammation contributes to the pathogenesis of many common diseases, such as diabetes, cardiovascular, metabolic, and autoimmune disease. Many studies have investigated the possible involvement of gonadal hormones in inflammatory process in the body. Indeed, several lines of evidence suggest that androgen can act as a modulator for inflammation in men [Citation1,Citation2]. Besides, human cross-sectional studies have suggested an inverse relationship between serum testosterone level and inflammatory markers in men with stable coronary artery disease (CAD) [Citation3], young men [Citation4], and older men [Citation5]. These findings reiterated that low testosterone levels might trigger the occurrence of inflammation-related diseases.
In addition, low testosterone levels have been associated with increase fat mass. Some scientists have speculated that adipose tissue functions as a complex endocrine organ rather than just a storage for energy [Citation6,Citation7]. Adipose tissue in inflamed condition expresses aromatase to convert testosterone to estradiol, thus decreasing testosterone levels [Citation8]. Excessive visceral fat is a cause of chronic inflammation because adipose tissue is a major source of pro-inflammatory mediators [Citation9]. A cross-sectional study demonstrated that low testosterone, lean body mass, as well as high fat mass, interleukin-6 (IL-6) and C-reactive protein (CRP) were observed in patients with cancer and cachexia as compared to non-cancer controls [Citation10]. Induction of inflammation was also observed in prostate cancer patients as indicated by a strong association between lipoprotein profile (cholesterol, high-density lipoprotein, and triglyceride [TG]) and androgens [Citation11].
The role of androgen as an anti-inflammatory hormone is further demonstrated by testosterone supplementation trial indicating a suppression of pro-inflammatory markers in either young or old hypogonadal men [Citation12]. Experimental studies also showed that pro-inflammatory cytokines, such as IL-6, tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) could inhibit testosterone secretion by modulating the hypothalamic-pituitary-gonadal axis [Citation2,Citation13]. The occurrence of hypogonadotropic hypogonadism syndrome with obesity in type 2 diabetes patients suggested that inflammation may play an important role as indicated by an increase in CRP level and its inverse relationship with plasma testosterone level [Citation14]. Understanding the association between androgens and inflammation is important because inflammation plays a vital role in the pathogenesis of many diseases [Citation15]. A significant association might justify the use of androgen replacement to improve the outcome of the patients with inflammatory disorders. This review aims to summarize the current data on the relationship between circulating testosterone levels and inflammatory markers in men. Evidence from human observation and animal studies will be presented. We hope to address the question as to whether testosterone substitution is able to alter pro-inflammatory state in men with chronic diseases.
Literature search
A literature search was performed using PubMed and EBSCOhost using keywords: “androgen” OR “testosterone” AND “inflammatory cytokines” OR “inflammatory markers.” The search was limited to primary studies including animal and human, published in English language. The search resulted in 14 and 20 relevant original research articles conducted in animals and humans.
Hypogonadism
A summary of the relationship between testosterone level and inflammatory markers and the effects of testosterone administration on inflammatory markers in hypogonadism animal studies and men were listed in .
Table 1. Summary of the association between testosterone level and supplementation with inflammatory markers in animals and humans with hypogonadism.
Negative relationship between testosterone and inflammatory markers
Evidence from animal studies
Kelly et al. utilized testicular-feminized mice (which express low endogenous testosterone and nonfunctional androgen receptor) to evaluate the effect of androgen status on inflammatory markers. This study found that the levels of TNF-α and IL-6 were significantly elevated in the testicular-feminized mice compared to the control group after 28 weeks. Only IL-6 was reduced by testosterone treatment (100 mg/mL) in the testicular-feminized mice, but not other serum cytokine concentrations such as IL-1β, IL-10, monocyte chemoattractant protein-1 (MCP-1), and TNF-α. Findings from this study suggested that testosterone was associated with a beneficial effect on inflammatory mediators [Citation16]. Similarly, in castrated Sprague Dawley rats (n = 48), Jia et al. indicated that androgens dose-dependently (0.25, 0.50, or 1.0 mg/kg) decreased the expression of TNF-α after 30 days of treatment. Testosterone was found to possess anti-inflammatory properties at these doses, which normalized or over-stimulated the growth of the prostate and seminal vesicles [Citation17].
Using adult male Sprague-Dawley rats as an animal model, Papadopoulos and Wardlaw examined the adrenocorticotropic hormone (ACTH) and corticosterone responses to IL-6 in the intact and orchidectomized rats. The results showed that IL-6 stimulated the release of ACTH and corticosterone and the release was attenuated by testosterone replacement therapy (TRT) [Citation18]. These findings suggested that testosterone restrained the response of the hypothalamic-pituitary adrenal (HPA) axis to inflammatory stimulus (IL-6) in the presence of gonadal steroids. A study by Wang et al. found that blocking the testosterone receptor by flutamide (10 mg) or depleting testosterone by castration in normal male rats improved myocardial function after acute ischemia reperfusion. The authors postulated that these effects might be attributed to the pro-inflammatory properties of endogenous testosterone, evidenced by the decrease in TNF-α, IL-1β, and IL-6 compared to the untreated controls [Citation19].
Inversely, a study by Dermitas et al. investigated the effects of etanercept (a TNF-α inhibitor) on testosterone concentrations with circulating and cavernosal levels of inflammatory markers in Wistar albino aged rats (24 months old). The results found that aged rats displayed significantly increased serum and cavernosal TNF-α, CRP, MCP-1, and intercellular adhesion molecule (ICAM-1) levels, while decreased serum testosterone levels compared with the controls. Treatment with the etanercept (0.8 mg/kg) was able to normalized the circulating and cavernosal concentrations of TNF-α, CRP, MCP-1, ICAM-1, and testosterone level in the treated aged group after 12 weeks [Citation20].
Evidence from human studies
In a cross-sectional study by Maggio et al., the relationship between testosterone and molecular markers of inflammation in older men (n = 473, aged >65 years old) from the InCHIANTI population (older persons living in the Chianti, Tuscany, Italy) was observed. A significant inverse relationship between testosterone and soluble IL-6 receptor (sIL-6r) levels (a soluble portion of the IL-6 receptor that may enhance the biological activity of IL-6) were found in this study but not with other markers of inflammation. This suggested that a close relationship between pro-inflammatory development and the decline in testosterone levels in aging men [Citation1]. In the subsequent year, another cross-sectional study by Maggio et al. found that testosterone was negatively regulated with inflammatory marker in older men (n = 467, age: >65 years). This study demonstrated that serum testosterone and bioavailable testosterone were gradually decreased with age in men from age 65 to 85 years. Inflammatory markers such as IL-6, TNF-α, IL-1β, and CRP were higher in older men. Soluble interleukin-6 receptor (sIL-6r) was inversely correlated with total testosterone [Citation5].
A study done by Bobjer et al. also reported the negative relationship between total testosterone level and inflammatory markers of macrophage inflammatory protein-1 alpha (MIP1α), macrophage inflammatory protein-1 beta (MIP1β), and TNF-α in men. Besides, men with subnormal levels of testosterone had significantly higher levels of TNF-α and MIP1α in serum [Citation4].
In a randomized single-blinded, placebo-controlled study, the levels of endogenous inflammatory cytokines in hypogonadal men (n = 27, aged 62 ± 9 years old) following testosterone replacement (Sustanon 100) for one month were observed. The results showed testosterone replacement shifted the cytokine balance by down-regulating pro-inflammatory cytokines (TNF-α and IL-1β) and concomitantly up-regulating an anti-inflammatory cytokine (IL-10) [Citation2]. The relationship between testosterone and cardiovascular inflammatory marker, high sensitivity C-reactive protein (hsCRP) in aging men (n = 467, mean age: 52 years old) was investigated by Kaplan et al. The study found that patients with hypogonadism had higher level of hsCRP. These results indicated that testosterone level had an inverse relationship with hsCRP, indicative of increased risk for cardiovascular diseases, in aging men [Citation21]. Besides, treatment with testosterone (parenteral testosterone undecanoate 1000 mg) intramuscularly for 30 weeks also was shown to restore plasma testosterone leading to the reduction of inflammatory markers such as IL-1β, TNF-α, and CRP in hypogonadal men (n = 184, aged 35–70 years old) with MetS [Citation22].
Negligible relationship between testosterone and inflammatory markers
Evidence from animal study
Although the aforementioned studies showed that testosterone had positive and negative effects on the production of inflammatory markers, several studies showed no effects. A study by Chin et al. evaluated the effects of testosterone deficiency and TRT on the circulating inflammatory cytokine level in orchidectomized rats. The results indicated that testosterone deficiency caused a significant increase in IL-6 and a non-significant increase in IL-1α, IL-1β, and TNFα. Supraphysiological testosterone enanthate (7 mg/kg/week) did not alter the cytokine levels in these rats. Researchers suggested that testosterone deficiency by itself might not induce strong inflammatory response, but it might exacerbate preexisting inflammatory conditions [Citation23].
Evidence from human studies
A double-blind placebo-controlled trials study by Ng et al. showed no significant effects on the levels of the important inflammatory markers including ICAM-1, CRP, and vascular cell adhesion molecule-1 (VCAM-1) in men (n = 73, aged >60 years old) with partial androgen deficiency (serum testosterone levels <15 nmol/L) after 3 month of treatment with DHT (70 mg daily transdermal) or recombinant human chorionic gonadotropin (rhCG; 250 mg twice weekly) [Citation24]. Their findings were consistent with a previous study by Dougherty et al., whereby inhibiting the aromatization of androgen to estrogen by anastrozole (1 mg daily or 1 mg twice weekly) did not appear to adversely affect inflammatory markers in men with mild hypogonadism (n = 37; age: 62–74 years old) [Citation25]. They demonstrated that no appreciable changes in either IL-6 or CRP from baseline in any treatment groups. Besides, authors suggested to prolong the intervention period to assess the effect of androgen replacement therapy on inflammation.
Kapoor and coworkers also investigated the effects of testosterone treatment on various inflammation markers (TNF-α, IL-6, and CRP) for 3 months in hypogonadal men (n = 20, age: >30 years old) with type 2 diabetes. The results suggested that administration of testosterone (Sustanon, 200 mg once every 2 weeks, intramuscularly) for two treatment phases of 3 months caused no change in IL-6, TNF-α, and CRP levels [Citation26]. In addition, Maggio et al. investigated the effects of transdermal testosterone treatment on inflammatory markers in elderly men (n = 108; age: >65 years old) for 36-months. Each subject was asked to wear a 60 cm2 testosterone patch, which delivered approximately 6 mg testosterone per day or a 60 cm2 placebo patch. This study found that there was no significant changes observed in inflammatory markers, such as CRP, TNF-α, soluble TNF-α receptor-1 (TNFR1), IL-6, and soluble IL-6 receptors (sIL6r and sgp130) [Citation27]. Thus, this study suggested that testosterone treatment after 36 months did not produce significant effects on inflammatory markers in older men.
Metabolic syndrome (MetS) and associated conditions
A summary of the relationship between testosterone level and inflammatory markers and the effects of testosterone administration on inflammatory markers in MetS and other associated conditions animal studies and men were listed in .
Table 2. Summary of the association between testosterone level and supplementation with inflammatory markers in animals and humans with metabolic disorders.
Negative relationship between testosterone and inflammatory markers
Evidence from animal studies
Vignozzi et al. developed a high-fat diet-induced MetS rabbit model with hypogonadism. These animals showed prostate inflammation with increased expression of several pro-inflammatory markers including interleukin-8 (IL-8), IL-6, IL-1β, and TNF-α. Treatment with testosterone (30 mg/kg) inhibited the expression of all these inflammatory mediators in the leporine prostate [Citation28]. Another study by Credit et al. determined the effects of endogenous androgens, such as testosterone, DHT, and androstenedione (AED) on the cardiovascular system in a rat model. Evaluation of the localized cytokines such as interleukin-1 (IL-1), IL-6, and TNF-α on these tissues has been measured. The findings showed that the control animals had a two-fold increase in IL-6 production in the ventricles over testosterone-treated animals and approximately five-fold increase over DHT- and AED-treated animals, while other inflammatory cytokines IL-1 and TNF-α were not differentially expressed in the ventricles of experimental animals when compared with the controls. The production of IL-6 in the ventricles was decreased in animals exposed to sustained androgenic steroids and the decrease could possibly lead to increased blood flow, resulting in better oxygenation of the myocardium and improved cardiac function [Citation29].
Evidence from human studies
In a case control study of 70 elderly men (control group: n = 32; mean age: 66.2 ± 7.3 years old and case group: n = 38; mean age: 64.6 ± 9.1 years old) selected based on Doppler’s method, Yang et al. investigated the relationship between androgen and inflammatory cytokines level in men with atherosclerosis. The study found that free testosterone correlated negatively with atherosclerosis risk in elderly men. Low free testosterone level was associated with increased level of inflammatory cytokines, such as CRP, IL-6, and soluble intercellular adhesion molecule-1 (sICAM-1). Thus, these findings suggested that low free testosterones were associated with inflammation and they were believed to affect the progression of atherosclerosis in elderly men [Citation30]. A study on the relationship between serum levels of inflammatory cytokines and testosterone in men (n = 69; age: 59 ± 1 years old) with stable CAD was conducted by Nettleship et al. The increase in IL-1β and IL-10 were observed in men with 1, 2, 3-vessel CAD, while IL-6 was observed to decrease only in men with 3-vessel CAD. Besides, a significant stepwise increase in level of IL-1β was detected in eugonadal (testosterone ≥12 nmol/L), borderline hypogonadal (12 > testosterone ≥7.5 nmol/L), and hypogonadal (testosterone <7.5 nmol/L) men. This study suggested that testosterone might regulate IL-1β activity in men with CAD [Citation3].
In a 12-month prospective study by Corrales et al., administration of testosterone enanthate (150 mg every 15 days, i.m.) in type-2 diabetic men (n = 13, aged 52–76 years old) with partial androgen deficiency was associated with an almost complete abrogation of spontaneous ex vivo secretion of the IL-β, IL-6, and TNF-α by both circulating peripheral blood monocytes and dendritic cells [Citation31]. These findings were in consistent with a prospective intervention study by Corrales and coworkers, whereby a significant decrease in pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, by monocytes and dendritic cells after 12 months of TRT was observed. The authors suggested that the long term effects of exogenous androgen treatment withdrawal should be studied [Citation32].
Other pathological conditions
A summary of the relationship between testosterone level and inflammatory markers and the effects of testosterone administration on inflammatory markers in other pathological conditions (cancer, hemorrhage/ischemia, infection, and wound healing) in animal models and men were listed in .
Table 3. Summary of the association between testosterone level and supplementation with inflammatory markers in animals and humans with other pathologies.
Negative relationship between testosterone and inflammatory markers
Evidence from animal studies
A study by Angele et al. demonstrated the effects of sex steroids (dihydrotestosterone [DHT] alone or combination of DHT and 17-estradiol) on the release of Th1 and Th2 cytokines by splenocytes in female and male castrated rats following trauma-hemorrhage. The results showed that the production of interleukin-2 (IL-2), interleukin-3 (IL-3), and interferon-γ (IFN-γ) by the castrated animals with trauma-hemorrhage were significantly decreased after treatment with DHT. Administration of DHT in male mice subjected to trauma-hemorrhage also increased the release of interleukin-10 (IL-10) compared to shams after 14 days of treatment. This study indicated that DHT might exhibit immunodepressive effects in castrated males and could be a useful approach for modulating the release of Th1 and Th2 cytokines following trauma-hemorrhage [Citation33].
Gilliver et al. established a rodent wound healing model to investigate the effect of MK-434 as an agent blocking the conversion of testosterone to DHT in dermal repair. The systemic 5α-reductase inhibition treatment (1 mg/day) accelerated the healing process by suppressing the IL-6 production at the wound. The findings suggested that the inhibition of DHT production for 10 days could be a potential therapeutic target to hasten wound healing in elderly men [Citation34].
In an in vivo study by Mendes et al., the investigators evaluated the role of hormonal therapy with testosterone on the expression of cytokines in plasma and ventral prostate in the alcohol-related disorders. In plasma, the levels of IL-6 and IL-10 were unchanged while TNF-α and transforming growth factor-1 beta (TGF-β1) were increased after treatment with testosterone cypionate (5 mg/kg). However, in ventral prostate, there was no significant difference in the levels of all inflammatory cytokines (IL-6, IL-10, TNF-α, and TGF-β1) [Citation35].
In a different study, Guo et al. examined the effects of testosterone administration (40 µg/g) on female C57BL/6NCrl mice injected weekly with heat-killed Brucella abortus (HKBA) to induce anemia with a chronic inflammation. The results found that testosterone supplementation for 6 weeks up-regulated hepatic expression of inflammatory markers (TNF-α), but down-regulated IL-6. The development of anemia as a result of HKBA administration through induction of erythropoietin could activate bone marrow production of erythroferrone, which may in turn down-regulate the hepatic production of hepcidin to meet the iron demand for accelerated erythropoiesis. This explained the alteration in the expression of hepatic cytokines, whereby IL-6, known to induce hepcidin, was suppressed, whereas TNF-α, known to suppress hepcidin, was elevated [Citation36].
Patil et al. further supported that acute high doses of testosterone promoted renal injury after renal ischemia reperfusion and acute low dose of testosterone was protective in male Sprague-Dawley rats. Their findings demonstrated that low-dose testosterone (20 µg/kg) increased intrarenal IL-10 and reduced intrarenal TNF-α. Testosterone at the dosage of 50 µg/kg increased IL-10 but failed to reduce TNF-α. Meanwhile, higher-dose of testosterone (100 µg/kg) significantly increased both IL-10 and TNF-α compared to other groups [Citation37].
Inversely, a study by Poutahidis et al. demonstrated that mice consuming Lactobacillus reuteri in their drinking water sustained serum testosterone levels via blocking the pro-inflammatory interleukin-17 (IL-17) signaling [Citation38]. This agrees with the study by Dermitas et al., whereby suppression of inflammation could improve testosterone levels.
Evidence from human studies
A cross-sectional study by Burney et al. was conducted to measure level of testosterone in male cancer patients with and without cachexia (n = 140, age: ≥18 years old) and determine the relationship between testosterone, inflammation, and symptom burden. Results found that patients with cancer-cachexia had lower bioavailable testosterone but higher IL-6 and CRP levels. This study provided the insight that increased inflammation and reduced testosterone were associated with heavier symptom burden among cancer patients with cachexia [Citation10].
In case control study by Kaparianos et al., the levels of testosterone and inflammatory markers in 80 male control smokers and 82 male of chronic obstructive pulmonary disease (COPD) smokers were compared. This study found that male COPD smokers had lower levels of total testosterone and free testosterone compared to the male non-COPD smokers. In addition, CRP level was inversely correlated with testosterone in the COPD group [Citation39].
Another study by Bini et al. examined the implications of inflammatory response during tuberculosis in the disturbed production of gonadal steroids. Their findings revealed that tuberculosis patients had lower level of testosterone together with augmented IFN-γ, IL-6, and transforming growth factor-beta (TGF-β). Testicular histological sections also showed high expression of IL-1β, TNF-α, IL-6, and IFN-γ [Citation40].
Negligible relathionship between testosterone and inflammatroy markers
Evidence from human studies
A study by Maggio et al. on androgen derivative therapy treatment in male prostate cancer patients (n = 58, age: 69.9 years old) for 12 months found no significant changes of median serum level for pro-inflammatory cytokines or anti-inflammatory cytokine such as IL-10 [Citation12]. A study performed by Zhao et al. investigated the association of endogenous testosterone in Chinese men (young, n = 289, mean age: 21.0 years old and elderly, n = 4,212; age: ≥50 years old) with systemic inflammatory markers using a separate-sample Mendelian randomization analysis to minimize reverse causality. In this study, they have found no association between endogenous testosterone with systemic inflammation markers (hsCRP). Thus, they concluded that their findings did not corroborate any anti-inflammatory effects of testosterone on chronic diseases resulting from reduced low-grade systemic inflammation [Citation41]. In a placebo-controlled double blind randomized trial, Huang and coworkers demonstrated the effects of testosterone replacement on metabolic and inflammatory markers in non-diabetic men (n = 64; age: 18–64 years old) for 14 weeks. They found that testosterone treatment (5 g once daily, transdermal gel) did not significantly change the level of inflammatory marker and was not related to the increase of testosterone concentrations [Citation42].
Discussion
Aside from its main function as a sex hormone in men, testosterone may play an important key in modulating the inflammation. Since chronic inflammation is the cause for various pathological conditions, such as heart disease and cancer, endogenous testosterone could predict these diseases. Inversely, excessive inflammation can affect the function of Leydig cell and reduce testosterone production since they are particularly sensitive to inflammation [Citation40]. The literature on the relationship between testosterone and inflammation was heterogenous, with studies demonstrating either beneficial or negligible effects of testosterone in reducing inflammation markers.
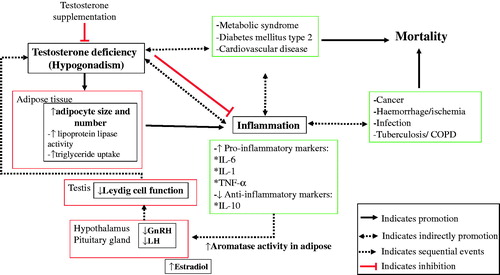
The multidirectional relationship between testosterone, inflammation and diseases is highly influenced by adipose tissue, which increases the activity of aromatase (enzyme involved in converting testosterone to estradiol) summarized in . This conversion directly inhibits the hypothalamic–pituitary axis and eventually decreased testosterone production. Visceral fat is an active secretory tissue producing inflammatory cytokines, adipokines, biochemical modulators, and other pro-inflammatory factors including IL-6, IL-1β, plasminogen activator inhibitor-1, and TNF-α that act as factors contributing to systemic and peripheral vascular inflammation and dysfunction. Adipose tissue also produces leptin that have a negative feedback effect on the hypothalamic–pituitary–gonadal axis through inhibition of gonadotropins on the Leydig cells of the testes, resulting in decreased androgen production. Reduced testosterone level in the tissues facilitates TG storage in adipocytes by increasing the activity of lipoprotein lipase. Elevated TG is an important risk factor that may contribute to the development of MetS, type 2 diabetes, and cardiovascular diseases. Inflammation also play an important role in other diseases such as cancer, hemorrhage, infection and tuberculosis. Concomitantly, increasing testosterone and decreasing estradiol levels through the use of agents like aromatase inhibitor will reduce inflammation and reduce the risk of several pathological conditions. TRT also improves glycemic control of diabetic patients by decreasing fasting blood glucose and hemoglobin A1c level [Citation43]. Finding also suggested that TRT in hypogonadal patients have a protective effect against high-grade prostate cancer in terms of lowering grading (Gleason score) and staging (tumor stage) [Citation44]. Testosterone also ameliorates features of MeS and decreases liver enzymes (alanine transaminase, aspartate transaminase and CRP) in patients [Citation45].
A study by Crisostomo and coworkers reported that testosterone enhanced inflammation through the increase in expression of active p38 and SPAK/JNK, which are the signaling proteins associated with myocardial inflammation in female and male castrated rats, after given a testosterone infusion before ischemia [Citation46]. However, in vitro study showed that toll-like receptor-4 expression in macrophages isolated from orchidectomized mice after testosterone replacement significantly decreased their inflammatory cytokine release compared with orchidectomized control [Citation47]. Toll-like receptors are crucial in alerting the immune system to trigger the cancer formation. Thus, this study suggested that testosterone decreased the inflammatory response in mice, which might affect various immune responses favorably. A study by Corcoran et al. further showed that physiological (10 nM) and pharmacological (100 nM) testosterone levels significantly reduced the gene and protein expression of TNF-α in human monocyte-derived macrophage derived from males and females subjects [Citation48].
Additionally, Bebo et al. also found that testosterone significantly decreased the interferon-c (IFN-c)/IL-10 ratio by decreasing IFN-c and Th1 pro-inflammatory cytokines as well as increasing IL-10 concentration in autoimmune encephalomyelitis in mice [Citation49]. This suggests that testosterone attenuates the IFN-c inflammatory pathway and enhances the IL-10 pathway. A similar study by Liva and Voskuhl examined the inflammatory status in the isolated rat splenic cells of male and female mice treated with DHT or placebo. Splenic cells from testosterone-treated rodents showed increased IL-10 secretion compared with those from placebo group [Citation50]. These findings proved that IL-10 was produced primarily by CD4+ T lymphocytes, which express the androgen receptor. Hence, testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. Thus, this study proved that testosterone can act directly via androgen receptors on CD4+ T lymphocytes to increase IL-10 gene expression [Citation50]. A different study by Bellido et al. demonstrated that an increase in either testosterone or DHT inhibited IL-6 production in osteoblastic cell lines dependent on the androgen receptor, leading to a reduction in inflammation. Since IL-6 has pro-inflammatory properties, it is an important mediator of bone loss [Citation51]. These findings were consistent with previous study by Hofbauer et al. that showed testosterone and DHT at a 107 mol/L concentration inhibit IL-6 mRNA expression in osteoblasts [Citation52]. Hence, apart from the direct and indirect effect through aromatization to estrogen, testosterone might influence bone health through regulating inflammation.
Existing evidence showed that testosterone has an inverse relationship with inflammation. Treatment with testosterone is speculated to modulate the inflammation status of the patients and improve their conditions. Treating hypogonadal men with testosterone was found to decrease the risk of mortality at about 39% with a hazard ratio (HR) of 0.61 (0.42–0.88) compared to untreated hypogonadal men [Citation53]. Additionally, in a study of hypogonadal men with type II diabetes, men who were not treated with testosterone were found to have a two-fold high mortality risk with a HR of 2.02 (1.2–3.4) compared to treated men [Citation54].
Conclusion
The current literature suggests that testosterone may possess anti-inflammatory effects in vivo. This is derived mainly from two observations, i.e. (1) testosterone deficiency is associated with increased inflammatory cytokine levels; (2) testosterone supplementation reduces inflammatory cytokines levels. Testosterone may also modulate the secretion of cytokines from adipose tissues and immune cells to achieve its anti-inflammatory effects. However, the magnitude of the anti-inflammatory effects of testosterone is debatable because some studies show a non-significant relationship. Hence, the role of testosterone in chronic inflammatory diseases could not be confirmed. In addition, suppression of inflammation is shown to upregulate the production of testosterone. The casual relationship between testosterone and inflammation can only be confirmed in a longitudinal study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Maggio M, Basaria S, Ceda GP, et al. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28(11 Suppl Proceedings):116–119.
- Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318.
- Nettleship JE, Pugh PJ, Channer KS, et al. Inverse relationship between serum levels of interleukin-1beta and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39:366–371.
- Bobjer J, Katrinaki M, Tsatsanis C, et al. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8:e61466.
- Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347.
- Coelho M, Oliveira T, Fernandes R. State of the art paper Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191–200.
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556.
- Fui MNT, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16:223–231.
- Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106:S5–S78.
- Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700–E709.
- Grosman H, Fabre B, Mesch V, et al. Lipoproteins, sex hormones and inflammatory markers in association with prostate cancer. Aging Male. 2010;13:87–92.
- Maggio M, Blackford A, Taub D, et al. Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl. 2006;27:725–728.
- Norata GD, Tibolla G, Seccomandi PM, et al. Dihydrotestosterone decreases tumor necrosis factor-α and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–554.
- Dandona P, Dhindsa S, Chaudhuri A, et al. Hypogonadotrophic hypogonadism in type 2 diabetes, obesity and the metabolic syndrome. Curr Mol Med. 2008;8:816–828.
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2008;65:S140–S146.
- Kelly DM, Sellers DJ, Woodroofe MN, et al. Effect of testosterone on inflammatory markers in the development of early atherogenesis in the testicular-feminized mouse model. Endocr Res, 2013;38:125–138.
- Jia YL, Liu X, Yan JY, et al. The alteration of inflammatory markers and apoptosis on chronic prostatitis induced by estrogen and androgen. Int Urol Nephrol. 2015;47:39–46.
- Papadopoulos AD, Wardlaw SL. Testosterone suppresses the response of the hypothalamic-pituitary-adrenal axis to interleukin-6. Neuroimmunomodulation 2000;8:39–44.
- Wang M, Tsai BM, Kher A, et al. Role of endogenous testosterone in myocardial proinflammatory and proapoptotic signaling after acute ischemia-reperfusion. Am J Physiol. Heart Circ Physiol. 2005;288:H221–H226.
- Demirtas Sahin T, Yazir Y, Utkan T, et al. TNF-alpha antagonism with etanercept enhances penile NOS expression, cavernosal reactivity, and testosterone levels in aged rats. Can J Physiol Pharmacol. 2017;96(2):1–8.
- Kaplan SA, Johnson-Levonas AO, Lin J, et al. Elevated high sensitivity C-reactive protein levels in aging men with low testosterone. Aging Male. 2010;13:108–112.
- Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73:602–612.
- Chin KY, Ima-Nirwana S. The effects of testosterone deficiency and its replacement on inflammatory markers in rats: a Pilot study. Int J Endocrinol Metab. 2017;15:e43053.
- Ng MK, Liu PY, Williams AJ, et al. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22:1136–1141.
- Dougherty RH, Rohrer JL, Hayden D, et al. Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol. 2005;62:228–235.
- Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602.
- Maggio M, Snyder PJ, De Vita F, et al. Effects of transdermal testosterone treatment on inflammatory markers in elderly males. Endocr Pract. 2014;20:1170–1177.
- Vignozzi L, Morelli A, Sarchielli E, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol. 2012;212:71–84.
- Credit SL, Benghuzzi HA, Tucci M, et al. Localization of cytokines in heart ventricular and apex tissues exposed to sustained delivery of AED, T, and DHT using a rat model. Biomed Sci Instrum. 2002;38:95–100.
- Yang Y-M, Lv X-Y, Huang W-D, et al. Study of androgen and atherosclerosis in old-age male. J Zhejiang Univ Sci B. 2005;6:931–935.
- Corrales JJ, Almeida M, Burgo R, et al. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189:595–604.
- Corrales JJ, Almeida M, Miralles JM, et al. Persistence of androgenic effects on the production of proinflammatory cytokines by circulating antigen-presenting cells after withdrawal of testosterone treatment in aging type 2 diabetic men with partial androgen deficiency. Fertil Steril. 2009;92:311–319.
- Angele MK, Knöferl MW, Ayala A, et al. Testosterone and estrogen differently effect Th1 and Th2 cytokine release following trauma-haemorrhage. Cytokine 2001;16:22–30.
- Gilliver SC, Ashworth JJ, Mills SJ, et al. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722–732.
- Mendes LO, Scarano WR, Rochel-Maia SS, et al. Testosterone therapy differently regulates the anti- and pro-inflammatory cytokines in the plasma and prostate of rats submitted to chronic ethanol consumption (UChB). Am J Reprod Immunol. 2014;72:317–325.
- Guo W, Schmidt PJ, Fleming MD, et al. Effects of Testosterone on Erythropoiesis in a Female Mouse Model of Anemia of Inflammation. Endocrinology 2016;157:2937–2946.
- Patil CN, Wallace K, LaMarca BD, et al. Low-dose testosterone protects against renal ischemia-reperfusion injury by increasing renal IL-10-to-TNF-α ratio and attenuating T-cell infiltration. Am J Physiol Renal Physiol. 2016;311:F395–F403.
- Poutahidis T, Springer A, Levkovich T, et al. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9:e84877.
- Kaparianos A, Argyropoulou E, Efremidis G, et al. Sex hormone alterations and systemic inflammation in a group of male COPD smokers and their correlation with the +138 insA/delA endothelin-1 gene polymorphism. A case-control study. Eur Rev Med Pharmacol Sci. 2011;15:1149–1157.
- Bini EI, D’Attilio L, Marquina-Castillo B, et al. The implication of pro-inflammatory cytokines in the impaired production of gonadal androgens by patients with pulmonary tuberculosis. Tuberculosis (Edinb). 2015;95:701–706.
- Zhao J, Jiang C, Lam TH, et al. Genetically predicted testosterone and systemic inflammation in men: a separate-sample Mendelian randomization analysis in older Chinese men. PLoS One. 2015;10:e0126442.
- Huang G, Travison T, Maggio M, et al. Effects of testosterone replacement on metabolic and inflammatory markers in men with opioid-induced androgen deficiency. Clin Endocrinol. 2016;85:232–238.
- Saad F, Yassin A, Almehmadi Y, et al. Effects of long-term testosterone replacement therapy, with a temporary intermission, on glycemic control of nine hypogonadal men with type 1 diabetes mellitus–a series of case reports. Aging Male. 2015;18:164–168.
- Yassin A, Salman M, Talib RA, et al. Is there a protective role of testosterone against high-grade prostate cancer? Incidence and severity of prostate cancer in 553 patients who underwent prostate biopsy: a prospective data register. Aging Male. 2017;20:125–133.
- Haider A, Gooren L, Padungtod P, et al. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Exp Clin Endocrinol Diabetes. 2009;118:167–171.
- Crisostomo PR, Wang M, Wairiuko GM, et al. Brief exposure to exogenous testosterone increases death signaling and adversely affects myocardial function after ischemia. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1168–R1174.
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432–437.
- Corcoran MP, Meydani M, Lichtenstein AH, et al. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206:217–224.
- Bebo BF, Jr., Schuster JC, Vandenbark AA, et al. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol. 1999;162:35–40.
- Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067.
- Bellido T, Jilka RL, Boyce BF, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95:2886–2895.
- Hofbauer LC, Ten RM, Khosla S. The anti‐androgen hydroxyflutamide and androgens inhibit interleukin‐6 production by an androgen‐responsive human osteoblastic cell line. J Bone Miner Res. 1999;14:1330–1337.
- Shores MM, Smith NL, Forsberg CW, et al. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058.
- Muraleedharan V, Marsh H, Kapoor D, et al. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169:725–733.