Abstract
The aim of this study is to investigate the expression of the luteinizing hormone/choriogonadotropin (LHCG) receptor in the human penis to see, if the luteinizing hormone (LH) effects are possible in the spongious and cavernous tissue of the penis. The number of men with erection disturbances increases significantly simultaneously with the elevated LH concentrations between 40 and 70 years. It is possible that the elevated LH concentrations may influence locally the erectile mechanisms. The precondition for this is the expression of LHCG receptors in the penis. Penile tissue was obtained from three patients undergoing total or partial penectomy due to a rectal cancer with secondary penile metastasis or squamous cell carcinoma of the penis. Immunohistochemistry was used for the detection of the LHCG receptor. Positive immunoreaction for LHCG receptors was discovered in the endothelial cells of cavernous spaces in the corpus cavernosum and corpus spongiosum penis, also in the endothelial cells of the capillary walls in all patients. Our results show that LHCG receptor is expressed in the spongious and cavernous tissue of the human penis. This finding suggests that LH can affect the spongious and cavernous tissue in human and play a significant role in the development of erectile dysfunction among the aging men.
Introduction
The luteinizing hormone/choriogonadotropin receptor (LHCGR) belongs to the group of glycoprotein hormone receptors (GpHRs) [Citation1,Citation2]. LH and hCG bind both to the LHCGR [Citation3]. Similar to other G-protein coupled receptors, LHCGR is anchored within the cell membrane by seven transmembrane domains [Citation4,Citation5]. Locating on the short arm of chromosome 2 (2p21), the human LHCGR gene consists of 11 exons and 10 introns, complemented by the primate-specific exon 6A [Citation6,Citation7]. Inactivating and disease-causing mutations of the LHCGR gene have been described [Citation8,Citation9]. Besides Leydig cells [Citation10,Citation11] the LHCGR is expressed by many cell types, including the corpus luteal cells of the ovary [Citation12], the decidua [Citation13], the myometrium [Citation14], the uterine vasculature [Citation15], the smooth muscle and endothelial cells of umbilical arteries and vein [Citation16], the embryonic stem cells [Citation17], the trophoblasts [Citation18], and some of the brain cells [Citation12,Citation19]. In the testis, LHGCRs are expressed during the fetal life, postnatally, at puberty, and also throughout adult life [Citation20]. This makes it possible for the pituitary LH to stimulate testosterone production in Leydig cells during the normal male embryogenesis and also after puberty, leading to induction and maintenance of secondary sexual features, including penile growth.
It has been previously shown that LHCGR is present in the mouse penis [Citation21]. Positive immunoreaction was found in the nuclei of urethral epithelium, in the endothelial cells of cavernous spaces in the corpus spongiosum and corpus cavernosum penis. The expression of LHCG receptors in the mouse penis was also confirmed by western blot analyses and by the quantitative RT-PCRs [Citation21].
As it is predicted that by 2025 roughly 322 million men are affected world-wide by erectile dysfunction [Citation22] and as hypogonadism [Citation23,Citation24] and subclinical hypogonadism (LH >6.0 IU/L and T > 9.8 nmol/L [Citation9,Citation25] and 31% of 41–50, 51–60, and 61–70-year-old men, respectively [Citation26]; LH >8.0 IU/L and T > 12 nmol/l [Citation25]), where the LH serum levels are higher than normally, are a frequent problem in the middle- and older-aged men, who also often have erectile dysfunction, it should be asked, whether the high LH levels of the aging men are involved in the development of impotence directly through actions on the penile tissue. As the prerequisite for this would be the expression of LHCG receptors in the penile tissue, the expression of LHCG receptors was studied in the human penile tissue in the present study to see, if LH action in this tissue is possible and if the age-associated increase in the LH levels could be involved in erectile dysfunction.
Materials and methods
Penile tissue was obtained from three patients undergoing partial or total penectomy either due to rectal cancer or squamous cell carcinoma of the penis. The patients were being treated at the Tampere University Hospital.
Two patients (66-year-old and 64-year-old) were undergoing total penectomy due to rectal cancer with secondary penile metastasis and one 83-year-old patient with squamous cell carcinoma of the penis was undergoing partial penectomy.
Samples from corpus cavernosum and corpus spongiosum penis were fixed in 4% formalin overnight at 4°C. After fixation, the samples were stored in 70% ethanol until embedding in paraffin.
Immunohistochemistry
The 5 µm sections were cut, deparaffinized and treated with 0.9% H2O2 to inactivate endogenous peroxidase. The sections were then treated with Dako REAL Antibody Diluent (S2022; Dako Denmark A/S, Glostrup, Denmark) to block nonspecific binding. After blocking, the sections were incubated with the rabbit polyclonal antibody to luteinizing hormone receptor (LHCGR) (C-term) (SP4594P, Acris Antibodies) overnight at 4 °C.
The immunoaffinity-purified antibody used was validated by Kokk et al. [Citation21] in the mouse using immunohistochemistry, western blotting, and qRT-PCR showing LHR antigens at M(r) = 97.4 and 78 kD. The usability of the antibody was confirmed by specific expression of LHR in the same spongious tissue in qRT-PCR. In the present study, the difficulty in obtaining human penile tissue prevented repeating the same with the human tissue, but the earlier validation of the antibody using several methods in the mouse together with the information of the producer’s data sheet (Acris Antibodies) about the usability of the antibody in immunohistochemistry in the human tissue was considered as sufficient for the present study.
Primary antibody dilution was 1:500. Visualization of the primary antibody was performed using the commercial kit Dako REAL™ EnVision™ Detection System, Peroxidase/DAB+, Rabbit/Mouse (K5007; Dako Denmark A/S, Glostrup, Denmark). Washing steps in-between were done in phosphate buffered saline (PBS) which contained 0.07% Tween 20 as the detergent. Toluidine blue (Applichem, Darmstadt, Germany) was used for background staining. No immunohistochemical staining was noted in negative controls where the primary antibody was omitted.
Results
Positive immunoreaction for LHCG receptors in the endothelial cells of cavernous spaces both in the corpus spongiosum penis () and the corpus cavernosum penis () was found in tissue samples of all patients. Positive immunoreaction was also present in the endothelial cells of capillary walls ().
Figure 1. Expression of LHCG receptors in the human penis. Positive cells are pointed by arrows. Note the presence of LHCG receptors in the endothelial cells of the cavernous spaces in corpus spongiosum penis (A) and corpus cavernosum penis (B). Positive immunoreaction is also present in the endothelial cells of the capillary wall (C). No positive cells were present in negative controls (D). All photographs were taken using the ×100 objective. The scalebar is on the lower right corner of the figures.
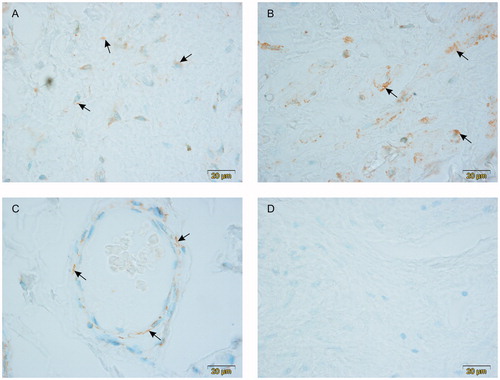
No positive cells were visible in negative controls ().
Discussion
The present results show that besides the mouse penis, LHCGR is also expressed in the human penis. At present, it is not yet clear, what functions LHCGR may have in the penis, but it is possible that the aging-associated increased serum LH concentrations [Citation26] regulate the function of the endothelial cells of the corpus spongiosum and cavernosum expressing the LHCGR. This finding is supported by the observations of Yu et al. [Citation27], who confirmed the influence of increased age and LH on the aging male symptoms and on the five-item version of the international index of erectile function scores.
Erectile dysfunction as an inability to achieve normal erection and to keep it for the sufficient time can be the result of many health conditions, including neurologic, psychogenic, vascular, urogenital, and hormonal abnormalities. Erectile dysfunction can also develop post-traumatically and after surgery [Citation28]. Potency disturbances possibly also have a genetic dimension, as e.g. expression of the DM1 protein kinase (DMPK) gene may be associated with many age-related signs, including hypogonadism and erectile disorders and there can be CTG repeat expansions in this gene affecting its function [Citation29].
Until now it has not been suggested that LH could directly affect the penile tissue of the aging men. This is, however, a serious alternative after it has been demonstrated that LHCG receptors are expressed in the endothelium of the spongious tissue of the penis in two species, the mouse [Citation21] and the human; that a significant proportion of the aging men are in a subclinical or clinical hypogonadism with their LH concentrations in the serum elevated [Citation25,Citation26,Citation30,Citation31]; and that LH influences the five-item version of the international index of erectile function scores [Citation27]. Functional tests would be needed to study this hypothesis further.
However, the late-onset hypogonadism may be a result of testicular failure or hypothalamic-pituitary failure. The first form is bound up with low T and high luteinizing hormone levels. The second form is often related with overweight, but also with chronic diseases (the metabolic syndrome, hypertension, inflammatory arthritis, diabetes, chronic obstructive lung disease, heart failure, etc.). The latter form is more common and shows low T and low or inappropriately normal LH [Citation32]. According to Bunch et al. [Citation33], 225 testosterone-deficient patients (testosterone <300 ng/dl) were found amongst the erectile dysfunction patients of their study and in 29 of them (12.9%) secondary hypogonadism based on a LH <13 mlU/ml was diagnosed. These findings may indicate that the suggested direct effect of high LH on the penile tissue is not the only factor involved.
If both of the above-mentioned forms of hypogonadism are equally associated with erectile dysfunction, is not known, but the present evidence together with the data of Yu et al. [Citation27] and Härkönen et al. [Citation26] would suggest that high LH associated with clinical or subclinical primary hypogonadism of the aging male might possibly affect the penile endothelial cells through the penile LHCGR and thus perhaps be a factor in the development of potency disturbances.
In fact, in our earlier preliminary studies, this suggestion was supported by the observation that there was a statistically significant positive correlation between s-LH and the reported severity of decreased potency among 61–70-year-old men (p < 0.05, n = 90; the subjects graded their potency disturbance in a form interview before their LH and T levels were measured; [Citation34]) and between s-LH and the reported severity of decreased frequency of morning erections in the same age group (p < 0.05, n = 90; [Citation34]), but not among the 40–50-years-old or the 51–60-years old men. Also these earlier observations together with the findings of Yu et al. [Citation27] and the present observations on the protein level LHCGR expression in the human penile endothelium would indicate that subclinical or clinical hypogonadism characterized by elevated LH may possibly affect the penile function as suggested. However, while in the so far published genome wide association studies for erectile dysfunction, LH or LHCGR have not been reported to be associated with erectile dysfunction [Citation35–37], the FSH receptor has been [Citation36] and as FSH increases with age like LH [Citation24], it is possible that the increased FSH leads to erectile dysfunction instead or together with the increased LH, but at present, there is no data about FSH receptor expression in the penile tissue available.
On the basis of the present knowledge, it cannot be excluded that the aging-associated high LH levels of the male may have an effect on the endothelial cells of the spongious tissue through the LHCG receptors. Furthermore, as the LH and FSH receptors belong to the same superfamily of G-coupled glycoprotein hormone receptors [Citation1,Citation2] with the same α subunit and 40% homology in the sequence of the ectodomains and 70% homology in the functionally significant serpentine domain of the genes of their β subunits [Citation38] and as it is known that chorionic gonadotrophin in high concentrations can inappropriately stimulate the FSH receptor [Citation39] and the TSH receptor [Citation40], it is not excluded that the same may also occur vice versa, if the FSH or the TSH levels are high. The role of high FSH may be strengthened by the observations of Santi et al. [Citation41], who reported a case of an atypical giant pituitary adenoma secreting follicle-stimulating hormone in 55-year-old patient with fatigue, loss of libido, and erectile disorder. It has also been reported that the wild-type LHCG receptors and the wild-type FSH receptors can form dimers [Citation42], suggesting a more complex interaction between these two G-protein-coupled transmembrane receptors. However, the present results demonstrate that the high circulating LH of the aging male could possibly bind to the LHCG receptor expressed by the penile endothelial cells and perhaps induce some effects in their functions. Experimental arrangements to test this possibility should be organized in the future.
Last but not the least, it should be remembered that the postmenopausal women as well as women approaching ovulation have high serum LH levels and the pregnant women have high levels of serum hCG, suggesting that high female LH or hCG concentrations in the vaginal excretions might bind to the penile LHCG receptors, thus possibly affecting the penile endothelial cell functions. However, the LHCG receptors are expressed by cells deep inside the penile tissue, suggesting that LH or hCG in the vaginal excretions may not reach these cells without active transport at some point between the penile surface and the spongious tissue [Citation43]. Further studies are indicated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ascoli M, Fanelli F, Segaloff DL. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev. 2002;23:141–174.
- Caltabiano G, Campillo M, De Leener A, et al. The specificity of binding of glycoprotein hormones to their receptors. Cell Mol Life Sci. 2008;65:2484–2492.
- Puett D, Angelova K, da Costa MR, et al. The luteinizing hormone receptor: insights into structure-function relationships and hormone-receptor-mediated changes in gene expression in ovarian cancer cells. Mol Cell Endocrinol. 2010;329:47–55.
- Rousseau-Merck MF, Misrahi M, Atger M, et al. Localization of the human luteinizing hormone/choriogonadotropin receptor gene (LHCGR) to chromosome 2p21. Cytogenet Cell Genet. 1990;54:77–79.
- Rousseau-Merck MF, Atger M, Loosfelt H, et al. The chromosomal localization of the human follicle-stimulating hormone receptor gene (FSHR) on 2p21-p16 is similar to that of the luteinizing hormone receptor gene. Genomics 1993;15:222–224.
- Atger M, Misrahi M, Sar S, et al. Structure of the human luteinizing hormone-choriogonadotropin receptor gene: unusual promoter and 5' non-coding regions. Mol Cell Endocrinol. 1995;111:113–123.
- Kossack N, Simoni M, Richter-Unruh A, et al. Mutations in a novel, cryptic exon of the luteinizing hormone/chorionic gonadotropin receptor gene cause male pseudohermaphroditism. PLoS Med. 2008;5:e88.
- Bruysters M, Christin-Maitre S, Verhoef-Post M, et al. A new LH receptor splice mutation responsible for male hypogonadism with subnormal sperm production in the propositus, and infertility with regular cycles in an affected sister. Hum Reprod. 2008;23:1917–1923.
- Latronico AC, Arnhold IJ. Inactivating mutations of the human luteinizing hormone receptor in both sexes. Semin Reprod Med. 2012;30:382–386.
- Eacker SM, Agrawal N, Qian K, et al. Hormonal regulation of testicular steroid and cholesterol homeostasis. Mol Endocrinol. 2008;22:623–635.
- Ingles SA, Liu SV, Pinski J. LHRH and LHR genotypes and prostate cancer incidence and survival. Int J Mol Epidemiol Genet. 2013;4:228–234.
- Bukovsky A, Indrapichate K, Fujiwara H, et al. Multiple luteinizing hormone receptor (LHR) protein variants, interspecies reactivity of anti-LHR mAb clone 3B5, subcellular localization of LHR in human placenta, pelvic floor and brain, and possible role for LHR in the development of abnormal pregnancy, pelvic floor disorders and Alzheimer's disease. Reprod Biol Endocrinol. 2003;1:46.
- Licht P, von WM, Berkholz A, et al. Evidence for cycle-dependent expression of full-length human chorionic gonadotropin/luteinizing hormone receptor mRNA in human endometrium and decidua. Fertil Steril. 2003;79:718–723.
- Phillips RJ, Tyson-Capper Née Pollard AJ, Bailey J, et al. Regulation of expression of the chorionic gonadotropin/luteinizing hormone receptor gene in the human myometrium: involvement of specificity protein-1 (Sp1), Sp3, Sp4, Sp-like proteins, and histone deacetylases. J Clin Endocrinol Metab. 2005;90:3479–3490.
- Toth P, Li X, Rao CV, et al. Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994;79:307–315.
- Rao CV, Li X, Toth P, et al. Novel expression of functional human chorionic gonadotropin/luteinizing hormone receptor gene in human umbilical cords. J Clin Endocrinol Metab. 1993;77:1706–1714.
- Gallego MJ, Porayette P, Kaltcheva MM, et al. The pregnancy hormones human chorionic gonadotropin and progesterone induce human embryonic stem cell proliferation and differentiation into neuroectodermal rosettes. Stem Cell Res Ther. 2010;1:28.
- Prast J, Saleh L, Husslein H, et al. Human chorionic gonadotropin stimulates trophoblast invasion through extracellularly regulated kinase and AKT signaling. Endocrinology 2008;149:979–987.
- Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. 2010;8:102.
- Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol. 1998;60:461–496.
- Kokk K, Kuuslahti M, Keisala T, et al. Expression of luteinizing hormone receptors in the mouse penis. J Androl. 2011;32:49–54.
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56.
- Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025.
- Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metab Clin Exp. 1997;46:410–413.
- Foresta C, Calogero AE, Lombardo F, et al. Late-onset hypogonadism: beyond testosterone. Asian J Androl. 2015;17:236–238.
- Härkönen K, Huhtaniemi I, Mäkinen J, et al. The polymorphic androgen receptor gene CAG repeat, pituitary-testicular function and andropausal symptoms in ageing men. Int J Androl. 2003;26:187–194.
- Yu XH, Zhao J, Zhang SC, et al. The impact of age, BMI and sex hormone on aging males' symptoms and the international index of erectile function scores. Aging Male. 2017;20:235–240.
- Celik O, Ipekci T, Akarken I, et al. To evaluate the etiology of erectile dysfunction: What should we know currently? Arch Ital Urol Androl. 2014;86:197–201.
- Brisson D, Houde G, St-Pierre J, et al. The pleiotropic expression of the myotonic dystrophy protein kinase gene illustrates the complex relationships between genetic, biological and clinical covariates of male aging. Aging Male. 2002;5:223–232.
- Chen RY, Ng KK. Self-referred older Asian males in a men's health clinic: the inter-relationships between androgens, metabolic parameters and quality of life measures. Aging Male. 2010;13:233–241.
- Li JH, Yu XH, Zheng JB, et al. The reproductive health indices and sex hormone levels in middle-aged and elderly Chinese men. Aging Male. 2016;19:143–147.
- Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202.
- Bunch TJ, Abraham D, Wang S, et al. Pituitary radiographic abnormalities and clinical correlates of hypogonadism in elderly males presenting with erectile dysfunction. Aging Male. 2002; 5:38–46.
- Pöllänen P, Mäkinen J, Irjala K, et al. Validation of two aging male symptom questionnaires, the Turku 3-question and the Heinemann questionnaires. CV%’s, hormone correlations, associations with diseases, medications, social factors and health behaviour. University of Turku. 2000.
- Hotaling JM, Waggott DR, Goldberg J, et al. Pilot genome-wide association search identifies potential loci for risk of erectile dysfunction in type 1 diabetes using the DCCT/EDIC study cohort. J Urol. 2012;188:514–520.
- Kerns SL, Ostrer H, Stock R, et al. Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:1292–1300.
- Kerns SL, Stock R, Stone N, et al. A 2-stage genome-wide association study to identify single nucleotide polymorphisms associated with development of erectile dysfunction following radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:e21–e28.
- Costagliola S, Urizar E, Mendive F, et al. Specificity and promiscuity of gonadotropin receptors. Reproduction. 2005;130:275–281.
- Ludwig M, Gembruch U, Bauer O, et al. Ovarian hyperstimulation syndrome (OHSS) in a spontaneous pregnancy with fetal and placental triploidy: information about the general pathophysiology of OHSS. Human Reprod. 1998;13:2082–2087.
- Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–433.
- Santi D, Spaggiari G, Casarini L, et al. Central hypogonadism due to a giant, “silent” FSH-secreting, atypical pituitary adenoma: effects of adenoma dissection and short-term Leydig cell stimulation by luteinizing hormone (LH) and human chorionic gonadotropin (hCG). Aging Male. 2017;20:96–101.
- Segaloff DL. Regulatory processes governing the cell surface expression of LH and FSH receptors. Subcell Biochem. 2012;63:113–129.
- Ghinea N, Mai TV, Groyer PMT, et al. How protein hormones reach their target cells. Receptor-mediated transcytosis of hCG through endothelial cells. J Cell Biol. 1994;125:87–97.
