Abstract
Aim: In this study our aim was to evaluate the nosocomial infections and to understand factors affecting the cost of used antibiotics in palliative care unit.
Materials and methods: Between 2016 and 2017, 113 patients were included in the study in palliative care unit of University of Health Sciences Bursa Yuksek Ihtisas Research and Training Hospital. Patients medical records were analyzed retrospectively for nosocomial infections, chronic diseases, presence of decubitis ulcers, opioid use, enteral, parenteral feedings, mortality and antibiotic cost.
Results: Nosocomial infections were observed in 74.3% of the cases and 92.0% of patients used antibiotics. The mean duration of antibiotic use was 23.13 ± 18.06 days; and the average antibiotic cost was 2009.72 ± 2153.37 TL. Length of stay, male sex, presence of decubitus ulcers, tracheostomy, enteral and parenteral nutrition significantly increased antibiotic cost. Antibiotic cost and mortality were not related statistically.
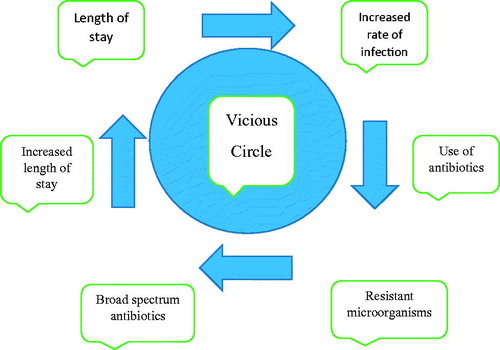
Conclusions: A vicious circle in palliative care involves the following order: length of stay, increased rate of infection, use of antibiotics, infection with resistant microorganisms, use of broad spectrum antibiotics, increased length of stay; all affecting each other. Therefore, using antibiotics for aggressive treatment of infections in palliative care is contraindicated as it opposes to real philosophy of palliative care.
Keywords:
Introduction
Palliative care (PC) is related to the quality of life of patients and their families associated with life-threatening illness, through the prevention and relief of suffering by early identification and treatment of pain and physical, psychosocial problems [Citation1]. The aim of PC is helping people for dying process [Citation2], with an increasing aging population, life-threatening chronic diseases and the need for PC is increasing [Citation3]. Terminally sick patients are very prone to infections. These patients have many symptoms and associated comorbid conditions. Infection increases this symptom burden and further reduces quality of life [Citation4]. Managing infection in the PC setting is very complex and has been increasingly evaluated [Citation5]. Appropriate management of infection results in palliative symptom control [Citation4]. Nosocomial infections result in morbidity, mortality and cost of care is greater than that would be expected from the patient’s underlying diseases alone. Case control studies clearly demonstrated that nosocomial infections significantly prolong hospitalization. Moreover, charges of the prolonged stay and resulting increased antibiotic use account for most of the costs related to nosocomial infection [Citation6]. The most common complications experienced by terminal patients are infections. Almost 90% of terminal patients hospitalized are treated with antibiotics during the week before their death [Citation7]. Two retrospective reviews reported that it is common that incurably dying patients receive empirically systemic antibiotics in their last weeks of life, even in patients with “do not resuscitate” orders [Citation8]. The goal of antibiotic therapy in PC should be symptom control, but the aim in the acute medical and surgical setting is the decrease of mortality and morbidity. When the decision to start treatment is made, it is helpful to be aware of pathogen and antibiotic susceptibility patterns to assist with antibiotic selection [Citation9]. But in PC there comes a time when treatment may do more harm than good as far as increasing resistance and antibiotic cost are concerned [Citation10]. There should be a consensus for structured guidelines about antibiotic use in this population [Citation11]. In this study, the aim was to evaluate nosocomial infections and to understand factors affecting the cost of used antibiotics in a palliative care unit (PCU).
Materials and methods
Between 2016 and 2017, 113 patients were included in the study in PCU of University of Health Sciences Bursa Yuksek Ihtisas Research and Training Hospital. Approval of ethics committee with the number 2011-KAEK-25 2018/03-10 was received. Patients medical records were analyzed retrospectively for nosocomial infections, isolated microorganisms, demographical features, chronic diseases, presence of decubitis ulcers, opioid use, enteral, parenteral feedings, mortality and antibiotic cost in TL(Turkish Liras) (1TL: 3,65 Dollars). General features of the patients were evaluated and the relation of antibiotic cost with these features was compared according to statistical methods mentioned below.
Statistical methods
For statistical analysis, NCSS (Number Cruncher Statistical System) 2007 (Kaysville, UT) program was used. Mann–Whitney U test was used for two group quantitative data comparison. Kruskal–Wallis test was used for three or more group and Bonferroni Dunn’s test was used for two groups comparison. Spearman’s correlation analysis was used for evaluation of correlation among variables. p < .05 was considered significant.
Results
The study was carried out in 2016–2017 in PCU of University of Health Sciences Bursa Yuksek Ihtisas Research and Training Hospital with a total of 113 patients 43.4% (n = 49) female and 56.6% (n = 64) male, respectively. The age ranged from 18 to 97 years, with an average of 67.03 ± 20.42 years. Length of stay ranged from 3 to 114 days with a mean of 30.22 ± 22.90 days (). For the nosocomial infections developed in patients the rate of urinary tract infection was 45.1% (n = 51), respiratory infection rate was 42.5% (n = 48), bloodstream infection rate was 16.8% (n = 19), skin and soft tissue infection rate was 27.4% (n = 31). Other infections were observed in 5.3% (n = 6) of the patients. While 25.7% (n = 29) of the cases did not have any infection; 30.1% (n = 34) had one infection, 29.2% (n = 33) had two infections and 15.0% (n = 17) had three or more than three infection types. The nosocomial infection rate of palliative care unit was 74.3% (). The rate of microorganisms causing infection in patients was 28.3% (n = 32) Klebsiella spp., 27.4% (n = 31) Pseudomonas spp., 25% (n = 22.1) Staphylococcus spp., 18.6% (n = 21) Acinetobacter spp with 18.6% (n = 21) E. coli 14.2% (n = 16) Candida spp. While the percentage of ESBL producing microorganisms was 32.7%, the rate of CRE and the MRSA was 23.9% and 15.9%, respectively (). 92.0% (n = 104) of the patients used antibiotics. The duration of antibiotic use ranged from 2 to 84 days, with an average of 23.13 ± 18.06 days; the cost of antibiotics ranged from 4 to 9713.2 TL and the average was found to be 2009.72 ± 2153.37 TL (). When the average antibiotic cost is evaluated according to nosocomial infections the average cost of antibiotic is 2342.47 ± 2199.25 TL in patients with urinary tract infection, 3061.93 ± 2372.72 TL in patients with respiratory infection, 3027.92 ± 2151.07 TL in patients with bloodstream infection, 3298.49 ± 2474.82 TL in patients with skin and soft tissue infection, 2736.75 ± 2753.01 TL in other infections. The average antibiotic cost of patients in terms of the number of infections who have one nosocomial infection was 1090.70 ± 1082.71, in patients with two infections was 2558.12 ± 2484.03 in patients with three or more infections 4318.14 ± 1872.43 TL (). A statistically significant difference was found between antibiotic cost and gender (p = .002; p < .01). Male cases had higher antibiotic cost than female. No statistically significant relationship was present between age and antibiotic cost. (p > .05). There was a statistically significant correlation with a level of 69.7% between length of stay and antibiotic cost (antibiotic cost increases as hospitalization time increases) (r:0.697; p = .001; p < .01) (). The antibiotic cost according to the number of decubitus ulcers shows statistically significant difference (p = .008; p < .01). As a result of binary comparisons; the antibiotic cost of patients with decubitus ulcers in two regions was higher than the cases without ulcers (p = .024; p < .05). No statistically significant difference was found in other binary comparisons (p > .05). According to the presence of decubitus ulcers, the antibiotic costs of the cases differ statistically (p = .008; p < .01); The antibiotic cost in patients with decubitus ulcers is higher than that in those with no decubitus ulcer (). There was no statistically significant difference in antibiotic cost according to the presence of Alzheimer's disease, malignancy, heart failure, diabetes, hypertension, hyperlipidemia, cerebrovascular diseases, coronary artery disease, Parkinson’s and chronic renal failure (p > .05). According to the presence of chronic obstructive pulmonary disease (COPD), the antibiotic costs of the cases were not statistically significant (p = .062; p > .05); but it was noteworthy that the antibiotic cost was high in the group with COPD. When chronic thyroid disease was present, the antibiotic costs of the cases were not statistically significant (p = .088; p > .05); but the antibiotic cost is high in the group with chronic thyroid disease. According to the presence of chronic liver disease, the antibiotic costs of the cases showed statistically significant differences. (p = .028; p < .05); in the group with chronic liver disease, the cost of antibiotics were lower (). Although there were no statistically significant differences in the antibiotic cost of the patients according to admission (p = .063; p > .05); the antibiotic costs of the patients admitted to the PCU from the intensive care unit (ICU) were higher than the cases taken from home. According to the presence of central catheter, the antibiotic costs of the cases were not statistically different (p = .073; p > .05); but antibiotic costs of patients with central catheters are higher than those without central catheter. When tracheostomy was present antibiotic cost was statistically significant (p = .001; p < .01); the cost of antibiotics in patients undergoing tracheostomy is higher than those without tracheostomy. According to the use of opioids, the antibiotic costs of the cases did not differ significantly (p > .05). According to the nutritional status, the antibiotic costs of the cases differed statistically (p = .001; p < .01) As a result of bilateral comparisons; the antibiotic cost of enteral and parenteral nutrition group was higher than enteral group (p = .001) and parenteral group (p = .010) (p < .05). No statistically significant difference was found between the antibiotic costs of enteral and parenteral groups (p > .05) There was no statistically significant difference in the antibiotic costs of the patients according to the mortality (p > .05) ().
Table 1. Distribution of demographic features.
Table 2. Distribution of nosocomial infections.
Table 3. Distribution of microorganisms leading to nosocomial infections.
Table 4. Distributions of antibiotic duration and cost.
Table 5. Evaluation of antibiotic cost according to nosocomial infections.
Table 6. Evaluation of antibiotic costs according to demographic features.
Table 7. Evaluation of antibiotic costs according to decubitus ulcer.
Table 8. Evaluation of antibiotic costs according to the presence of chronic diseases.
Table 9. Evaluation of antibiotic costs according to some features.
Discussion
In this study, 74.3% patients had nosocomial infection and 92% of the patients used antibiotics and average age of the patients was 67.0 with an average hospital stay of 30.2 days. In 15% of the cases at least three or more different nosocomial infections were observed. Rate of infection is 74.3% in our PCU which is higher compared to previous studies. In a previous study the PC infection rate was 50% with an average stay of 29.8 days [Citation9]. In another study from a PC the mean age was 70.6 and length of stay was 27.2 days. With the increase in elderly population, the need and stay for PC also increase [Citation12]. Generally average length of stay in PC is around one month in many units. Thompson et al. performed a retrospective study with 145 patients of which 69.8% had infection, and 51.6% were treated empirically and Pereira J showed that 71.6% of diagnosed infections were treated with antibiotics [Citation9,Citation13]. Most frequent infection was urinary system infection and following infections in order were respiratory infection, bloodstream infection, skin and subcutaneous infection with percentages of 45.1%, 42.5%, 16.8% and 27.4%, respectively. In a previous study of Macedon et al. most frequent infection was urinary system infection followed with respiratory infection [Citation14]. Nagy et al. in a study in PC reported that most frequent infection was urinary system infection and secondly respiratory infection. Mortality was between 19 and 39% [Citation15]. In this study, overall mortality was 23.9%. In another study in PCU most frequent infection was urinary system infection followed by respiratory infection, skin and subcutaneous infection and blood stream infection with percentages of 39.2%, 36.5%, 12.2% and 5.4%, respectively [Citation9]. Rate of skin and subcutaneous infection in our study is 27.4% which is high compared to previous studies in the literature probably due to decubitus ulcers with a rate of 66%. This should be further evaluated in the future studies. Most frequently isolated microorganisms in our PCU in order were; Klebsiella spp., Pseudomonas spp., Staphylococcus spp., Acinetobacter spp., E. coli and Candida spp. In a previous study from a PCU most frequently isolated microorganisms were E. coli, Staphylococcus spp. and Enterococcus spp [Citation9]. In a study of hospice patients E. coli was the predominant organism cultured [Citation4]. In many PCU and ICU mostly resistant pathogens, MRSA, CRE and ESBL positive microorganisms are frequently isolated due to underlying chronic diseases, immunosuppressive old patients. Multidrug-resistant organisms are organisms resistant to one or more classes of antimicrobials. As a result these multidrug-resistant organisms complicate infection management in PC [Citation16,Citation17]. In our study ESBL, CRE and MRSA rates were 32.7%, 23.9% and 15.9%, respectively. As a result, infections with resistant microorganisms are leading to excessive antibiotic use in PC. End of life treatment is related with infections with resistant bacteria. Patients dying receive more antibiotics and develop more resistant microorganisms [Citation18]. These patients may represent a reservoir of resistant bacteria in the ICU. Oneschkuk et al. observed intensive antibiotic use in PC till last week of life [Citation19]. Antibiotics can be used for both symptom control and prolong life. Therefore, strategical aim for use of antimicrobials in PC should be different from other service patients. Costs of antibiotics are increasing due to prolonged and unnecessary use in PCU leading to increased stay and total cost of the patients. In our PCU 92.0% of patients used antibiotics with an average of 23.13 ± 18.06 days and average cost of 2009.72 ± 2153.37 TL. Stiel et al. observed 63.8% of the patients in PC used antibiotics [Citation20]. There are also many studies reporting frequent antibiotic use in end stage cancer patients in PC [Citation15]. Juthani et al. observed that close to 90% of hospitalized patients with advanced cancer receive antimicrobials during the week prior to death in a PCU [Citation7]. Treatment of infections with antibiotics especially in case of sepsis can increase the quality of the palliative cancer patients especially in last weeks of life. According to this previous study excessive antibiotics are somewhat worth using compared to harmful cost effects opposing to some literature [Citation21]. Although the most frequent infection was urinary system infection the highest average antibiotic cost compared to other nosocomial infections was skin and subcutaneous infection with an average cost of 3298.49 ± 2474.82 TL in our PC. This was directly related to decubitus ulcers. When patients had three or more nosocomial infection average antibiotic cost reached to 4318.14 ± 1872.43 TL. Antibiotic costs in male patients were more than female patients reaching to a statistical difference. There was no significant relation between age and antibiotic cost in our study. However White et al. found a relation between age and antibiotic use before [Citation22]. Length of stay was related significantly with increased antibiotic cost in our PC. Albrecht et al. observed that use of antibiotics increased the survival and increased length of stay led to more infections [Citation11]. In previous studies, economical analysis related to costs of nosocomial infections has been reported between 1018 and 2280 $. Jarvis et al. reported that the estimated average costs of nosocomial infections were 4947 $ for each pneumonia [Citation23–25]. Our study is unique for PC infections and resulting antibiotic cost. Antibiotic cost is also increasing in the presence of decubitus ulcers reaching to a statistical difference. This is because of skin and subcutaneous infections complicating the decubitus ulcers. It leads to highest average antibiotic cost mentioned before. As far as the underlying diseases of PC patients were concerned in spite of not reaching to a statistical significance patients with COPD in our study had relatively high average antibiotic cost of 2851.33 ± 2379.18 TL. In Varol et al. study from Turkey showed that the cost and use of antibiotics were higher than usual in COPD exacerbations requiring hospitalizations [Citation26]. Patients admitted to our PC from ICU had higher average antibiotic cost compared to patients admitted from home reaching to a statistical difference. This is probably due to intensive and broad spectrum antibiotic use before admission to ICU predisposing to patients to infections with multidrug-resistant organisms in PC. Result is of course increased cost of antibiotics. Strategies that aim to reducing costs in the ICU are of great interest [Citation27], because prolongation of hospitalization results in increased cost and adversely affects economic well-being [Citation28]. Patients with central catheter had higher average antibiotic cost of 2199.47 ± 2049.42 TL. Moreover, when patients had enteral and parenteral feeding it was observed that antibiotic cost was higher as these are known risk factors for developing nosocomial infection. Development of serious blood stream infections in patients receiving total parenteral nutrition have been known for long time in literature [Citation29]. The use of tracheostomy in palliative care can be a useful component to a patient’s airway control [Citation30]. In our study, patients with tracheostomy had higher average antibiotic cost reaching to a statistical difference, clinically indicated. Tracheostomy is an effective procedure in the management of many conditions but respiratory infections can occur [Citation31]. This can lead to high antibiotic use and cost like it was in our study reaching to 2492.90 ± 2110.12 TL. According to the use of opioids, the antibiotic costs of the cases did not differ significantly. According to the mortality status in our study, the antibiotic costs of the living cases were not higher. This shows that use of antibiotics do not prolong the life of patients. In a previous study, hospital mortality rate of infected patients receiving antimicrobial treatment was statistically greater than the hospital mortality rate of the remaining patients [Citation32]. Terminal sick patients have many infections. But in PC, the use of antibiotics can be an ethical dilemma. Deciding whether to treat, withhold, or withdraw the antibiotic treatment for an infection is very difficult. Antibiotics generally have two main benefits, longer survival and symptom relief. Therefore, physicians prescribe antimicrobials when treating terminally ill patients [Citation14]. White et al. observed that there was no mortality difference in PC between infected or uninfected patients [Citation22]. Vitetta et al. observed that infection is not the direct cause of death in terminally ill hospice patients [Citation4]. Reinbot et al. observed that survival was not affected by the presence of infection or the use of antimicrobials in terminal patients [Citation33]. In this study, mortality was also not related to infection and duration of use of antibiotics.
Limitations
Limitation of our study was the distribution and analysis of types of antibiotics used, but instead of evaluating this fact we tried to concentrate on duration and the average cost of antibiotic use in PC as far as economical burden was concerned.
Conclusions
Most important observation of this study is to show a vicious circle which can happen in PC (). This circle involves in order; length of stay, increased rate of infection, use of antibiotics, infection with resistant microorganisms, use of broad spectrum antibiotics, increased length of stay all affecting each other. As a result antibiotic cost increases more and dying process is harmed. When antibiotics were given until death, the indication should be reconsidered because of a possibly undesirable prolongation of the dying process. Therefore, antibiotic use should be for symptom relief more than being therapeutic, earliest possible discharge of patient should be provided, homecare of the patients should be encouraged. Using antibiotics for aggressive treatment of infections in PC is contraindicated as it opposes to real philosophy of PC which is expressed as; PC should not shorten the life and should not prolong the painful life.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Sepúlveda C, Marlin A, Yoshida T, et al. Palliative care: the World Health Organization’s global perspective. J Pain Sympt Manag. 2002;24:91–96.
- Chochinov HM. Dignity-conserving care – a new model for palliative care: helping the patient feel valued. JAMA. 2002;287:2253–2260.
- Davies E, Higginson IJ, World health organization. Better palliative care for older people. Copenhagen, Denmark: WHO Regional Office for Europe; 2004.
- Vitetta L, Kenner D, Sali A. Bacterial infections in terminally ill hospice patients. J Pain Sympt Manag. 2000;20:326–334.
- Datta R, Juthani-Mehta M. Burden and management of multidrug-resistant organisms in palliative care. Palliat Care. 2017;10:1178224217749233.
- Wakefield DS, Helms CM, Massanari RM, et al. Cost of nosocomial infection: relative contributions of laboratory, antibiotic, and per diem costs in serious Staphylococcus aureus infections. Am J Infect Control. 1988;16:185–192.
- Juthani-Mehta M, Malani PN, Mitchell SL. Antimicrobials at the end of life: an opportunity to improve palliative care and infection management. JAMA. 2015;314:2017–2018.
- Ahronheim JC, Morrison RS, Baskin SA, et al. Treatment of the dying in the acute care hospital. Advanced dementia and metastatic cancer. Arch Intern Med. 1996;156:2094–2100.
- Pereira J, Watanabe S, Wolch G. A retrospective review of the frequency of infections and patterns of antibiotic utilization on a palliative care unit. J Pain Symptom Manage. 1998;16:374–381.
- Schofield P, Carey M, Love A, et al. ‘Would you like to talk about your future treatment options?’Discussing the transition from curative cancer treatment to palliative care. Palliat Med. 2006;20:397–406.
- Albrecht JS, McGregor JC, Fromme EK, et al. A nationwide analysis of antibiotic use in hospice care in the final week of life. J Pain Symptom Manage. 2013;46:483–490.
- Dincer M, Kahveci K, Doger C. An examination of factors affecting the length of stay in a palliative care center. J Palliative Med. 2018;21:11–15.
- Thompson AJ, Silveira MJ, Vitale CA, et al. Antimicrobial use at the end of life among hospitalized patients with advanced cancer. Am J Hosp Palliat Care. 2012;29:599–603.
- Macedo F, Nunes C, Ladeira K, et al. Antimicrobial therapy in palliative care: an overview. Supportive Care Cancer. 2018;26:1361–1367.
- Nagy-Agren S, Haley HB. Management of infections in palliative care patients with advanced cancer. J Pain Sympt Manag. 2002;24:64–70.
- Hidron AI, Edwards JR, Patel J, et al. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996–1011.
- Siegel JD, Rhinehart E, Jackson M, et al. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35:S165–S193.
- Levin PD, Simor AE, Moses AE, et al. End-of-life treatment and bacterial antibiotic resistance: a potential association. Chest. 2010;138:588–594.
- Oneschuk D, Fainsinger R, Demoissac D. Antibiotic use in the last week of life in three different palliative care settings. J Palliat Care. 2002;18:25–28.
- Stiel S, Krumm N, Pestinger M, et al. Antibiotics in palliative medicine – results from a prospective epidemiological investigation from the HOPE survey. Support Care Cancer. 2012;20:325–333.
- Helde-Frankling M, Bergqvist J, Bergman P, et al. Antibiotic treatment in end-of-life cancer patients – a retrospective observational study at a palliative care center in Sweden. Cancers. 2016;8:84.
- White PH, Kuhlenschmidt HL, Vancura BG, et al. Antimicrobial use in patients with advanced cancer receiving hospice care. J Pain Symptom Manage. 2003;25:438–443.
- Andersen BM. Economic consequences of hospital infections in a 1,000-bed university hospital in Norway. Infect Control Hosp Epidemiol. 1998;19:805–807.
- Haley RW, Schaberg DR, Von Allmen SD, et al. Estimating the extra charges and prolongation of hospitalization due to nosocomial infections: a comparison of methods. J Infect Dis. 1980;141:248–257.
- Yalcin A, Hayran M, Ünal S. Economic analysis of nosocomial infections in a Turkish university hospital. J Chemother. 1997;9:411–414.
- Varol Y, Varol U, Başer Z, et al. The cost of COPD exacerbations managed in hospital. Turk Toraks Dergisi/Turk Thorac J. 2013;14:19–23.
- Khandelwal N, Benkeser D, Coe NB, et al. Patterns of cost for patients dying in the intensive care unit and implications for cost savings of palliative care interventions. J Palliative Med. 2016;19:1171–1178.
- Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996;17:552–557.
- Michel L, McMichan J, Bachy J. Tracheostomy and indwelling central venous line: a hazardous combination? Intensive Care Med. 1979;5:83–86.
- Chan T, Devaiah AK. Tracheostomy in palliative care. Otolaryngol Clin North Am. 2009;42:133–141.
- Gotsman M, Whitby J. Respiratory infection following tracheostomy. Thorax. 1964;19:89.
- Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–474.
- Reinbolt RE, Shenk AM, White PH, et al. Symptomatic treatment of infections in patients with advanced cancer receiving hospice care. J Pain Sympt Manag. 2005;30:175–182.