Abstract
Aim: To investigate the effect of tadalafil in rats administered with daily dutasteride.
Methods: Twenty-four Sprague–Dawley male rats were allocated to three groups as control (group C), dutasteride (group D) and dutasteride plus tadalafil (group D + T). After a month of treatment, serum samples were obtained from rats to measure dihydrotestosterone and total testosterone. Nitric oxide (NO) synthase (NOS) immunoreactivity and levels of NOS enzyme isoforms, NO and cyclic guanosine monophosphate (cGMP) were evaluated in the harvested penile tissues. Also, corporal smooth muscle and collagen were examined.
Results: Staining intensities of neuronal NOS and endothelial NOS were significantly lower in group D (p < .05). They were similar between group C and group D + T. Immunoreactivity of inducible NOS was observed higher in group D than group C (p = .01) whereas group D + T had the highest iNOS (p<.001). ELISA revealed similar outcomes in terms of NOS enzyme isoform levels. The mean of smooth muscle to collagen ratio was the lowest in group D (p < .001) and it was similar among group C and group D + T (p = .072). Group D had the lowest cGMP and NO levels (p < .05) and they did not differ between group C and group D + T (p>.05). Group D and group D + T had significantly decreased dihydrotestosterone and increased testosterone, compared to group C (p < .001). They were similar between group D and group D + T.
Conclusion: Daily treatment with tadalafil improves dutasteride-induced changes in rat penis.
Introduction
Lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) are prevalent among the aging men [Citation1,Citation2]. Treatment with 5 alpha-reductase (5AR) inhibitors (5ARIs) is commonly administered to the men with BPH-LUTS and it is associated with sexual dysfunction [Citation3–5].
Testosterone (T) turns into a more active compound dihydrotestosterone (DHT) by 5AR enzyme [Citation6]. Two isoforms of this enzyme are present: type 1 and type 2 [Citation7,Citation8]. Dutasteride is one of the two 5ARI drugs used in the management of BPH-LUTS [Citation7–9]. This agent inhibits both isoenzymes and decreases DHT concentrations in serum and prostatic tissue by 95% [Citation10]. The initial research on the efficacy of dutasteride has reported sexual adverse events with a rate of 7.3% for erectile dysfunction (ED), 4.2% for reduced sexual desire, and 2.2% for ejaculatory dysfunction [Citation11]. In another study, long-term dutasteride treatment resulted in decrease in International Index of Erectile Function (IIEF) scores and worsened ED [Citation12]. However, the exact mechanism of these side effects has not been elucidated yet.
Penile erection is initiated by nitric oxide (NO) which is generated by NO synthase (NOS) enzyme [Citation13].There are three types of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS). Among those, nNOS and eNOS have respectively an initiating and maintaining role on penile erection while mission of iNOS has not been fully explained yet [Citation14]. NO is synthesized from l-arginine by NOS and it activates guanylate cyclase enzyme, leading to increase of cyclic guanosine monophosphate (cGMP) levels in the endothelial smooth muscle cells [Citation13]. These events facilitate penile corporal smooth muscle relaxation, increase blood flow into the corpora cavernosa, and consequently result in a firm erection [Citation15].
Androgens are essential for activity and expression of NOS and restore those which decrease in corpus cavernosum of castrated rats [Citation16]. It has been demonstrated that chronic administration of dutasteride has harmful effect on erectile function by decline in response to in vivo nerve stimulation and molecular alterations such as a reduction in nNOS [Citation17,Citation18].
Phosphodiesterase type 5 (PDE5) inhibitors are frequently prescribed class of drugs to men with ED [Citation19,Citation20]. They prevent cGMP hydrolysis, leading to higher cGMP levels inside the smooth muscle cells, which is associated with improved erectile function [Citation19,Citation20]. Uniquely, PDE5 is localized to the corpus cavernosum and thus PDE5 inhibitors facilitate erection without significant systemic vasodilation [Citation21]. Tadalafil is an effective PDE5 inhibitor, which is used for the treatment of ED [Citation22]. It is the only PDE5 inhibitor that has once daily form with 5 mg dosage in men [Citation23]. Tadalafil is also indicated in the treatment of BPH-LUTS [Citation24]. Coadministration of tadalafil and 5ARIs has provided more benefit on erectile functions in men with BPH-LUTS, compared with those alone [Citation25,Citation26].
Although these studies have shown the useful effect of tadalafil on ameliorating sexual dysfunction due to 5ARI treatment, there is no data on the mechanism clarifying this phenomenon. The purpose of current study was to investigate the mechanism underlying the preventative effect of tadalafil in rats treated with daily dutasteride.
Methods
Animals
Present study was performed after obtaining Institutional Animal Care and Use Committee approval with the number of 2014/128-39 in the animal lab of Istanbul Bagcilar Training and Research Hospital, Turkey. A total of 24 adult Sprague–Dawley male rats were equally divided into three groups of 8. Rats were allowed unlimited water, fed standard chow, and were kept at 22 °C with light and dark periods of 12 h. Daily for a month, rats were administered orally with a placebo of 5 ml/kg tap water in the control group (group C), with dutasteride (0.5 mg/rat) dissolved in 5 ml/kg tap water in group D and dutasteride solution plus 5 mg/rat tadalafil (5 mg tadalafil dissolved in 1 ml tap water) in group D + T.
At the end of the protocol, all rats were sacrificed between 8 and 10 am. First, the rats were anesthetized using a combination of 5 mg/kg xylazine, followed by 65 mg/kg ketamine administered intramuscularly 15 min later. Blood samples were collected to evaluate serum androgen hormone concentrations and serum was separated after centrifugation at 3500 rpm for 7 min. Each rat was shaved and positioned supinely. A suprapubic vertical incision was used to access and excise the penis down to the level of the pubic symphysis. Subsequently, pentobarbital dosed at 150 mg/kg was administered. The distal 1/3 of the resected penile tissue was used for immunohistochemical analysis whereas the proximal 2/3 part was sectioned in phosphate-buffered saline in a petri dish placed on ice (NaCl–phosphate; 0.2 M, pH 7.29) for enzyme-linked immunosorbent assay (ELISA) analysis.
Histopathological analyses
The distal portions of the rat penis were fixed in 10% buffered formaldehyde solution for a day. Paraffin blocks were made and embedded tissues were cut to a thickness of 4 mm. Lysine coated slides were prepared and immunohistochemical analyses with nNOS (ab3511, polyclonal, IgG isotype, UK), iNOS (ab204017, polyclonal, IgG isotype, UK), and eNOS (ab50010, polyclonal, IgG isotype, UK) antibodies were performed. All antibodies were diluted with a 1:100 ratio. Sections were examined for staining intensity by a pathologist (SA) who was blinded to the rats’ group. Semi-quantitative scoring was used where 0 = none, 1 = mild, 2 = moderate and 3 = intense staining.
Moreover, contents of collagen and smooth muscle in corpus cavernosum were determined with Masson’s trichrome staining. Using image analysis program (Image-Pro Plus 4.5 software, Media Cybernetics, USA), areas of smooth muscle and collagen were examined in 10 fields of each tissue section and evaluated quantitatively.
NOS enzyme, NO and cGMP levels
Cavernosal tissues were excised, and the urethral tissue was excluded. The cavernosal tissue was diced into small pieces in cold PBS and homogenized using a homogenizer (Ultra Turrax Type T25-B, IKE Labortechnic, Germany) at 16,000rpm for 3 min on ice in buffer. Afterwards, it was re-homogenized with a glass homogenizer on ice and was subjected to two freeze-thaw cycles followed by ultrasonication. The resulting homogenate was centrifuged at 5000 rpm for 30 min at −20 °C. Levels of NOS isoforms (nNOS, eNOS, and iNOS), NO and cGMP were assessed using ELISA kits (Cloud-Clone Corp., Houston, TX, USA) at 450 nm using a microplate spectrophotometer reader (BioTek, Winooski VT, USA) in 100 µl homogenate.
Serum androgen levels
Electrochemiluminescence immunoassay was used to measure DHT and total T (Roche Hitachi Cobas 8000; Roche Science, Mannheim, Germany) levels.
Statistics
Kruskal–Wallis procedure including Bonferroni corrected Mann–Whitney U test was used for the analysis of ordinal variables. Continuous data were analyzed by one-way analysis of variance (ANOVA) with post-hoc Tukey’s test. IBM SPSS statistics version 21 was used for data analysis. Results were presented as mean ± standard deviation and statistical significance was determined as a p value of .05 or less.
Results
Histopathological analyses
Semi-quantitative immunohistochemical staining intensity scoring of penile tissues revealed that group D had markedly reduced nNOS levels compared to group C (0.87 ± 0.64 vs. 2.12 ± 0.35, p = .001) whereas coadministering tadalafil with dutasteride was associated with improved nNOS levels in group D + T (1.87 ± 0.64) which was similar to group C (p = .505) (, ). Corporal eNOS results were similar with nNOS outcomes in terms of significant differences. Moreover, iNOS levels were the highest in group D + T (p < .001) and they were higher in group D compared with group C (p = .01). Mean smooth muscle to collagen (SM/C) ratio was the lowest in group D (p < .001) whereas it was similar among group C and group D + T (p = .072).
Figure 1. (A–M). Immunohistochemical studies (×200) and Masson’s trichrome (MT) staining (×40) results in the cavernosal tissue. nNOS staining with decreased localization to the nerves of the corpus cavernosum from group C (A) to group D (B) and with improved localization as similar to group C (A) in group D + T (C). Reduced eNOS staining in cavernous sinus endothelial cells was observed in group D (E) compared to group C (D). Group D + T (F) had similar eNOS immunoreactivity with group C (D). iNOS staining enhanced in the corpus cavernosal smooth muscle of group D (H) and group D + T (I) as the greatest compared to group C (G). Markedly decreased smooth muscle content and increased collagen were observed in Masson’s trichrome staining of group D (L). No difference in the ratio of SM/C was observed between group C (K) and group D + T (M).
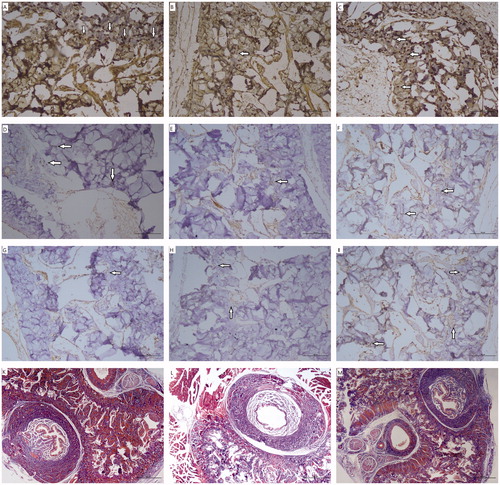
Table 1. Outcomes of mean intensity of nitric oxide synthase (NOS) isoenzymes in immunohistochemical staining and smooth muscle to collagen (SM/C) ratios.
NOS enzyme, NO and cGMP levels
Comparison of the levels of NOS enzyme isoforms, NO and cGMP are summarized in . Group D had lower nNOS levels than group C (p < .001). Simultaneously administration of dutasteride with tadalafil was able to improve nNOS levels in group D + T, which were significantly higher than those in group D (p < .014). Group D also had the lowest eNOS (p < .001) levels whereas group D + T had the highest iNOS levels (p < .001). No difference in eNOS levels was observed between group C and group D + T (p = .135). Both cGMP and NO were the lowest in group D (p < .05) and coadministering tadalafil with dutasteride resulted in cGMP and NO levels similar to the controls (p>.05).
Table 2. Comparison of the levels of nitric oxide (NO) synthase (NOS) isoenzymes, NO and cyclic guanosine monophosphate (cGMP) in groups by ELISA.
Serum androgen concentrations
DHT levels markedly decreased in both group D and group D + T compared to group C (p < .001). On the other hand, group D and group D + T had increased T concentrations compared to group C (p < .001 and p = .001, respectively) (). Both DHT and T levels were similar between group D and group D + T (p = .942 and p = .661, respectively).
Table 3. Mean serum androgen levels of the groups.
Discussion
Our study revealed that dutasteride was associated with reduction in nNOS, eNOS staining intensity and SM/C ratio along with an increase in iNOS staining intensity. We also demonstrated that nNOS and eNOS intensity in immunohistochemical staining and SM/C ratio in Masson’s trichrome staining improved when rats were treated with dutasteride plus tadalafil. Moreover, coadministration of dutasteride with tadalafil resulted in much more increase in iNOS staining intensity. According to the ELISA analysis dutasteride caused decline in nNOS and eNOS enzyme expression levels and coadministered tadalafil with dutasteride prevented to decrease nNOS and eNOS enzyme levels, provided consequently preservation of NO and cGMP decline in our study. Present findings revealed that DHT significantly reduced in groups administered dutasteride while total T significantly increased in those.
A study conducted by Pinsky et al. revealed that dutasteride administration of 4 weeks in adult rats resulted in reduction of induced relaxation responses and increase of induced contraction responses as well as increased collagen deposition and decreased nNOS expression [Citation17]. However, some of these alterations seem to be reversible as these impairments were restored after 2 weeks of washout period [Citation18]. On the other hand, cholinergic and adrenergic contractile responses were partially restored in the rats treated with long-term dutasteride, even after the cessation of the dutasteride administration [Citation18]. In another study investigating whether the dutasteride associated changes in rat penis depend on duration of treatment, dutasteride administration of both 4 and 8 weeks harmed corporal smooth muscle content and morphological alterations could not be restored after washout period in both groups [Citation27]. However, reduced erectile responses were regained during washout period in the 4-week treatment group despite remained depressed in the 8-week group [Citation27].
Dutasteride resulted in enhanced iNOS staining intensty in the study of Pinsky et al. [Citation17]. Earlier reports revealed that iNOS levels increased to prevent fibrosis and oxidative process in penile tissues of experimental models with iNOS knockout [Citation28] and Peyronie’s disease [Citation29,Citation30]. Dutasteride caused elevation in iNOS intensity and protein levels more likely as body’s protective response secondary to detrimental effect of dutasteride on penile erection in our study. Also, body’s protective response against dutasteride was supported and improved via much more increase in the iNOS levels and intensity in our study by tadalafil. Similarly, Ferrini et al. demonstrated the protective effect of vardenadil on corporal fibrosis after cavernosal nerve resection by causing a two-fold increment in iNOS levels in their study [Citation31].
Androgens are essential for activity and expression of NOS in erection physiology and it was shown to decrease nNOS mRNA levels in castrated rats [Citation16]. Androgen replacement with T or DHT provided effectively restoration of those. Of them, DHT was superior to T [Citation16]. When DHT production was inhibited with finasteride, both erectile response and nNOS amounts decreased despite giving T replacement to castrated rats [Citation16]. Dutasteride-induced ED is more likely linked to decline in DHT, thereby causing reduced eNOS, nNOS and NO levels in the penile corpus cavernosum. In our study, dutasteride caused significant reduction both in serum DHT and eNOS, nNOS, NO and cGMP levels in rat penis compared to control rats. Serum androgen levels remained unchanged with the coadministration of tadalafil with dutasteride compared to rats treated only dutasteride. Tadalafil improved NO, cGMP, smooth muscle content and all of the NOS enzyme isoforms in rats that were under dutasteride treatment. These findings show that androgens are not responsible for the healing mechanism in our study.
Sildenafil-induced relaxation of corpus cavernosum smooth muscle was observed in rats treated with dutasteride compared to control in organ-bath, although dutasteride resulted in reduction of induced relaxation responses and increase of induced contraction responses as well as increased collagen deposition and decreased nNOS expression [Citation17]. In a study conducted by Da Silva et al., both dutasteride treatment and coadministration of orally given sildenafil with dutasteride for a period of 40 days resulted in detrimental morphological changes in rat penis [Citation32]. They could not carry out the mechanism how PDE5 inhibitors improve the erection in patients with 5ARI induced ED [Citation32]. We found an amelioration in the structural content and SM/C ratio of rat penis with the concomitant administration of tadalafil and dutasteride. This different result may depend on the dosage of administered PDE5 inhibitor. Because Da Silva et al. gave dosage of 0.5 mg/kg/day dutasteride to the rats whilst 30 mg/kg/day sildenafil dosage [Citation32]. We gave a dosage of 0.5 mg/rat/day dutasteride based on the previous studies [Citation17,Citation18] and 5 mg/rat/day tadalafil. Also, we carried out that concomitant administration of tadalafil and dutasteride ameliorated NOS enzyme isoforms, consequently increasing NO and cGMP.
PDE5 inhibitors do not benefit to men with testosterone deficiency to recover erectile function [Citation33]. Testosterone replacement therapy could restore PDE5 activity in castrated rabbits [Citation34]. By contrast, rats treated with dutasteride [Citation17] like in ours have still abundantly testosterone which may maintain PDE5 activity. In another study conducted by Ahn et al. udenafil (another PDE5 inhibitor) modulated nNOS and eNOS expressions which were significantly decreased in streptozotocin-induced diabetic rats [Citation35]. There was no androgen deprivation or insufficiency in rats of both our study and the study conducted by Ahn et al. Udenafil also improved in vivo amplitude of cavernous nerve stimulation [Citation35]. Considering the NO-cGMP signaling pathway, we infer from these findings by combining with our results that NOS genes may be stimulated by tadalafil or any PDE5 inhibitors. Also, PDE5 inhibitors enhance the amount of cGMP by inhibition of cGMP degradation as well-known role.
As for clinical studies, Casabe et al. demonstrated that coadministration of tadalafil with finasteride improved significantly BPH-LUTS compared with coadministered finasteride and placebo with few sexual adverse events [Citation36]. Moreover, erectile functions were significantly better after the treatment of tadalafil plus finasteride compared with placebo plus finasteride treatment in men having ED during admission [Citation36]. In a similar study, treatment with coadministration of tadalafil with finasteride for up to 6 months improved IIEF scores more than placebo plus finasteride [Citation25]. Additionally, in a study conducted in South Korea, men treated with dutasteride plus tadalafil for BPH-LUTS had similar results in improvement of IPSS, Qmax and PVR compared with patients receiving combination therapy of dutasteride and tamsulosin [Citation37]. Also, IIEF scores increased in patients treated with dutasteride plus tadalafil whilst decreased in those treated with combination therapy of dutasteride and tamsulosin [Citation37]. Tadalafil treatment also provided significant improvement in IIEF scores of the patients under dutasteride treatment for BPH-LUTS [Citation26].
There are some inherent drawbacks of current study. We did not evaluate phosphorylated eNOS, NOS enzymatic activity, NO bioavailability and NOS mRNA expression levels in corpus cavernosum. Also, in vivo erectile activity and in vitro contractile and relaxant responses of cavernosal smooth muscle in organ bath were not investigated due to our inadequate lab.
Conclusion
Inhibition of PDE5 with chronic tadalafil treatment may be a useful therapeutic strategy to prevent dutasteride-induced ED by enhancing expression of nNOS, eNOS and iNOS, causing elevation in NO and cGMP.
Acknowledgements
The authors wish to thank chief staff of animal lab of Istanbul Bagcilar Training and Research Hospital, Mrs Duygu Sultan Cevik, for helping us during applying anesthesia to the rats.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urol Clin N Am. 2016;43(3):289–297.
- Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Sci Rep. 2017;7(1):7984.
- Liu L, Zhao S, Li F, et al. Effect of 5alpha-reductase inhibitors on sexual function: a meta-analysis and systematic review of randomized controlled trials. J Sex Med. 2016;13(9):1297–1310.
- Erdemir F, Harbin A, Hellstrom WJ. 5-alpha reductase inhibitors and erectile dysfunction: the connection. J Sex Med. 2008;5(12):2917–2924.
- Maeda T, Kikuchi E, Hasegawa M, et al. A prospective longitudinal survey of erectile function status in symptomatic benign prostatic hyperplasia patients treated with dutasteride. Aging Male. 2016;19(2):111–116.
- Harris G, Azzolina B, Baginsky W, et al. Identification and selective inhibition of an isozyme of steroid 5 alpha-reductase in human scalp. PNAS. 1992;89(22):10787–10791.
- Bramson HN, Hermann D, Batchelor KW, et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J Pharmacol Exp Ther. 1997;282(3):1496–1502.
- Andriole GL, Kirby R. Safety and tolerability of the dual 5alpha-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur Urol. 2003;44(1):82–88.
- Qian X, Yu G, Qian Y, et al. Efficacy of 5 α-reductase inhibitors for patients with large benign prostatic hyperplasia (> 80 mL) after transurethral resection of the prostate. Aging Male. 2015;18(4):238–243.
- Clark RV, Hermann DJ, Cunningham GR, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179–2184.
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60(3):434–441.
- Traish A, Haider KS, Doros G, et al. Long-term dutasteride therapy in men with benign prostatic hyperplasia alters glucose and lipid profiles and increases severity of erectile dysfunction. Horm Mol Biol Clin Investig. 2017;30(3):1–16.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. The urologic clinics of North America. Urol Clin North Am. 2005;32(4):379–395.
- Giuliano F, Rampin O. Neural control of erection. Physiol Behav. 2004;83(2):189–201.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142.
- Park KH, Kim SW, Kim KD, et al. Effects of androgens on the expression of nitric oxide synthase mRNAs in rat corpus cavernosum. BJU Int. 1999;83(3):327–333.
- Pinsky MR, Gur S, Tracey AJ, et al. The effects of chronic 5-alpha-reductase inhibitor (dutasteride) treatment on rat erectile function. J Sex Med. 2011;8(11):3066–3074.
- Oztekin CV, Gur S, Abdulkadir NA, et al. Incomplete recovery of erectile function in rat after discontinuation of dual 5-alpha reductase inhibitor therapy. J Sex Med. 2012;9(7):1773–1781.
- Huang SA, Lie JD. Phosphodiesterase-5 (PDE5) inhibitors in the management of erectile dysfunction. Peer Rev J Formul Manage. 2013;38(7):407–419.
- Lue TF. Erectile dysfunction. N Engl J Med. 2000;342(24):1802–1813.
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338(20):1397–1404.
- Padma-Nathan H, McMurray JG, Pullman WE, et al. On-demand IC351 (Cialis) enhances erectile function in patients with erectile dysfunction. Int J Impot Res. 2001;13(1):2–9.
- Xu WD, Liu ZY, Ye HM, et al. [Efficacy and safety of long-term small-dose tadalafil in the treatment of erectile dysfunction]. Zhonghua Nan ke Xue. 2011;17(6):531–534.
- Gratzke C, Bachmann A, Descazeaud A, et al. EAU guidelines on the assessment of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2015;67(6):1099–1109.
- Glina S, Roehrborn CG, Esen A, et al. Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med. 2015;12(1):129–138.
- Ozkidik M, Gokce MI, Yaman O. Efficacy of tadalafil treatment on erectile dysfunction in patients under dutasteride treatment: a prospective non-randomized comparative study. Turk J Urol. 2018;44(4):294–297.
- Sung HH, Yu J, Kang SJ, Chae MR, et al. Persistent erectile dysfunction after discontinuation of 5-alpha reductase inhibitor therapy in rats depending on the duration of treatment. World J Mens Health. 2019;37(2):240–248.
- Ferrini MG, Rivera S, Moon J, et al. The genetic inactivation of inducible nitric oxide synthase (iNOS) intensifies fibrosis and oxidative stress in the penile corpora cavernosa in type 1 diabetes. J Sex Med. 2010;7(9):3033–3044.
- Vernet D, Ferrini MG, Valente EG, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide Biol Chem. 2002;7(4):262–276.
- Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide Biol Chem. 2002;6(3):283–294.
- Ferrini MG, Davila HH, Kovanecz I, et al. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68(2):429–435.
- Da Silva MHA, Medeiros JL, Jr., Costa WS, et al. Effects of the dutasteride and sildenafil association in the penis of a benign prostatic hyperplasia animal model. Aging Male. [cited 2019 Aug 20]; [7 p.]. DOI:10.1080/13685538.2019.1653839
- Gooren LJ, Saad F. Recent insights into androgen action on the anatomical and physiological substrate of penile erection. Asian J Androl. 2006;8(1):3–9.
- Traish AM, Park K, Dhir V, et al. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140(4):1861–1868.
- Ahn GJ, Chung HK, Lee CH, Kang KK, et al. Increased expression of the nitric oxide synthase gene and protein in corpus cavernosum by repeated dosing of udenafil in a rat model of chemical diabetogenesis. Asian J Androl. 2009;11(4):435–442.
- Casabe A, Roehrborn CG, Da Pozzo LF, et al. Efficacy and safety of the coadministration of tadalafil once daily with finasteride for 6 months in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia. J Urol. 2014;191(3):727–733.
- Park H, Park N. 1092 Combination therapy with dutasteride and tadalafil in men with moderate-to-severe benign prostatic hyperplasia. Eur Urol Suppl. 2013;12(1):e1092.
