Abstract
Aim: We investigated whether low plasma free testosterone (FT) levels could predict cardiovascular events (CVE) in Japanese men with coronary risk factors.
Methods: Male patients with classical coronary risk factors who had undergone serum FT testing were enrolled. New incidences of CVE were retrospectively investigated among all eligible participants based on their medical records.
Results: Overall, 466 male outpatients with coronary risk factors without a previous history of CVE were identified. Throughout the follow-up period (median = 92 months), 126 CVE occurred. The Kaplan–Meier survival analysis according to the tertiles of plasma FT levels revealed that patients with the lowest FT tertile (<6.5 pg/mL) had a higher likelihood of developing CVE than those with the highest tertile (>9.3 pg/mL) (p<.01). Multivariate analysis showed that increased frequency of CVE was observed with lower FT tertiles, independent of other coronary risk factors, with hazard ratios of 0.617 (95% CI, 0.389–0.976; p=.030) and 0.524 (95% CI, 0.309–0.887; p=.016) for the second and highest tertile relative to the lowest FT tertile, respectively.
Conclusion: Among Japanese men with coronary risk factors, a lower FT level was a predictor for the development of cardiovascular diseases independent of other coronary risk factors and age.
Introduction
Serum testosterone levels in men have been found to decline with age [Citation1]. Moreover, a study revealed that free testosterone (FT) levels among Japanese men showed strong correlation with age and decreased with aging [Citation2]. Testosterone deficiency has often been associated with age-related diseases, such as erectile dysfunction, osteoporosis, depression, cognitive impairment, and frailty [Citation3]. In addition, several previous cross-sectional and prospective studies have found an inverse relationship between testosterone levels and coronary risk factors, including obesity, hypertension, dyslipidemia, and diabetes mellitus (DM) [Citation4–7]. In particular, metabolic syndrome, which is as a cluster of metabolic factors, has been a well-known risk factor for cardiovascular diseases (CVDs) and stroke. Studies have shown that testosterone deficiency is related to the development of CVDs [Citation8,Citation9], and testosterone levels were negatively correlated with the severity of coronary artery disease in patients undergoing elective coronary angiography [Citation10]. Therefore, potential prevention of the development of CVDs via the treatment of metabolic factors and metabolic syndrome, which are significantly associated with testosterone deficiency, has been a clinical concern worldwide.
Furthermore, an epidemiological study demonstrated that community-dwelling older men with low testosterone levels showed a higher likelihood of death due to CVDs, independent of multiple risk factors and several lifestyle habits [Citation11]. However, this issue remains unclear in Asian populations. Moreover, very few studies have currently investigated the impact of low testosterone conditions on clinical outcome among patients with several risk factors. Therefore, the present retrospective survey aimed to examine the association between baseline testosterone levels and incidence of CVDs in Japanese patients with coronary risk factors and attempted to determine whether low baseline testosterone level is an independent predictor for CVDs.
Materials and methods
Study population
Between 2007 and 2009, 535 male patients aged 37–89 years at baseline with classical coronary risk factors, including hypertension, dyslipidemia, DM, and current cigarette smoking, underwent serum FT testing at our hospital for the screening of the late-onset hypogonadism (LOH) syndrome. At the initial visit, blood was collected between 09:00 and 11:00 to measure serum FT levels using radioimmunoassay (DPC Free Testosterone Kit, Mitsubishi Kagaku Iatron, Tokyo, Japan).
Hypertension, dyslipidemia, and DM were diagnosed according to the Japanese diagnostic criteria [Citation12–14] or treatment with any medications for such diseases. Patients with a history of CVDs at baseline, including stroke, coronary heart disease, and peripheral arterial disease, were excluded from analysis. Moreover, malignancies were excluded considering their potential significant influence on the clinical course. Among the 535 patients who underwent plasma FT measurement between 2007 and 2009, 466 were eligible for the analysis after excluding 11 and 58 patients with cancer and a history of CVDs, respectively. Thereafter, follow-up data from the 466 patients were collected.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Kanazawa University Hospital Ethics Committee (approval no. 2020-074). Written informed consent was obtained from each subject.
Study design and protocol
Medical records of all eligible patients were retrospectively investigated for new incidences of cardiovascular events (CVE) from baseline visit until 2019. The primary endpoints of the present study included CVE such as stroke, coronary artery disease, and peripheral arterial disease. Cardiovascular events were diagnosed by cardiologists based on clinical courses, physical examinations, laboratory tests, and radiological examinations, whereas new CVE were determined based on medical records.
Initially, all patients were divided into three groups according to serum FT tertiles at baseline, following which new incidences of CVE until 2019 were compared among the groups. Furthermore, we attempted to identify the predictors for the development of CVE by analyzing baseline characteristics.
Statistical analysis
First, the Kolmogorov–Smirnov test was used to assess parameter distributions, and the findings revealed that data in all parameters were not normally distributed. Thereafter, the data were divided into three groups based on their serum FT level (<6.5 pg/mL, 6.5–9.3 pg/mL, >9.3 pg/mL), following which they were compared using the Kruskal–Wallis H-test. For evaluating categorical data and correlations between age and serum FT levels, respectively, chi-squared tests and Spearman’s coefficient analyses were conducted. New incidences of CVE were determined using the Kaplan–Meier plots and log-rank tests. Hazard ratios (HRs) for CVE were analyzed using multivariate Cox proportional hazards regression. All statistical analyses were performed using SPSS (Ver. 25.0, SPSS Inc., Chicago, IL) with a p value of <.05 indicating statistical significance.
Results
In our cohort of 466 participants, the median age was 66 years (interquartile = 15 years, range = 37–89 years) and the median serum FT level was 8.0 pg/mL (interquartile = 4.2 pg/mL, range = 0.6–22.9 pg/mL). Biochemical hypogonadism was observed in 396 cases (84.9%) according to the Japanese criteria (FT < 11.8 pg/mL).
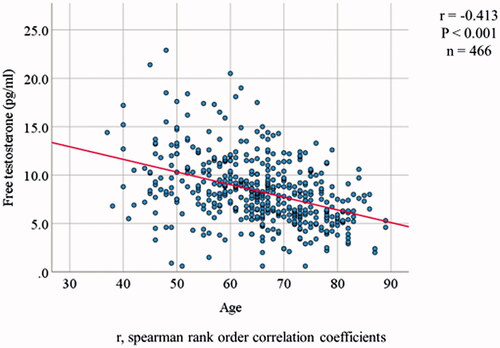
Baseline characteristics of the participants according to FT tertiles are summarized in . The age decreased as FT tertile increased, showing a strong inverse correlation between age and FT levels (). Participants with the lowest FT tertile showed a higher frequency of developing dyslipidemia compared with those with higher FT tertiles. No significant difference in DM was observed between the groups, although those with lower FT tertiles had increased HbA1c levels. Moreover, body mass index, hypertension, DM duration, and smoking status did not differ between the groups.
Table 1. Patients' baseline characteristics by tertile group of plasma free testosterone.
Throughout the follow-up period (median = 92 months, interquartile = 95 months, range = 0–146 months), a total of 126 CVE occurred (). Accordingly, 56 participants developed coronary artery disease, including 10 with myocardial infarction and 27 with angina pectoris; 26 cases received percutaneous coronary intervention and three underwent with coronary artery bypass grafting. Of the 24 cases of stroke, 20 were due to cerebral infarction, whereas four were due to cerebral hemorrhage. Incidences of CVE increased with decrease in FT tertiles. Moreover, Kaplan–Meier’s analysis according to FT tertiles revealed that patients with the lowest FT tertile experienced significantly more CVE compared with those with higher FT tertiles after adjusting for age (p<.01 using the log-rank test) ().
Figure 2. Cumulative survival of cardiovascular events by tertile group of plasma concentration of free testosterone.
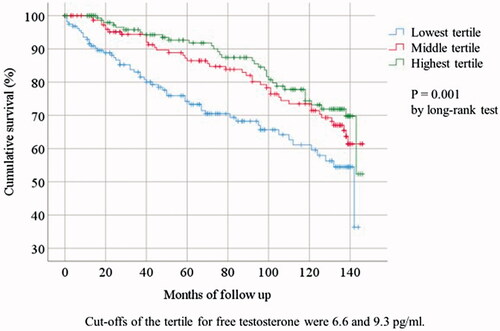
Table 2. Cardiovascular events by tertile of plasma free testosterone.
Multivariate Cox proportional hazards models demonstrated that age, lower baseline FT levels, hypertension, dyslipidemia, longer DM duration, and current cigarette smoking were independent predictive factors for the occurrence of CVE (). Increased frequency of CVE were noted with lower FT tertiles, independent of other coronary risk factors, with HRs of 0.617 (95% CI, 0.389–0.976; p=.030) and 0.524 (95% CI, 0.309–0.887; p=.016) for the second and highest tertile relative to lowest tertile, respectively.
Table 3. Multivariate analyses of factors associated with cardiovascular events.
Discussion
The present study suggested that lower FT values were significantly correlated with the future occurrence of CVE throughout a median follow-up duration of 92 months among Japanese patients with coronary risk factors. To the best of our knowledge, this was the largest study to demonstrate that testosterone deficiency was an independent predictor for CVE within the Japanese population.
The study participants exhibited poor coronary risk profiles, including hypertension, hyperlipidemia, DM, and cigarette smoking as previously reported [Citation11,Citation15,Citation16]. Indeed, the present study found that age, hypertension, longer DM duration, and hyperlipidemia were independent risk factors for CVE – all of which have been reported to be significantly associated with testosterone deficiency. However, although our patients received medical interventions for the aforementioned coronary risk factors from other physicians, their FT levels remained an independent risk factor for CVE. Therefore, lower FT levels may have a direct negative effect on the cardiovascular system regardless of the presence of various metabolic factors. These findings highlight the importance of evaluating FT levels in the management of patients with cardiovascular risk factors.
Numerous investigations have shown associations between low testosterone levels and the incidence or increased risk of mortality from CVDs. A cross-sectional study found that men with total testosterone (TT) levels in the highest quartile (550 ng/dL) had a smaller risk of CVE than men with TT levels in the lower quartiles (HR: 0.70, 95% CI: 0.56–0.88), despite adjusting for traditional cardiovascular risk factors and excluding men with known CVDs at baseline [Citation17]. Moreover, a study by Yeap et al. found that in a cohort of 3443 men aged >70 years, lower TT (HR: 1.99, 95% CI: 1.33–2.99) and FT (HR: 1.69, 95% CI: 1.15–2.48) levels predicted an increased incidence of ischemic stroke following adjustments for risk factors of ischemic stroke and age [Citation18]. With regard to mortality, a longitudinal study of 2599 men with and without LOH syndrome demonstrated that during the follow-up period of 3.0–5.7 years, patients with moderate and severe hypogonadism had a threefold (HR 2.9, 95% CI, 1.2–7.1) and eightfold (HR 8.5, 95% CI, 3.0–23.8) higher risk of death from CVDs compared with men without LOH, respectively [Citation11]. Additionally, when Hyde et al. investigated causes of death in 605 patients from a 5-year prospective study (n = 12,203), low FT levels at baseline were significantly associated with mortality from all causes (HR: 1.62, 95% CI: 1.20–2.19) and CVDs (HR: 1.71, 95% CI: 1.12–2.62) [Citation15]. Conversely, in a retrospective study of 1470 men with documented low TT levels and previous myocardial infarction, Oni et al. proved that normalization of TT levels with testosterone replacement therapy (TRT) was associated with decreased all-cause mortality compared with those with non-normalized TT levels (HR: 0.76, 95% CI: 0.64–0.90) and the untreated group (HR: 0.76, 95% CI: 0.60–0.98) [Citation19]. Considering that most of the aforementioned studies were conducted on Caucasians, the current study investigated the relationship between endogenous testosterone and CVE in Asians, with our results suggesting the clinical importance of measuring testosterone levels in Caucasians as well as Asians.
Meanwhile, numerous previous studies have found no correlation between low testosterone levels and cardiovascular deaths or events [Citation20]. This discrepancy may be attributed to differences in population characteristics, such as age, race, presence of other coronary risk factors, follow-up duration, and serum testosterone cut-off levels. Moreover, most studies investigating the correlation between testosterone levels and CVE were conducted on Caucasians, whereas very few studies have targeted Asian populations. Therefore, the present study, which included a relatively large number of subjects, may be of considerable significance.
Certain mechanisms may be responsible for the ability of endogenous testosterone to prevent future CVE in men with several coronary risk factors. A previous study reported that baseline TT levels were inversely correlated with changes in intra-abdominal fat after 7 years and proposed that lower baseline TT levels were independent predictors of visceral obesity [Citation21]. Further, studies have reported close associations between testosterone deficiency and other metabolic factors. Accordingly, a cross-sectional analysis of 2470 men aged >70 years without diabetes demonstrated tapering declines in TT and FT levels in a homoeostatic model and suggested that lower TT levels were associated with insulin resistance [Citation22]. Tsujimura et al. demonstrated a lower serum TT level was associated with higher levels of HbA1c in the study comprising 1150 Japanese men [Citation7]. We also showed HbA1c value of the subjects in the highest FT tertile was the lowest significantly in , whereas the prevalence and the duration of DM among the patients in that group were the highest. However, the number and the period of DM between the groups were not different significantly. Mäkinen et al. revealed that low testosterone levels were associated with potentially deleterious lipid profiles, including high total cholesterol and triglyceride and low high density lipoprotein cholesterol levels [Citation23]. Moreover, a longitudinal study including 702 middle-aged men showed that low testosterone levels independently predicted the development of metabolic syndrome and DM after a follow-up period of 11 years, with odds ratios of 2.23 and 2.28, respectively [Citation24]. On the other hand, in 77 hypogonadal men with a history of CVD who received TRT, Haider et al. observed that weight, waist circumference, lipid pattern, glycemic control, blood pressure, heart rate, and pulse pressure, which are cardio-metabolic parameters, all improved significantly and sustainably in up to 8 years [Citation25]. Therefore, low testosterone levels may contribute to an increased risk of CVE by promoting new metabolic risk factors or worsening existing coronary risks factors.
Furthermore, several basic studies have found that testosterone itself has a vasodilatory effect on arteries independent of the endothelium and nitrogen monoxide [Citation26–28]. Moreover, the anti-inflammatory and cardioprotective effects of testosterone might be involved in the final processes leading to CVE [Citation29]. Indeed, men with lower FT levels reportedly have significantly higher levels of highly sensitive C-reactive protein (hsCRP), which is a biomarker of chronic inflammation in systemic vessels and a likely predictor for CVE, compared with those with higher FT levels [Citation30]. Conversely, one previous study demonstrated that long-term testosterone replacement can result in a continuous decrease in hsCRP levels over 5 years [Citation31]. Therefore, testosterone may exert a direct positive effect on the prevention of CVE. Accordingly, measurement of FT levels should be included as a clinical intervention for patients with cardiovascular risk factors.
The present study has some limitations worth noting. Primarily, the evaluation of serum FT levels using radioimmunoassay has typically been considered unreliable, considering that the guidelines from the European Academy of Andrology [Citation3], American Urological Association [Citation32], and British Society for Sexual Medicine [Citation33] currently recommend relying on serum TT levels for the diagnosis LOH syndrome. Moreover, the European Academy of Andrology suggests the calculation of FT levels based on TT, sex hormone-binding globulin, and albumin levels [Citation3]. However, a large epidemiological study conducted in Japan found that serum FT levels, but not TT levels, significantly correlated with aging – possibly a unique finding that is a characteristic of Japanese individuals – and noted a favorable correlation (R2=0.4238) between calculated FT and analog FT levels measured using a radioimmunoassay [Citation2]. Such favorable correlations have also been confirmed in another Japanese study [Citation34]. Hence, Japanese guidelines recommend the use of serum FT levels to diagnose LOH. It should be noted that between 2007 and 2009, when our patients underwent serum FT testing, only one type of kit for measuring FT levels was commercially available in Japan. In addition, the observational nature of this retrospective study will inevitably produce results that are lower in quality than those of longitudinal studies. Moreover, our findings are not applicable to community-based populations considering that this study only included participants with coronary risk factors. For example, one epidemiological study found that healthy Japanese men in their 50s and 60s had a mean serum FT level of 12.0 pg/mL and 10.3 pg/mL, respectively [Citation2]. However, in the present study, the mean plasma FT levels of the 384 participants in their 50s and 60s were 9.8 and 8.6 pg/mL, respectively, which were considerably lower than those in the healthy men. Moreover, our participants who had coronary risk factors often received medications that could have been altered during the follow-up period, which may have influenced their testosterone levels and cardiovascular risk. However, these biases were not investigated in the present study. Our results were based on only single measurements of FT at baseline. Two measurements of testosterone on separate days are recommended [Citation3]. Therefore, further prospective studies including multiple FT calculation and several evaluations throughout the follow-up period are required to clarify the correlation between testosterone levels and the occurrence of CVE among patients with coronary risk factors.
Conclusions
The present study found that low serum FT levels were an independent predictor for the occurrence of CVE in middle-aged Japanese men with coronary risk factors. The study findings suggest the importance of evaluating FT levels in the management of patients with cardiovascular risk factors and provide evidence supporting the protective role of endogenous testosterone against the development of CVD in elderly men.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Muller M, den Tonkelaar I, Thijssen JH, et al. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149(6):583–589.
- Iwamoto T, Yanase T, Koh E, et al. Reference ranges of total serum and free testosterone in Japanese male adults. Nihon Hinyokika Gakkai Zasshi. 2004;95(6):751–760.
- Corona G, Goulis DG, Huhtaniemi I, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European Society of Endocrinology. Andrology. 2020;8(5):970–987.
- Blaya R, Thomaz LD, Guilhermano F, et al. Total testosterone levels are correlated to metabolic syndrome components. Aging Male. 2016;19(2):85–89.
- Grosman H, Rosales M, Fabre B, et al. Association between testosterone levels and the metabolic syndrome in adult men. Aging Male. 2014;17(3):161–165.
- Grossmann M, Ng Tang Fui M, Cheung AS. Late-onset hypogonadism: metabolic impact. Andrology. 2020;8(6):1519–1529.
- Tsujimura A, Miyagawa Y, Takezawa K, et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology. 2013;82(4):814–819.
- Akishita M, Hashimoto M, Ohike Y, et al. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210(1):232–236.
- Liao PW, Wu CC, Chen KC, et al. Testosterone threshold for increased cardiovascular risk in middle-aged and elderly men: a locally weighted regression analysis. J Sex Med. 2016;13(12):1872–1880.
- Li L, Guo CY, Jia EZ, et al. Testosterone is negatively associated with the severity of coronary atherosclerosis in men. Asian J Androl. 2012;14(6):875–878.
- Pye SR, Huhtaniemi IT, Finn JD, et al. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99(4):1357–1366.
- American Association of Dental Examiners. Committee to Develop Guidelines on Sexual Boundaries. American Association of Dental Examiners guidelines on unprofessional conduct involving sexual boundary violations: the report of the AADE Committee to Develop Guidelines on Sexual Boundaries. Chicago (IL): American Association of Dental Examiners; 2007.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252.
- Teramoto T, Sasaki J, Ueshima H, et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14(4):155–158.
- Hyde Z, Norman PE, Flicker L, et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab. 2012;97(1):179–189.
- Jones TH, Kelly DM. Randomized controlled trials – mechanistic studies of testosterone and the cardiovascular system. Asian J Androl. 2018;20(2):120–130.
- Ohlsson C, Barrett-Connor E, Bhasin S, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58(16):1674–1681.
- Yeap BB, Hyde Z, Almeida OP, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94(7):2353–2359.
- Oni OA, Dehkordi SHH, Jazayeri MA, et al. Relation of testosterone normalization to mortality and myocardial infarction in men with previous myocardial infarction. Am J Cardiol. 2019;124(8):1171–1178.
- Arnlöv J, Pencina MJ, Amin S, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;145(3):176–184.
- Tsai EC, Boyko EJ, Leonetti DL, et al. Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord. 2000;24(4):485–491.
- Yeap BB, Chubb SA, Hyde Z, et al. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study. Eur J Endocrinol. 2009;161(4):591–598.
- Mäkinen JI, Perheentupa A, Irjala K, et al. Endogenous testosterone and serum lipids in middle-aged men. Atherosclerosis. 2008;197(2):688–693.
- Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036–1041.
- Haider A, Yassin A, Haider KS, et al. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: observational, real-life data from a registry study. Vasc Health Risk Manag. 2016;12:251–261.
- O'Connor EK, Ivey JR, Bowles DK. Differential effects of androgens on coronary blood flow regulation and arteriolar diameter in intact and castrated swine. Biol Sex Differ. 2012;3(1):10.
- Jones RD, English KM, Jones TH, et al. Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci (Lond). 2004;107(2):149–158.
- Tep-Areenan P, Kendall DA, Randall MD. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Br J Pharmacol. 2002;135(3):735–740.
- Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78(3):432–437.
- Shigehara K, Konaka H, Ijima M, et al. The correlation between highly sensitive C-reactive protein levels and erectile function among men with late-onset hypogonadism. Aging Male. 2016;19(4):239–243.
- Traish AM, Haider A, Doros G, et al. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. Int J Clin Pract. 2014;68(3):314–329.
- Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200(2):423–432.
- Hackett G, Kirby M, Edwards D, et al. British Society for sexual medicine guidelines on adult testosterone deficiency, with statements for UK practice. J Sex Med. 2017;14(12):1504–1523.
- Taya M, Koh E, Izumi K, et al. Comparison of testosterone fractions between Framingham Heart Study participants and Japanese participants. Int J Urol. 2014;21(7):689–695.