Abstract
Background
We aimed to investigate the efficacy and tolerability of ultra-hypofractionated radiotherapy (UHRT) in the treatment of low and intermediate-risk prostate cancer patients.
Methods
This retrospective study was conducted using data derived from 44 patients who underwent UHRT, and toxicity assessment and clinical response were investigated. Treatment consisted of 35–36.25 Gy in 5 fractions using stereotactic ablative radiotherapy (SABR) with the Linac-based delivery system.
Results
The median duration of follow-up was 52 months (8–68 months) and the median age was 71.5 years (54–85 years). Twenty-seven patients were assigned as intermediate-risk, whereas 17 patients had low-risk. The 5-year overall survival rate was 87.8%, while the 5-year biochemical recurrence-free survival (bRFS) rate was 97.4%. Acute grade 3 genitourinary (GU) side effect was not observed in any patient, whereas acute gastrointestinal (GI) system grade 3 side effect was seen in 6.8% of the patients. Late grade 3 GU and GI side effects were seen in 4.6% and 6.8% of the patients, respectively. In patients with planning target volume (PTV) ≥85 ml, acute grade ≥2 GU side effects were more common (p=.034).
Conclusion
Our data demonstrated that UHRT administered with volumetric arc therapy (VMAT) can be recommended for selected patients with low-intermediate risk prostate cancer. Further prospective, multicentric, controlled trials on larger series are warranted to reach more accurate conclusions.
Introduction
Prostate cancer is a frequent malignancy and constitutes a remarkable cause of cancer-related mortality in men [Citation1]. Therapeutic alternatives for low and intermediate-risk non-metastatic prostate cancer involve active surveillance, radical prostatectomy, and radiotherapy [Citation2]. Stereotactic ablative radiotherapy (SABR) is based on the administration of biological effective radiotherapy in ≤5 fractionated doses and it is utilised in the treatment of localised prostate cancer [Citation3]. Relevant publications demonstrated that the alpha/beta ratio is low (1.5–3.0 Gy) in slow-proliferating prostate cancer cells. The low alpha/beta ratio is consistent with the increased therapeutic ratio and indicates a higher sensitivity of tumour cells for hypofractionated radiotherapy [Citation4,Citation5]. Hypofractionation is supposed to shorten the total duration of treatment and provides satisfactory outcomes as conventional external beam radiation therapy (EBRT) [Citation6,Citation7]. This method has been approved by The American Society for Radiation Oncology (ASTRO) as an effective and reliable treatment mode for selected low and intermediate-risk prostate cancer patients in the setting of clinical trial [Citation8].
A dose increase in radiotherapy for prostate cancer has been preferred attributed to its advantages to enhance therapeutic outcomes [Citation9–11]. Amplified doses are associated with higher tissue toxicities involving late gastrointestinal (GI) and late genitourinary (GU) system toxicities [Citation9,Citation12]. Developments in the planning and delivery of RT allowed administration of highly conformal radiation dose via intensity-modulated radiotherapy (IMRT). RapidArc (RA, Varian Medical System, Palo Alto, CA, USA) is a volumetric arc therapy (VMAT) that can introduce IMRT doses with single or multiple rotations [Citation13].
As a consequence of its obvious dosimetric benefits over three-dimensional conformal radiotherapies (3D-CRT), IMRT has arisen as the most frequent form of administration of radiotherapy (RT) for prostate cancer [Citation14]. The acceptance of IMRT as a standard treatment modality for EBRT of prostate cancer provided both a better tumour control by amplification of dose for target tissue and less likelihood of GI and GU toxicities [Citation15,Citation16]. Robotic-based radiosurgery system has been extensively studied in the majority of studies investigating prostate cancer treatment and no significant difference was noted between the outcomes using gantry linear accelerator or robotic-based radiosurgery system (Cyberknife). Both IMRT and Cyberknife yielded high conformal dose distribution and similar dosimetric results and coverage of PTV. Moreover, rotational IMRT techniques such as VMAT are associated with better PVT homogeneity compared to the cyberknife treatment plan. [Citation17] The rectum and urinary bladder were exposed to lower doses after use of IMRT techniques [Citation18,Citation19]. Besides, image guidance during EBRT allows meticulous follow-up and correction of errors associated with patient positioning. In circumstances such as prostate cancer, internal organ movement is a critical aspect for positioning and target localisation [Citation20], image guidance enables the delivery of higher doses safely per fractionation. In addition, VMAT is an effective modality in terms of monitor units and duration of treatment compared to fixed field IMRT [Citation21].
During image-guided IMRT (IG-IMRT), using fiducial markers within the prostate gland allow visualisation of the exact position of the gland, as improves the positional accuracy of the target and also provide significant reduction in PTV margins [Citation22].The studies comparing the use or not of fiducial markers IG-IMRT techniques show reduced treatment-related urinary and GI toxicity for the groups with fiducial markers [Citation23,Citation24].
Despite the dose-escalated IG-IMRT provides better biochemical and locoregional control, the prostate-rectum proximity contributes to acute and late GI side effects and perirectal spacers are under investigation to reduce the risk of rectal side effects related to RT. A phase III trial reported improved urinary and rectal quality outcomes of hydrogel spacer user group versus non-user, however in a systematic review there was not found significantly difference between spacer and no-spacer groups of patients within the first year of follow-up as of the limited benefits in long-term quality of life and high cost, routine usage of spacer was not recommended [Citation25,Citation26].
We aimed to share our experience with SABR, which was administered in five fractions using IMRT, in low and intermediate-risk prostate cancer patients. Our treatment results, efficacy, and safety profile of this method was presented together with a brief review of current literature.
Materials and methods
Study design
This retrospective study was performed in the radiation oncology department of a single center. The approval of the local institutional review board was obtained before the study (23.06.2020/48670771-514.10) and all patients had given informed consent for the use of data and the treatment delivery. The information was gathered from the clinical database of our hospital. Medical records of 44 patients diagnosed with low and intermediate-risk prostate cancers were reviewed. All data were checked for integrity.
Data were collected from 44 adult patients (≥18 years of age) who received SABR with VMAT as the primary treatment for prostate adenocarcinoma between February 2013 and March 2019. Eligible patients were no previous prostate surgery and histologically proven adenocarcinoma with diagnosed with low or intermediate-risk prostate cancer as for the National Comprehensive Cancer Network (NCCN) and D’Amico criteria, including Gleason score ≤7; staged T1c–T2c, and prostate specific antigen (PSA) level ≤20 ng/ml [Citation27].
All patients had an Eastern Cooperative Oncology Group performance status between 0–2.
Patients who underwent transurethral resection of the prostate less than 6 weeks before radiotherapy, previous history for radiotherapy, high-risk patients, detection of lymph node involvement, and distant metastases on imaging were excluded from the study.
Treatment plan
Patients were prepared for treatment with a comfortably full bladder and empty rectum in the supine position daily. The target definition was based on computerised tomography (CT) in conjunction with the support of magnetic resonance imaging (MRI) for a more precise delineation of the anatomical configuration of the prostate, rectum, urinary bladder, and bulb of penis. Immobilisation of pelvis was achieved by a custom vacuum lock bag during the simulation and treatment.
The clinical target volume (CTV) was considered for the prostate alone in low-risk patients. For intermediate-risk patients, the proximal third of seminal vesicles was also included in the target. The PTV was defined as CTV, and an additional margin of 5 mm in every direction except 3 mm posteriorly. The guidelines declared in the Radiation Therapy Oncology Group (RTOG) were followed contouring the organs such as the rectum, urinary bladder, penil bulb, and femoral heads that are under risk during treatment [Citation28]. The patients were treated with 6 mV photons using two arcs VMAT plan. Total dose 35–36.25 Gy was prescribed to the 95% of the PTV and it was applied in 5 fractions using RapidArc (Varian Medical Systems, Palo Alto, CA, USA). The VMAT was planned using Aria 11.0 system. The treatment was delivered using daily image guidance with cone-beam CT (CBCT). If necessary, couch repositioning was performed after the automatic matching of CBCT images to reference planning CT scans, followed by manual adjustments. Matching was conducted on the prostate gland and other soft tissue structures. During plan optimisation, the dose-volume constraints for normal tissues had priority over PTV in case of overlapping regions. The target coverage needed was V95%>99% on CTV and 95% on PTV.
The dose-volume objectives for optimisation of dose over organs at risk were as follows: V18 Gy <35% for rectum; V28 Gy < 10% (15%), V32 Gy <5% (10%); D1%<35 Gy; for bladder D1%<35 Gy for other organs such as femoral heads, bulb of penis and other healthy tissue.
The plan was established as for the RapidArc technique with two arcs with the collimator angle set to ± 30°. The maximum dose rate was 2,400 MU/min. Before the delivery of treatment, daily image acquisition with CBCT was taken in each fraction. The treatments were applied over 5 consecutive days for the first 17 patients and on alternating day subsequently [Citation29].
Androgen deprivation therapy
Intermediate-risk patients received luteinizing hormone-releasing hormone (LHRH) agonist as an androgen deprivation therapy (ADT) with the duration of 6 months so as if the treatment was employed 3 months prior to RT as a neoadjuvant treatment and concurrent ADT in 3 months. According to NCCN recommendation; the patients with one or more intermediate-risk factors such as T2b-T2c, Gleason score of 7 and PSA 10–20 ng/ml received ADT [Citation27]. The patients with low-risk group were not treated with ADT. Neoadjuvant hormonal therapy aims to reduce tumour bulk and treat micrometastatic disease together with the primary lesion [Citation30].
Outcome measures
Digital rectal examination, clinical assessment, laboratory data before treatment and during follow-up including toxicity assessment and clinical response as reflected in PSA levels were noted. The follow-up was carried out every three months for the first two years and every six months thereafter. Biochemical failure was described as for the Phoenix definition of PSA rise of ≥2 ng/mL above the nadir [Citation31]. Findings regarding GI and GU toxicity were noted regularly during follow-up visits as for the Common Terminology Criteria for Adverse Events (CTCAE) V.4 classification [Citation32]. Acute toxicity was defined as adverse event occurring within 90 days after completion of SABR.
Statistical analysis
All of our data were analysed using IBM Statistical Package for Social Sciences v.15 program (SPPS Inc., Chicago, IL, USA). Descriptive data were presented as mean and standard deviation or median (interquartile range) values for quantitative variables, while categorical variables were expressed as number (%). Since quantitative variables did not display normal distribution, two independent groups were compared using the Mann–Whitney U-test. Kruskal Wallis test was employed to compare more than two groups, Subgroup analyses were performed with Mann–Whitney U-test and data were interpreted using Bonferroni correction. The ratios in groups were compared using chi-square test. A p-value of less than .05 was considered statistically significant.
Results
The UHRT was completed in 44 patients without any cessation attributed to toxicity or side-effects. The median duration of follow-up was 52 months (range: 8–68 months) and the median age was 71.5 years (range: 54–85 years). The numbers of patients having T2a, T2b, and T2c stages were 28 (63.6%), 12 (27.3%), and 4 (9.1%), respectively (). Thirty-two patients (72.7%) had Gleason score of 6, while 12 cases (27.3%) had Gleason score of 7.Twenty-seven patients were assigned as intermediate-risk prostate cancer of whom 14 (31.8) had favourable risk, and 13 (29.5) had unfavourable risk disease, whereas 17 patients had low-risk. ADT was administered in 23 of 27 patients with intermediate-risk. Four cases who did not receive ADT had undergone either coronary angioplasty and stent procedure or coronary artery bypass surgery for ischaemic heart disease recently. A total of 26 patients received 35 Gy/5 fractions (fx), whereas the remaining group had a total dose of 36.25 Gy/5 fx.
Table 1. Patients and treatment characteristics.
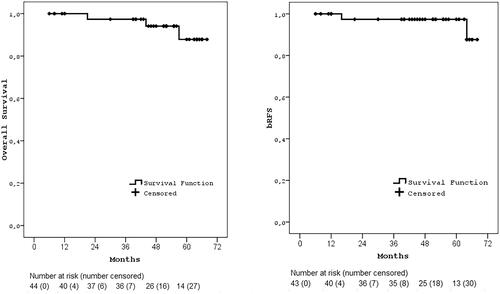
In only two patients, PSA recurrence was detected on the 16th month received 36.25 Gy and 64th month received 35 Gy RT without any local failure or distant metastasis. The 5-year cumulative overall survival rate in our patient population was 87.8%, while the 5-year bRFS rate in our patient population was estimated as 97.4% (). The 5-year bRFS rates were 100% and 96.2% for low and intermediate-risk group, 100% vs. 95.5% for non-ADT and ADT group, and 100% vs. 95.2% in the groups receiving 5 consecutive day vs alternating day treatment, respectively.
Figure 1. Kaplan-Meier survival curves. a) Overall survival, b) bRFS (biochemical recurrence-free survival).
In our series, acute GU toxicities with grade of 2 were noted in 15 (34.1%) while grade 3/4 toxicity was not observed. On the other hand, acute GI toxicities of grade 2 in 4 (9.1%), and grade 3 in 3 (6.8%) patients were detected but grade 4 was not seen. Three patients experienced with grade 3 GI acute toxicity had hematochezia within in the first 90 days in the consecutive day group, treated with argon plasma coagulation. The most common GU side effects were urinary frequency and dysuria, while rectal discomfort was the main GI complaint. Late grade 2 and 3 GU side effects were observed in 3 (6.8%), 2 (4.5%), and grade 2 and 3 GI side effects were observed in 1 (2.3%) and 3 (6.8%) patients, respectively. Grade 4 GU and GI late toxicity was not observed in any patient ().
Table 2. Toxicities by grades.
Before treatment, median PSA level was 9.125 ng/mL (range 2–18.41 ng/mL). The median PSA level was 0.035 ng/mL (range 0–3.02 ng/mL) and it was achieved at median 12 months (7–28 months) after completion of radiotherapy.
There was no statistically significant difference between the baseline PSA values of patients who received treatment daily and alternating days (p=.754) A decreasing trend was detected in firstly measured PSA levels after completion of RT, while 12th month, and PSA values were significantly lower in patients receiving treatment for 5 consecutive days compared to those of patients receiving treatment on alternating days (p=.091, p=.028 and p=.013) (). In the five consecutive day group, receiving hormonotherapy was higher than alternating day group (70.6% vs 40.7%, p=.054).
Table 3. Comparing PSA levels and Grade ≥3 toxicities between 5 consecutive day and alternating day.
There was no significant difference between the two groups in terms of early and late GU toxicity, but early grade ≥3 GI toxicity was almost significantly higher in the group of patients treated for 5 consecutive days (17.6% vs. 0%, p=.051).Compared the groups of patients receiving ADT and non-ADT with acute and late GI–GU grade ≥3 toxicity, no statistical significance was found ().In the ADT group, the median PTV was found to be larger than non-ADT group (115.90 cc vs 98.50 cc, p=.059).
Table 4. Comparing grade ≥3 toxicity between non-ADT and ADT group.
The average prostate volume of 44 patients included in the study was 48.6 cc (36–75.5 cc) and the median CTV was 50.2 cc (36–75.5 cc) and PTV was 110.3 cc (80.3–152.5). In the group with PTV ≥85 cc. Acute grade ≥2 and GU side effects were statistically significantly higher (0% vs 45.2%, p=.034) (). In addition, acute GI grade ≥2, late GI and late GU side effects were observed more frequently but not statistically significantly.
Table 5. Comparing grade ≥2 toxicity between PTV <85 and PTV ≥85.
Discussion
In our clinic we attempted a study to investigate the efficacy and safety of SABR using VMAT with total dose of 35–36.25 Gy, which is the most commonly used in the literature, in patients with low and intermediate risk prostate cancer.
As a standard technique of the EBRT for prostate cancer, IMRT improves tumour control by both intensification of dose directed to the target volume and diminution of GU and GI toxicities [Citation15,Citation16].
Dosimetric studies revealed that isocentric volumetric treatment performed with gantry linear accelerator preserved rectum better and due to superior coverage for target volume, the duration of treatment is shorter than robotic-based surgery. The importance of image guidance during EBRT must not be overlooked. It allows the detection and correction of errors associated with the positioning of the patient. In this manner, image guidance facilitates safe delivery of high doses of radiotherapy per fraction and to achieve precise localisation which may be otherwise hindered by internal organ mobilisation [Citation20]. In comparison to fixed field IMRT, VMAT constitutes the most effective treatment in terms of monitor units and duration of treatment [Citation21].
Comparative studies focussing on conventional fractionation and UHRT in low-risk prostate cancer patients displayed better rates of control together with lower GI and GU toxicities with UHRT [Citation33,Citation34].
In the HYPO-RT-PC study, they compared UHRT with conventional fractionation with a median follow-up for 52 months in 1,200 intermediate and high-risk patients. The study demonstrated that SABR was non-inferior to CRT in terms of biochemical recurrence and late toxicity at 5 years follow-up [Citation35].
A meta-analysis published in 2019 yielded 5-year bRFS rates of all patients was 95.3% while 96.7% and 92.1% in low-risk and intermediate-risk prostate cancers, respectively and their median duration of follow-up was 39 months [Citation36]. Our results agree with the outcomes in the meta-analysis.
Our results are also consistent with the Menkarios et al. [Citation37] study as acute GU toxicity during treatment was grade 2 in 29%, and grade 3 in 4%. No grade 4 acute GU toxicity occurred. Acute GI toxicity was grade 0 in 62%, grade 1 in 27%, grade 2 in 11%, and no grade 3 or 4 occurred during treatment.
In a prospective SABR study, Zimmermann et al. [Citation38] applied 9 Gy up to a total of 45 Gy, and maximal late GU toxicities occurred in 27.5% (grade 2) and 3.8% (grade 3/4) and maximal late GI toxicities in 17.5% (grade 2) and 12.5% (grade 3) of cases. Our toxicity rates were lower, since the fraction dose in our study was lower than the fraction dose in this prospective study.
In the study of Katz et al. [Citation39] performed SABR with Cyberknife, and 515 patients received treatment every day, and applied a total dose of 35 Gy and 36.25 Gy RT. With a median follow-up of 84 months, the 8-year bRFS was 95.3% and 93.5% for patients with 35 Gy vs 36.25 Gy in low and intermediate prostate cancer. In the publications of the same authors comparing treatment-related toxicity, grade 3 acute toxicity was not observed after a minimum of 12 months of follow-up, while late grade 1, 2 and 3 GU side effects were seen in 4%, 2%, 0% in 35 Gy group, while 4.8%, 5.8%, 0.5% in 36.25 Gy group, respectively. [Citation40] When the groups receiving 35 Gy and 36.25 Gy were compared, late grade 1 and 2 rectal toxicities were found in 4.2% and 0% vs 5.3% and 2.9% of the patients, respectively. As in our study, no difference was found between 35 Gy and 36, 25 Gy in terms of bRFS and toxicity. Due to the low number of events, the difference may become apparent when the follow-up time is longer.
Our results indicated that the patients receiving 5 consecutive day treatment had remarkably lower PSA on 12 and 24 months, as well as diminished nadir PSA and lesser time to achieve nadir PSA levels. This result can be explained by the fact that the number of intermediate risk prostate ca patients in the consecutive day treatment group and the rate of those receiving hormonotherapy were high.
A comparison of side effects between groups receiving 5 consecutive day treatment and treatment on alternating day, revealed that acute grade 3 GI toxicity was higher than the literature [Citation29]. Although there was no statistical difference between groups, the result was attributed to the small number of patients. In our preliminary outcomes; long-term side effects were found to be similar with the previous studies. For the reason of acute GI toxicities was found to be higher in consecutive fractionation group, alternating day schedule was applied after then as recommended in ASTRO guideline [Citation8].
When the PTV <85 cc and PTV ≥85 cc were compared in the patients included in our study; it was found that in the group with larger PTV acute grade ≥2 GU side effects were more numerous, and in addition, there was a tendency to increased grade ≥2 acute GI, late GI and GU side effects. . In the recently published study of Franseze et al., they reported that patients with large CTV correlated with acute GI side effects [Citation41]. In the current study, we think that since 70.5% of the patients (n = 31) had larger PTV (≥85 cc), we had higher toxicity rates such as grade 2 and 3 GU side effects. Additionally, all grade 3toxicities were observed in the ADT group. This can be explained by the larger PTV in the ADT group, as the SV was added to the CTV in the intermediate-risk group.
Our results demonstrated that this treatment modality was a practical and well-tolerated option with a 5-year bRFS rate was 97.4% and we suggest that SABR in selected patient group whom have lower PTV, which is administered in several fractions in high doses, can improve the therapeutic gain in prostate cancer, where alpha/beta ratio is low.
The strengths of the current study involve data integrity and adequate duration of follow-up for assessing side effects. However, limitations such as retrospective design, data collected from a single-center, and scarce number of patients must be remembered during the extrapolation of our data to larger populations.
In conclusion, our data demonstrated that UHRT administered with VMAT can be an option for selected patients with low and intermediate-risk prostate cancer. Single-arm prospective trials on low-intermediate risk patients remind a satisfactory biochemical control and low toxicity; however, the duration of follow-up is limited. Therefore, further prospective, multicentric, controlled trials on larger series are warranted to reach more accurate conclusions.
Statement of Ethics
The approval of the local institutional review board was obtained before the study (23.06.2020/48670771-514.10) and all patients had given informed consent for the use of data and the treatment delivery.
Author contributions
Design: STD, HA, TB. Acquisition of data: STD, EU. Analysis or interpretation: TB, HA. Literaturesearch: STD, EU, TB.Writing: STD.Editing: EU, HA.Approval: STD, HA.
Disclosure statement
Authors declare that there is no conflict of interest in this article.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7–34.
- Mohler JL, Kantoff PW, Armstrong AJ, et al. Prostate cancer, version 2. J Natl Compr Canc Netw. 2014;12(5):686–718. 2014;
- Mantz C. A phase II trial of stereotactic ablative body radiotherapy for low-risk prostate cancer using a non-robotic linear accelerator and real-time target tracking: report of toxicity, quality of life, and disease control outcomes with 5-year minimum follow-up. Front Oncol. 2014;4:279.
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited - an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963–974.
- Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17–e24.
- King C. Stereotactic body radiotherapy for prostate cancer: current results of a phase II trial. Front Radiat Ther Oncol. 2011;43:428–437.
- Kishan AU, King CR. Stereotactic body radiotherapy for low- and intermediate-risk prostate cancer. Semin Radiat Oncol. 2017;27(3):268–278.
- Morgan SC, Hoffman K, Loblaw DA, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO and AUA evidence-based guideline. J Urol. 2019;201(3):528–534.
- Beckendorf V, Guerif S, Le PE, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of Getug 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063.
- Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487.
- Peeters STH, Heemsbergen WD, Koper PCM, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996.
- Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239.
- Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310–317.
- Yu T, Zhang Q, Zheng T, et al. The effectiveness of ıntensity modulated radiation therapy versus three-dimensional radiation therapy in prostate cancer: a meta-analysis of the literatures. PLoS One. 2016;11(5):e0154499.
- Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three‐dimensional conformal radiotherapy and intensity‐modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129.
- Zelefsky MJ, Fuks Z, Happersett L, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55(3):241–249.
- Lin YW, Lin KH, Ho HW, et al. Treatment plan comparison between stereotactic body radiation therapy techniques for prostate cancer: non-isocentric cyberknife versus isocentric RapidArc . Phys Med. 2014;30(6):654–661.
- Macdougall ND, Dean C, Muirhead R. Stereotactic body radiotherapy in prostate cancer: is Rapidarc a better solution than cyberknife? Clin Oncol. 2014;26(1):4–9.
- Scobioala S, Kittel C, Elsayad K, et al. A treatment planning study comparing IMRT techniques and cyber knife for stereotactic body radiotherapy of low-risk prostate carcinoma. Radiation Oncol. 2019;14:143.
- Dang A, Kupelian PA, Cao M, et al. Image-guided radiotherapy for prostate cancer. Transl Androl Urol. 2018;7(3):308–320.
- Quan EM, Li X, Li Y, et al. A comprehensive comparison of IMRT and VMAT plan quality for prostate cancer treatment. Int J Radiat Oncol Biol Phys. 2012;83(4):1169–1178.
- Paluska P, Hanus J, Sefrova J, et al. Utilization of cone beam CT for reconstruction of dose distribution delivered in image-guidedradiotherapyofprostatecarcinoma—bonylandmarksetupcompared to fiducial markers setup. J Appl Clin Med Phys. 2013;14:4203.
- Zelefsky MJ, Kollmeier M, Cox B, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:125–129.
- Sveistrup J, afRosenschöld PM, Deasy JO, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol. 2014;9:44.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017;97:976–985.
- Forero D, Dendukuri N, Almeida N. Hydrogel spacer to reduce rectal toxicity in prostate cancer radiotherapy: a health technology assessment. (Report No. 82). Montreal (QC): Technology Assessment Unit (TAU) of the McGill University Health Centre (MUHC); 2018.
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505.
- Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;76(2):361–368.
- Alongi F, Mazzola R, Fiorentino A, et al. Phase II study of accelerated Linac-based SBRT in five consecutive fractions for localized prostate cancer. Strahlenther Onkol. 2019;195(2):113–120.
- Payne H, Mason M. Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: recent developments. Br J Cancer. 2011;105(11):1628–1634.
- Thompson A, Keyes M, Pickles T, et al. Evaluating the phoenix definition of biochemical failure after 125I prostate brachytherapy: can PSA kinetics distinguish PSA failures from PSA bounces? Int J Radiation Oncol Biol Physics. 2010;78(2):415–421.
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676.
- Musunuru HB, Quon H, Davidson M, et al. Dose-escalation of five-fraction SABR in prostate cancer: toxicity comparison of two prospective trials. Radiother Oncol. 2016;118(1):112–117.
- Loblaw A, Pickles T, Crook J, et al. Stereotactic ablative radiotherapy versus low dose rate brachytherapy or external beam radiotherapy: propensity score matched analyses of Canadian data. Clin Oncol. 2017;29(3):161–170.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395.
- Jackson WC, Silva J, Hartman H, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys. 2019;104(4):778–789.
- Menkarios C, Vigneault E, Brochet N, et al. Toxicity report of once weekly radiation therapy for low-risk prostate adenocarcinoma: preliminary results of a phase I/II trial. Radiat Oncol. 2011;6(1):112.
- Zimmermann M, Taussky D, Menkarios C, et al. Prospective phase ii trial of once-weekly hypofractionated radiation therapy for low-risk adenocarcinoma of the prostate: late toxicities and outcomes. Clin Oncol (R Coll Radiol). 2016 ;28(6):386–392. Jun
- Katz A, Formenti SC, Kang J. Predicting biochemical disease-free survival after prostate stereotactic body radiotherapy: risk-stratification and patterns of failure. Front Oncol. 2016;6:168.
- Katz AJ, Santoro M, Ashley R, et al. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 2010;10:1.
- Franzese C, Badalamenti M, Di Brina L, et al. Linac-based Stereotactic Body Radiation Therapy for Low and Intermediate-Risk Prostate cancer: long-term results and factors predictive for outcome and toxicity. Strahlenther Onkol. 2020;196(7):608–616.
