Abstract
Purpose
Multiparametric magnetic resonance imaging (mpMRI) targeted biopsy has emerged as an augmentation to systematic prostate biopsy (SBx) with improved diagnostic accuracy. The purpose of this study was to determine whether biopsy modality impacted management of prostate cancer (PCa).
Methods
We performed a retrospective review of patients with newly diagnosed non-metastatic PCa at our institution (2014–2020). Either ultrasound-guided 12-core SBx or SBx plus ≥1targeted biopsy cores from identifiable lesions on mpMRI were performed. Patients were managed with active surveillance (AS), radiation therapy (RT), or radical prostatectomy (RP). Multivariate logistic and multinomial regression analyses were performed.
Results
Of 578 patients, 221(38%) proceeded with AS, 121(21%) received RT, and 236(41%) underwent RP. Median age and prostate-specific antigen (PSA) were 65.4 years and 7.2 ng/mL, respectively. On multivariate analysis, biopsy type did not predict decision to pursue treatment (p=.951). On multinomial regression analysis, biopsy type did not predict selection of AS over RP (p=.973) or RT over RP (p=.813). Alternatively, age, grade group, and PSA were significant predictors of management selection.
Conclusions
Biopsy technique did not impact management for patients with new PCa diagnosis. Despite paradigm shifts in obtaining tissue diagnosis, age, PSA, and grade group remain valuable indices for shared decision-making and counseling patients with PCa.
Keywords:
1. Introduction
Clinical suspicion for prostate cancer (PCa), such as elevated prostate-specific antigen (PSA) or abnormal digital rectal exam (DRE), warrants further investigation via prostate biopsy to obtain tissue diagnosis [Citation1]. While MRI-guided biopsy is commonly utilized for the detection of other solid-organ tumors, PCa has traditionally been diagnosed using a randomly sampled or template-guided systematic 12-core transrectal ultrasound-guided biopsy (systematic prostate biopsy [SBx]) [Citation2,Citation3]. However, there is concern that SBx may over diagnose clinically insignificant cancer, under diagnose clinically significant prostate cancer (csPCa) and carries a false negative rate as high as 47% [Citation4–6]. In addition, without an identifiable target, many men undergo unnecessary transrectal prostate biopsies leading to increased healthcare costs and potentially life-threatening morbidity [Citation2].
Recently, multiparametric magnetic resonance imaging (mpMRI) targeted biopsy has emerged as an adjunct to SBx that improves diagnostic accuracy for patients with prostate lesions visible on imaging. When compared to SBx alone, the addition of mpMRI targeted prostate biopsy has been shown to detect a greater ratio of clinically significant to clinically insignificant cancer [Citation2,Citation5,Citation7]. However, studies have shown that SBx is not obsolete and thus is often used in combination with a targeted biopsy [Citation8]. In the past, patient age, race, PSA, and Gleason score have been studied to determine their influence on the decision to proceed with watchful waiting, active surveillance (AS), or treatment [Citation9–11]. However, to the best of our knowledge, we are not aware of an investigation into the impact of combined biopsy (SBx and targeted biopsy) on subsequent PCa management.
With this paradigm shift in the approach to detection and tissue diagnosis of PCa, our primary outcome was to determine whether there was a significant impact of the type of biopsy on treatment choice for men treated for PCa at our institution. Furthermore, we studied other clinicopathologic factors as secondary outcomes to evaluate their effect on clinical decision-making in the setting of shifts to a targeted prostate biopsy.
2. Materials and methods
2.1. Study population and data
After obtaining Institutional Board Review approval, we performed a retrospective review of men who underwent either systematic or combined prostate biopsy with subsequent newly diagnosed non-metastatic PCa between January 2014 and March 2020. We included patients who chose one of three standard of care management options at our institution: AS, radiation therapy (RT) (external beam radiation and/or brachytherapy with or without androgen deprivation therapy), or radical prostatectomy (RP). Patients who had received other PCa treatment such as prostate ablation was excluded from the analysis. Patients who did not receive treatment within 1 year of their PCa diagnosis but were still being followed by our institution were included in the AS cohort for the purpose of analysis. Patient demographic and clinical characteristics, as well as prostate biopsy results were recorded. csPCa was defined as ≥ Grade group (GG) 2 disease.
2.2. Image acquisition and interpretation
Pelvic mpMRI was obtained using either a 1.5 Tesla and endorectal coil or 3-Tesla whole-body units (Signa HDx GE Healthcare, Waukesha, WI). We utilized a dedicated prostate imaging protocol in line with literature recommendations outlined by the Prostate Imaging-Reporting and Data System: 2015, Version 2.0.9 (PI-RADS-v2) [Citation12]. All imaging studies were read by genitourinary radiologists with specialization in interpreting mpMRI of the prostate.
2.3. Biopsy protocol
SBx was performed using a sextant template to capture 12 random prostate biopsy cores transrectally under ultrasound guidance. Combined biopsy consisted of combined SBx and MRI targeted fusion biopsy (FBx) (ARTEMIS™, Eigen Inc., Grass Valley, CA) which incorporates both rigid and elastic fusion. FBx was defined as ≥1 targeted cores taken from all prostatic lesions concerning for possible malignancy following a pre-biopsy pelvic mpMRI. In our cohort, all patients that received a pre-biopsy pelvic mpMRI received a targeted MRI/US FBx. Indications for pelvic mpMRI included abnormal DRE or elevated PSA. Pathologic analysis of biopsy specimens was performed by dedicated genitourinary pathologists at our institution.
2.5. Statistical analysis
Statistical analysis was performed by using STATA version 13.0 (StataCorp LP, College Station, TX). Descriptive analysis was performed on patients’ characteristics and pathology results. Kruskal–Wallis Rank Sum Test was used to compare distribution of continuous variables. Pearson chi-square test and Fisher’s exact test were used to compare proportions of categorical variables. Multivariate logistic regression and multinomial regression analyses models were prepared to control for possible confounding clinical and demographic variables and evaluate the impact of type of biopsy on the treatment choice. Continuous variables were divided into the categories for regression analysis. Age was categorized into three categories <60 years, 60–70 years, and older than 70 years. PSA was divided into three categories of 0–10, 10–20, and >20 ng/ml. Prostate volume was divided into 0–40, 40–60, and >60 cc. RP was defined as base outcome for multinomial logistic regression analysis. Statistical significance was defined as p<.05 for both analyses.
3. Results
3.1. Patient characteristics
We reviewed a total of 578 men with a median age of 65.4 years (IQR 60–70.6) and PSA of 7.2 ng/ml (IQR 5.2–11.4). Of the total cohort, 221 (38%) patients chose AS, 121 (21%) patients received RT, and 236 (41%) patients underwent RP. Demographic and clinicopathologic parameters are shown in .
Table 1. Clinical, demographic, and pathologic data of total cohort (n = 578).
csPCA was found in 413 (73%) total patients. Of these, 84 (20%) were on AS, 112 (27%) underwent RT, and 217 (53%) underwent RP. Several baseline differences between PCa management subgroups are detailed in and include age, prostate volume, PSA, and GG (p<.001).
3.2. Role of biopsy type in predicting active surveillance versus treatment
A multivariate logistic regression was performed to evaluate if biopsy technique predicted whether patients chose AS or treatment. After controlling for age, PSA, GG, gland volume, DRE findings, family history, and race, biopsy type (OR = 1.02, 95% CI = 0.62–1.66, p=.951) did not predict the decision to pursue treatment over AS. However, younger age, higher PSA, and GG favored treatment over AS ().
Table 2. Multivariate logistic regression (n = 578).
3.3. Role of biopsy type in treatment type selection
Multinomial regression analysis was performed to identify factors predicting AS and RT over RP as the base outcome. Biopsy type did not predict the management selection of AS over RP (Coefficient = 0.01; 95% confidence interval [CI] − 0.51–0.53, p=.973) or RT over RP (Coefficient = 0.06; 95% CI −0.45–0.57, p=.813) after controlling for clinicopathologic parameters (). Biopsy type also did not predict the management selection of RT over AS (Coefficient = 0.05; 95% CI −0.54–0.64, p=.863) after controlling for clinicopathologic parameters.
Table 3. Multinomial logistic regression (n = 578) (radical prostatectomy = base outcome).
4. Discussion
The advancement and utilization of prostate mpMRI has led to an improvement in the diagnostic accuracy of prostate biopsy. With the acquisition of a clearer tissue diagnosis the decision to proceed with one management pathway over another has become more informed. To our knowledge and to date, there has not been a study investigating a possible relationship between biopsy type and subsequent treatment selection. We sought to determine if the decision to pursue treatment is impacted by the type of prostate biopsy men undergo for the diagnosis. The results of our single institutional review suggest that once PCa is diagnosed, subsequent management selection is not dictated by the differences in diagnostic biopsy techniques and is determined by the standard clinicopathologic parameters. We found that age, pathology, and PSA continue to be important clinical characteristics that impact management in our cohort.
Given the validated and improved ability of detection of mpMRI targeted biopsy for the detection of csPCa [Citation2,Citation5], we hypothesized that there may be an alteration in how various degrees of disease were managed. As Shoji et al. posited the improvement of technology and the continued development of the targeted biopsy should allow for treatment options to be more specific for each individual with localized PCa [Citation13]. While it is certainly possible that targeted biopsy leads to a more individualized management we were unable to substantiate this claim at this time. Although targeted biopsy increases the ratio of detection of clinically significant to clinically insignificant PCa, it is likely that the role of biopsy technique is summative to patient comorbidities, ancillary testing, consultant expertise, and shared decision-making when it comes to choosing AS or treatment.
Specific clinicopathologic characteristics have been shown to impact care for patients with localized PCa [Citation9]. Our analysis demonstrated that age, GG, and PSA are significant indices of PCa management, which is consistent with that reported in the literature [Citation10,Citation11]. GG is used as a measure of disease severity and is an important part of determining whether or not to pursue treatment [Citation10]. In a study by Bergh et al., the decision to pursue treatment with curative intent was more likely based on younger patient age and three times more likely for patients showing intermediate-risk PCa. On the other hand, treatment with curative intent was less frequently chosen with increasing age, with a 12% decrease in 5-year increments [Citation11]. Our cohort demonstrated similar findings, as increasing age was correlated with a lower probability of RP (). In addition, PSA is used in current practice to drive management selection as evidenced by several AS programs who utilize a threshold of PSA of 10 ng/ml as the upper limit for appropriate management with AS [Citation14]. We identified similar results, as higher PSA values lead to a greater inclination to pursue more invasive management.
Figure 1. Results from multivariate logistic regression analysis demonstrating the effect of age on management selection. A line graph demonstrating the probability of a certain preference of management selection for prostate cancer based off of age.
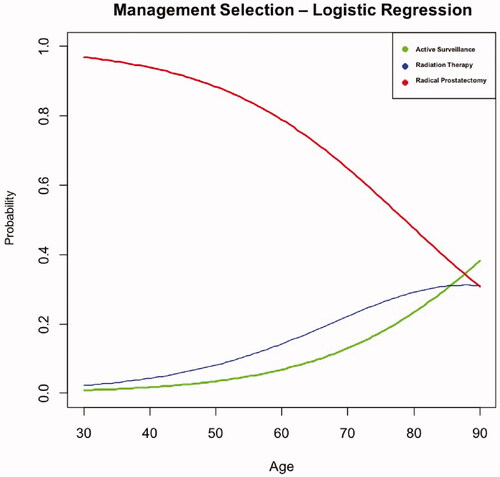
Despite the trend amongst urologists to utilize mpMRI and targeted prostate biopsy [Citation2,Citation5,Citation7], biopsy technique perhaps does not play as dominant a role in clinician and patient decision-making when managing a new diagnosis of PCa. Urologists assuredly continue to rely on standard clinicopathologic factors and their clinical judgment to navigate patient counseling. Gwede et al. explored additional factors that impact shared decision-making and discussed the importance of cognitive factors in the selection of PCa management [Citation15]. The authors suggested that physician bias toward a specific treatment and the patient’s perception of the management type can be more influential for managing patients with PCa diagnosis. As the care pathway becomes more patient-selective, and treatment begins to be segregated into whole versus partial gland therapy, imaging for the purposes of more precisely targeting treatment may begin to be used more frequently to guide management [Citation13,Citation16].
There are several limitations to our single institutional retrospective review. First, the potential for selection bias and lack of data on potential confounders impacting management selection (e.g. patients’ preferences, education level, and genitourinary function) associated with the retrospective nature of this study must be noted. Second, five different urologists with various levels of training, experience with FBx, and methods of counseling represented the clinicians included in our review; therefore, clinician bias and their impact on the results of the prostate biopsy is noted. However, we believe that current urologic practice is such that not all urologists obtain mpMRI, and those that do may handle imaging findings differently depending on prostate and patient specific clinical factors as previously discussed. Lastly, we did not include patients with newly diagnosed PCa who received prostate ablation, which may impact the interpretation of our results regarding prostate biopsy’s impact on the decision treat and which modality to use. We deliberately limited our analysis to more established standard of care management to facilitate the generalizability of our findings.
5. Conclusion
In spite of shifts toward targeted prostate biopsy for the diagnosis of PCa, management selection of newly diagnosed PCa is not based on the biopsy modality after controlling for clinicopathologic parameters. Prospective and randomized study will be required to elucidate if the benefits of mpMRI targeted prostate biopsy independently influence the management of PCa.
Ethical approval
Our study follows tenets of Declaration of Helsinki.
Disclosure statement
The authors report no conflict of interest. All authors read and approved the final manuscript. Abhinav Sidana designed the study. Brandon A. Levin, Daniel J. Lama, and Abhinav Sidana drafted the manuscript. Tianyuan Guan and Marapelli Rao ran analysis on data.
References
- Hoge C, Maynor S, Guan T, et al. A comparison of cancer detection rates between template systematic biopsies obtained using magnetic resonance Imaging-Ultrasound fusion machine and freehand transrectal Ultrasound-Guided systematic biopsies. J Endourol. 2020;34(10):1095–1098.
- Ahmed HU, Bosaily AE-S, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822.
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion–guided biopsy with Ultrasound-Guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–397.
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-Targeted or standard biopsy for Prostate-Cancer diagnosis. N Engl J Med. 2018;378(19):1767–1777.
- Sidana A, Watson MJ, George AK, et al. Fusion prostate biopsy outperforms 12-core systematic prostate biopsy in patients with prior negative systematic biopsy: a multi-institutional analysis. Urol Oncol Semin Orig Investig. 2018;36(7):341.e1-341–e7.
- Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65(4):809–815.
- van den Bergh RCN, van der Poel HRM, Minhaj Siddiqui S, et al. Magnetic resonance imaging/ultrasound–fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2014;65(6):e106–e107.
- Sugano D, Sidana A, Calio B, et al. MRI-targeted biopsy: is systematic biopsy obsolete? Can J Urol. 2017;24(4):8876–8882.
- Moses KA, Orom H, Brasel A, et al. Racial/ethnic disparity in treatment for prostate cancer: does cancer severity matter. Urology. 2017;99:76–83.
- Brunese L, Mercaldo F, Reginelli A, et al. Formal methods for prostate cancer gleason score and treatment prediction using radiomic biomarkers. Magn Reson Imaging. 2020;66:165–175.
- Bergh RC, Loeb S, Moyer VA, et al. Current impact of age and comorbidity assessment on prostate cancer treatment choice and over/undertreatment risk. World J Urol. 2017;35:587–593.
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol. 2016;69(1):16–40.
- Shoji S, Hiraiwa S, Hanada I, et al. Current status and future prospective of focal therapy for localized prostate cancer: development of multiparametric MRI, MRI-TRUS fusion image-guided biopsy, and treatment modalities. Int J Clin Oncol. 2020;25(4):509–520.
- Kinsella N, Helleman J, Bruinsma S, et al. Active surveillance for prostate cancer: a systematic review of contemporary worldwide practices. Transl Androl Urol. 2018;7(1):83–97.
- Gwede CK, Pow‐Sang J, Seigne J, et al. Treatment decision‐making strategies and influences in patients with localized prostate carcinoma. Cancer. 2005;104(7):1381–1390.
- Calio B, Kasson M, Sugano D, et al. Multiparametric MRI: an opportunity for focal therapy of prostate cancer. Semin Roentgenol. 2018;53(3):227–233.
