ABSTRACT
Little is known about the information parents of children with cancer find when searching for clinical trials information on YouTube. Thus, this study aimed to analyse the content, quality and reliability of YouTube videos focused on clinical trials for paediatric cancer. A descriptive cross-sectional design was used, and YouTube was searched using the phrases ‘clinical trials for children with cancer’ and ‘paediatric cancer clinical trials’. Videos that met inclusion criteria were assessed using the instruments Global Quality Scale and DISCERN. About half of the examined videos were in the GQS excellent-quality group and exhibited a total of 84,804 views. The mean time for videos was 5.7 minutes, they originated from the US or UK, were uploaded after 2016, and had a cancer centre/foundation or children hospital as video source. Half of them were focusing on early experimental trials and had a positive tone. Twenty percent were classified as useful without serious shortcomings, almost 50% as misleading with serious shortcomings, and 30% as inappropriate sources of information. In conclusion, most YouTube videos on paediatric cancer trials are not very informative and fall short of what could ethically be required regarding their facilitation of informed decision-making.
Introduction
The survival rate for children with cancer has increased in recent years, reaching as high as 80% in developed countries (Allemani et al., Citation2018; Pritchard-Jones & Hargrave, Citation2014). Most treatment advances and improved survival rates in cancer are due to findings from clinical trials. Approximately 5% of adults and 50% of paediatric of patients below the age of 15 participate (Bond & Pritchard, Citation2006; Saletta et al., Citation2014).
Even though the informed consent procedure should support parents in their decision-making process, studies indicate that parents often have difficulties understanding the information provided or find it overwhelming (Chappuy et al., Citation2005; Chappuy et al., Citation2010; Eder et al., Citation2007). Not surprisingly, Internet and social media are therefore essential sources of health communication (Chou et al., Citation2009; Gage-Bouchard et al., Citation2017), as well as a specific source of cancer information to many parents (Dominguez & Sapina, Citation2015; Gage & Panagakis, Citation2012; Phillips et al., Citation2019). Although online searches for clinical trials continue to increase (Dolinsky & Metz, Citation2006; Patel et al., Citation2010), the resulting information is usually on a reading level beyond the literacy capabilities of the average reader. This is regardless of whether information is searched from national authorities (Godskesen et al., Citation2018), from a Google search (Hillyer et al., Citation2020), or from the webpages of NCI-designated Cancer Centres in the US (Monaco & Krills, Citation2003).
YouTube is an increasingly popular social media platform where users can share, upload and view videos. With over five billion videos watched per day (Android Authority, Citation2019) and more than two billion unique users monthly (Business of Apps, Citation2020), there is the potential to reach parents through visual means. Parents use the Internet to seek support and information related to their children, and they frequently search much more for health-related topics than the general population (Phillips et al., Citation2019). Studies have examined the quality of YouTube information on children’s health issues but, unfortunately, many videos have poor quality, are neither accurate nor free of bias, and are often anecdotal in nature (Clerici et al., Citation2012; Madathil et al., Citation2015; Strychowsky et al., Citation2013). Those videos are neither editorially assessed, nor under the surveillance of any authorised organisation, which results in many uploaded health information videos generally having poor validity, by presenting non-evidence based ‘facts’ with a potential to mislead or even harm people (Haslam et al., Citation2019).
Since children are relatively unable to protect their own interests and are not capable of giving informed consent, and thus at an increased risk of being harmed. Therefore, specific protections to safeguard children’s rights and welfare in research are often called for (CIOMS, Citation2016). One such protective measure would be to safeguard that parents receive correct information about what their children’s participation could entail. It is of particular importance that the parents grasp that clinical trials only have potential value as an intervention and that it is uncertain whether it will prove beneficial for participants or future patients. In some cases, parents might enrol their child because they perceive a trial as having the purpose of providing them with the best available treatment (the so-called therapeutic misconception).
Little is known about the kind of information parents of children with cancer find when searching for clinical trials information on YouTube. Thus, this study aimed to analyse the content, quality and reliability of YouTube videos focused on clinical trials for paediatric cancer. The research questions were: (i) Does the examined video address the ethical uncertainty inherent in research? (ii) Is the given information balanced and unbiased? (iii) Are both benefits and risks from participation clearly described? (iv) Is the voluntariness of participation in a trial emphasised?
Material and methods
Design
A descriptive, cross-sectional study design was applied to analyse information on clinical trials for paediatric cancer on YouTube.
Inclusion and exclusion criteria
The inclusion criteria for the videos were: spoken in English; focusing on clinical trials for paediatric cancer information; posted 2010 or later; ≤20 minutes, ≥100 views, and with good audio-visual quality and flow (i.e., having a Global Quality Scale value ≥3). Videos in other languages than English, with academic or non-relevant other purposes, posted before 2010, >20 minutes, <100 views, or with a poor Global Quality Scale value (<3) were excluded, as these were believed not to attract that many viewers and therefore not having much impact.
Search strategy
Using the search phrases ‘clinical trials for children with cancer’ and ‘paediatric cancer clinical trials’, a search was conducted (on July 3 and 6 2020) in the English language, using filters for ‘videos’ and ‘view count’; thus, videos were listed according to popularity.
Assessment of quality
The resulting list of videos was evaluated by TG according to audio-visual quality and flow in the next step. To be included, videos needed to have good or moderate audio-visual quality and flow, according to the Global Quality Scale (GQS) (Bernard et al., Citation2007). The GQS is an evaluation tool for Internet resources with a five-point scale, from the lowest score of 1 up to 5 ().
Table 1. Global quality scale.
The overall audio-visual quality, flow, ease of use and quality of each video were assessed. Acceptable videos were defined as those that have either excellent audio-visual quality and flow, good quality and flow, or moderate-quality and flow.
Analysis instrument and procedure
The rating for video content reliability used a modified version of the DISCERN instrument (Charnock et al., Citation1999; DISCERN, Citation1997). The original DISCERN instrument consists of 15 specific questions and a final question about the overall evaluation. DISCERN was initially designed for assessment of written consumer health information on treatment choices but has been used to appraise online health information (Charnock & Shepperd, Citation2004) and in a modified form for video analysis (Khatri et al., Citation2020; Kocyigit et al., Citation2019; Radonjic et al., Citation2020; Ranade et al., Citation2020). The DISCERN instrument questions were modified for our purposes, being changed slightly to reflect an emphasis on video material, research participation (rather than treatment), and research ethical issues (HHS.gov, Citation2018) (see ).
Table 2. A modified version of DISCERN instrument (original in parenthesis when a change has been made).
The modified version used in this study consists of 15 questions and aims at providing a comprehensive valuation of YouTube videos. Since videos on clinical trials for paediatric cancer should prepare parents for the informed consent process and include at least some explanation of the potential benefits and risks, the nature of voluntariness, the uncertainty inherently present in research, and be presented in an objective and unbiased way, questions 6, 8, 10, 11 and 15 were of specific interest.
For ranking, each question is ranked on a scale of 1 to 5, where 5 is given if the quality criterion was completely fulfilled, and 2, 3, or 4 are assigned when the video meets the criterion only partially. Lastly, 1 was assigned if the quality criterion was not fulfilled at all (following handbook available at http://www.discern.org.uk/).
The total score of all 15 questions varies between 75 and 15 points and is classified as ‘excellent with minimal shortcomings’ between 75 and –61 points, ‘good with no serious shortcomings’ between 60 and 46 points, ‘low with serious shortcomings’ between 45 and 31 points, and as ‘inappropriate source of information’ for ≤30 points. Mean and SD are reported for each question and for the total score.
Two researchers (TG and SE), experienced in clinical trial research ethics, ranked the first twelve sampled videos cooperatively to calibrate for accuracy and consistency. Next, TG ranked fourteen videos in line with the modified DISCERN instrument without uncertainty. In uncertain cases SE crosschecked the ranking independently to ensure consistent and intersubjective rankings.
The videos included in this study were evaluated in detail, with noted characteristics that included the country of origin, length, the date of upload, number of views and ‘thumbs up’ or ‘thumbs down’ (a way for viewers to convey like or dislike of the video). The sources for the videos were categorised as: research centre/hospital/foundation with both testimonies from patient/caregiver and professionals speaking, research centre/hospital/foundation where a professional speaks; individual from the general public; or news broadcaster.
Clinical trial phases discussed were categorised into three groups: early trial phases, expressed as experimental therapy or innovative clinical trials for children whose cancer has recurred or whose standard treatment failed; clinical trials in general, no phase explicitly mentioned; or educational, phases I, II and III addressed independently. The videos were also categorised as cognitive or affective in style. Videos mainly using facts and relevant information based on research, presented in a non-persuasive style were coded as cognitive. Videos using a lot of anecdotal and/or testimonial information and more emotionally driven information were coded as affective (Meyer, Citation2008). Videos were coded as unbalanced and biased when focusing on a single, particular trial, presenting evidence from isolated patient cases without any reference to reliable sources of information or external assessments, or when presented from a personal point of view in a sensationalist way. Note that there were no difficult cases somewhere in between whose classification was hard to determine. The rating is more thoroughly described in the DISCERN instrument instructions (DISCERN, Citation1997).
Results
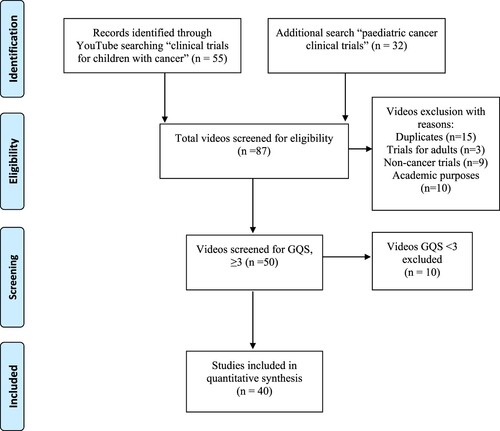
The search process resulted in eighty-seven videos. Thirty-seven of these were excluded with reason (see for the criteria) and the resulting 50 were screened according to GQS criteria. After excluding 10 videos because of poor audio-visual quality and flow, 40 unique videos with acceptable audio-visual quality and flow remained. Of the 40 videos, 22 of them were in the excellent quality group, and 18 were in the good quality or moderate quality group, respectively, and thus included in the analysis ().
Table 3. Characteristics of YouTube videos related to clinical trials for paediatric cancer.
The mean time of the videos was 5.7 min, had their origin from the US (83%) or UK (15%), and were uploaded between 2016 and 2019 (50%) while the rest were uploaded from 2010 to 2016. Ninety-two percent had a cancer centre/foundation or children’s hospital as source. Almost half (45%) included both patients/caregivers and professionals as speakers. 38% were discussing clinical trials for paediatric cancer in general without mentioning any specific trial phase, whereas some videos (12%) were educational and explicitly mentioned all trial phases. Half of the videos focused on early experimental trials, and 55% were affective in style with a positive, hopeful tone. Of the 84,804 viewers who rated the videos, 406 gave ‘thumbs up’, and 67 ‘thumbs down’, so they can in that respect be considered to be perceived of as useful by most viewers.
The ratings using the DISCERN instrument are shown in . The quality of the videos, expressed by the mean overall DISCERN rating scores, was generally low on a scale of 1 to 5, with 5 being the maximum. None of the videos was classified as excellent, 20% (8/40) were classified as useful without serious shortcomings, 48% (19/40) as misleading with serious shortcomings, and 32% (13/40) were even rated as an inappropriate source of information ().
Figure 2. Mean rating per question using the DISCERN tool on a scale from 1 to 5, with 5 being the maximum.
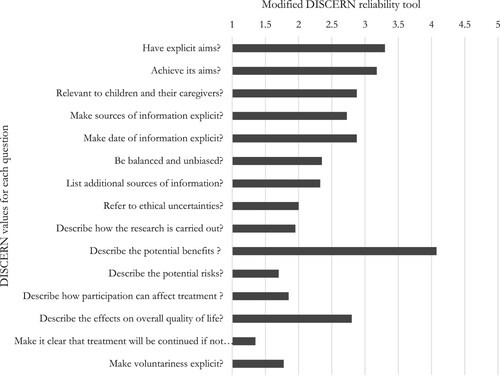
The detailed analysis of the DISCERN values for each question revealed major shortcomings. Information given in videos about clinical trials were frequently unbalanced and biased (question 6; mean 2.3, min-max 1–4, Standard Deviation [SD] 1.0, as well as lacking an explication of the ethical uncertainty inherent in research, question 8; mean 2.0, min-max 1–5, SD 1.2). Potential benefits were disproportionately overrepresented and praised (question 10; mean 4.0 min-max 3–5, SD 0.7), while the risks were mentioned in a condensed form or not referred to at all (question 11; mean 1.7 min-max 1–3, SD 0.8). Voluntariness of participation was not emphasised explicitly; in fact, most videos did not mention this at all (question 15; mean 1.7 min-max 1–5, SD 1.2) (see and ).
The mean scores using the DISCERN tool were low in this study (). None of the videos attained maximum possible scores on DISCERN, indicating that the quality of information on clinical trials for children on YouTube is questionable. The three lowest scores for these videos concerned whether the video discussed potential risks of participation, the consequences of no treatment and voluntariness.
Discussion
To our knowledge, this is the first study to describe the characteristics of the most viewed recent YouTube videos related to clinical trials for paediatric cancer. The average quality of YouTube videos on clinical trials for paediatric cancer was low when scored using DISCERN. This finding is in line with other studies in the quality of YouTube videos of clinical trials (Hillyer et al., Citation2018), idiopathic clubfoot videos (Ranade et al., Citation2020) and paediatric tonsillectomy videos on YouTube (Strychowsky et al., Citation2013).
Most videos in this study had what might seem like a short duration (mean 5.7 min). However, according to YouTube standards, this is not short. Research indicates that most viewers prefer instructional and information videos within the range of 3 to 6 minutes (TechSmith, Citation2021). About half of the videos in this study were anecdotal, subjective and affective in style. Unscientific content was disproportionately overrepresented and most of the focus was on early experimental cancer trials. This is not unique to our study, one study on clinical trials videos by Hillyer et al. (Citation2018) found most cancer trials videos to be short (6.6 min), and frequently using an effective communication style with a positive tone. Further, they concluded that videos on cancer trials also tend to emphasise benefits of participation and present the content as testimonials in a mix of both patient and physician testimony (Hillyer et al., Citation2018). This is in line with our results, which found that nearly half of the videos included professionals and patients/caregivers. Hillyer et al. found that almost half of all the examined YouTube videos informed about phase I trials. This is comparable to our results, which indicated that half of the videos on clinical trials for paediatric cancer had a focus on experimental therapy or innovative early trials for patients whose cancer has recurred or whose standard treatment has failed. The focus on experimental therapy or innovative early trials is important to note since classic phase I cancer trials are primarily aiming to study how much of the drug can safely be given without too many or too severe adverse effects; those trials are probably of little therapeutic benefit for participating children (Kim et al., Citation2008; Morgenstern et al., Citation2014). Therefore, such trials for paediatric cancer present complex clinical and ethical challenges (Howie & Peppercorn, Citation2014). Ethical challenges concern e.g., parents consenting on behalf of their child for enrolment in a trial aiming to assess toxicity (Hazen et al., Citation2015). Therefore, a clear understanding of the aim of a clinical trial, and the experimental characteristics of the study, and the potential risks and benefits of participation, are essential for the ethical conduct of clinical trials in children (Bond & Pritchard, Citation2006). Children who participate in these clinical trials are patients who need complex medical care, as well as individuals who require protection from research risks.
Looking at children’s participation in research, it is complex from both a legal and ethical standpoint and having different regulations in different countries makes it even more challenging (Dove et al., Citation2013). Children’s involvement in the decision process on whether to enrol in a clinical trial has been reported to be low and children may have only a limited understanding of the research. Unguru et al. studied children aged 7 to 18 with cancer and enrolled in studies (Unguru et al., Citation2010). About half of the children did not know or could not recall having had a role in deciding to participate in a study. An obvious remedy to this is to emphasise that children have a right to receive age-appropriate and understandable information. Children should take an active part in the decision-making process and give their assent to participate in research, defined as a willingness to participate in research expressed by those too young to give informed consent, but who are mature enough to understand the proposed research in general and what participation entails for them as subjects (Swartling et al., Citation2009). The videos examined in this study are directed towards the parents, serving a specific enrolment purpose primarily or in general trying to inform about the usefulness of clinical trials.
Since the YouTube videos are primarily for the parents, it falls upon them to include their children in deliberation over possible enrolment in a trial. An important ethical aspect of children’s participation in clinical trials concerns this fact; that the parents, as legal guardians, are supposed to give consent for their children. From a legal point of view, children are not autonomous and cannot approve informed consent themselves. As noted above, however, the ethical goal is to include the children in the decision-making process and respect both their autonomy and integrity. This serves relational autonomy; where decisions made by parents for their child concerning participation in a clinical trial can be seen as a relational enterprise where parents and staff strive to get assent from the child as a decision is made (Lindemann et al., Citation2019; Matar et al., Citation2020).
Thus, the videos on YouTube are important ethically, as they are to provide the first foundation for a shared informed decision-making process that expresses deep relational concerns. It is then an obvious and major ethical problem if experimental and innovative paediatric phase I trials on YouTube use an affective and positive tone bolstered in hope talk.
As video is a medium well suited for providing information for the young, it is also disappointing that quality videos suitable for them are not available.
The videos present an ethical problem
It is important that patients and parents make a free and informed choice about their participation. Half of the studied videos were often overly affective or biased, and omitted or downplayed important research ethical issues. Therefore, they risk being misleading about what participation entails and distort the subsequent informed consent process (Godskesen et al., Citation2018). Behavioural psychology offers a theoretical frame explaining why people may bring with them misconceptions when asked to consent to a trial. If people find themselves in unfamiliar situations with high-stake outcomes and limited time available, reasoning starts from intuitions that might be mistaken and too positive (Kahneman, Citation2013). People are ‘programmed to believe the best’ and susceptible to confirmation bias (Gilbert et al., Citation1990). First impressions influence our judgement and if we come across what we perceive as a positive value, we view subsequent lists of values or characteristics as positive as well. This is known as the ‘halo effect’ (Fernan et al., Citation2018) or ‘priming’ (Mussweiler & Strack, Citation2000). Such mechanisms of the mind explain why information in consent forms can fail in promoting (the difficult) balancing of benefits and costs and instead be read and understood out of a focus on the positive (Gilovich et al., Citation2002). Instead, an ideal YouTube video on clinical trials for paediatric cancer should properly prepare for the informed consent process and include at least some explanation of the potential for both benefits and risks while conveying the uncertainty of research.
Prospective trial participants should get clear and unbiased information. Guidelines on how to produce appropriate key information about clinical trials have previously been published (HHS.gov, Citation2018); however, most YouTube videos on clinical trials for paediatric cancer seem not to adhere to these principles. Of course, such online information is not part of the informed consent process and therefore not scrutinised by IRBs or research ethics committees. Yet, from the perspective of research ethics parents and children should be cognizant of the limitations of YouTube videos, given the importance of such information for the informed consent process.
Implications for practice
Filtering and integrating useful, reliable, and valid sources of health information is a complex cognitive action that is becoming increasingly difficult due to the significant amount of online information available. As the young are more likely to search for anecdotal healthcare information (Lagan et al., Citation2010), appropriate counselling on the quality of these sources is particularly important for such patients and their parents when in contact with trial centres and cancer clinics. Therefore, researchers could as part of the informed consent process caution parents about misleading information on clinical trials for children on YouTube. They could help them obtain reliable information to understand clinical trials better and support their decision to participate or not. Further, healthcare providers should be encouraged to create and upload short informational videos with good visual quality to provide patients and their caregivers with qualitative and accurate information about participation in paediatric cancer trials. Important factors that can promote and sustain quality videos on YouTube have been identified in an integrative review (Haslam et al., Citation2019) and should be carefully considered.
Limitations and further research
Some study limitations should be noted. First, related to the cross-sectional nature of data analysis, findings from our study on YouTube present only a snapshot of information available about clinical trials for paediatric cancer at a specific time. Second, we included only videos after 2010 and with >100 views. Thus, the inclusion of videos uploaded earlier than 2010 and videos with <100 views could give other results, affecting a minor group of viewers. Additionally, non-English language videos were excluded, which reduces the generalisability of our results to videos in other languages. Studies on using the Internet to offer parents guidance on understanding clinical trials are required to inform such efforts.
Conclusion
YouTube has rapidly become one of the essential interactive educational modalities. However, the results in this study indicated that the quality and scientific accuracy of videos about clinical trials for paediatric cancer is insufficient. YouTube videos are therefore poor sources for accurate information on paediatric cancer trials. Other studies that have examined the quality of clinical trial information on the Internet have had similar results. Since Internet is a vital source of information about cancer for parents, YouTube should be considered an important educative tool that could play a significant role in offering high-quality information on clinical trials for children. For this purpose, healthcare providers should be encouraged to upload short informational videos where account for varying levels of understanding of medical information has been taken, with good visual quality, to provide patients and their caregivers with qualitative and accurate information about paediatric cancer trials.
| Abbreviations | ||
| GQS | = | global quality scale |
| IRB | = | institutional review board |
| SD | = | standard deviation |
Availability of data and material
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Acknowledgements
We thank the two anonymous reviewers whose suggestions helped improve and clarify this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Tove Godskesen
TG designed the study and collected data. TG and SE reviewed the videos and analysed the data. TG and SE authored the first version of the paper. SFH and ATH contributed with text and comments on the manuscript. All authors were involved in discussions and revisions of the paper. All authors approved the final version.
References
- Allemani, C., Matsuda, T., Di Carlo, V., Harewood, R., Matz, M., Niksic, M., Bonaventure, A., Valkov, M., Johnson, C. J., Esteve, J., Ogunbiyi, O. J., Azevedo, E. S. G., Chen, W. Q., Eser, S., Engholm, G., Stiller, C. A., Monnereau, A., Woods, R. R., Visser, O., … Group, C. W. (2018). Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet, 391(10125), 1023–1075. https://doi.org/10.1016/S0140-6736(17)33326-3
- Android Authority. (2019). YouTube in numbers: Monthly views, most popular video, and more fun stats!. Retrieved June 29, from https://www.androidauthority.com/youtube-stats-1016070/
- Bernard, A., Langille, M., Hughes, S., Rose, C., Leddin, D., & Veldhuyzen van Zanten, S. (2007). A systematic review of patient inflammatory bowel disease information resources on the World Wide Web. The American Journal of Gastroenterology, 102(9), 2070–2077. https://doi.org/10.1111/j.1572-0241.2007.01325.x
- Bond, M. C., & Pritchard, S. (2006). Understanding clinical trials in childhood cancer. Paediatrics & Child Health, 11(3), 148–150. https://www.ncbi.nlm.nih.gov/pubmed/19030270
- Business of Apps. (2020). YouTube revenue and usage statistics. https://www.businessofapps.com/data/youtube-statistics/
- Chappuy, H., Baruchel, A., Leverger, G., Oudot, C., Brethon, B., Haouy, S., Auvrignon, A., Davous, D., Doz, F., & Treluyer, J. M. (2010). Parental comprehension and satisfaction in informed consent in paediatric clinical trials: A prospective study on childhood leukaemia. Archives of Disease in Childhood, 95(10), 800–804. https://doi.org/10.1136/adc.2009.180695
- Chappuy, H., Doz, F., Blanche, S., Gentet, J. C., Pons, G., & Treluyer, J. M. (2005). Parental consent in paediatric clinical research. Archives of Disease in Childhood, 91(2), 112–116. https://doi.org/10.1136/adc.2005.076141
- Charnock, D., & Shepperd, S. (2004). Learning to DISCERN online: Applying an appraisal tool to health websites in a workshop setting. Health Education Research, 19(4), 440–446. https://doi.org/10.1093/her/cyg046
- Charnock, D., Shepperd, S., Needham, G., & Gann, R. (1999). DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. Journal of Epidemiology and Community Health, 53(2), 105–111. https://doi.org/10.1136/jech.53.2.105
- Chou, W. Y., Hunt, Y. M., Beckjord, E. B., Moser, R. P., & Hesse, B. W. (2009). Social media use in the United States: Implications for health communication. Journal of Medical Internet Research, 11(4), e48. https://doi.org/10.2196/jmir.1249
- CIOMS. (2016). Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-related Research Involving Humans. Geneva 2016. https://cioms.ch/wp-content/uploads/2017/01/WEB-CIOMS-EthicalGuidelines.pdf
- Clerici, C. A., Veneroni, L., Bisogno, G., Trapuzzano, A., & Ferrari, A. (2012). Videos on rhabdomyosarcoma on YouTube: An example of the availability of information on pediatric tumors on the web. Journal of Pediatric Hematology/Oncology, 34(8), e329–e331. https://doi.org/10.1097/MPH.0b013e31825886f8
- DISCERN. (1997). Quality criteria for consumer health information. Retrieved June 30, from http://www.discern.org.uk/discern_instrument.php
- Dolinsky, C., & Metz, J. M. (2006). Palliative radiation therapy in oncology. Anesthesiology Clinics, 24(1), 113–128, viii-ix. https://doi.org/10.1016/j.atc.2005.11.002
- Dominguez, M., & Sapina, L. (2015). Pediatric cancer and the internet: Exploring the gap in doctor-parents communication. Journal of Cancer Education, 30(1), 145–151. https://doi.org/10.1007/s13187-014-0700-4
- Dove, E. S., Avard, D., Black, L., & Knoppers, B. M. (2013). Emerging issues in paediatric health research consent forms in Canada: Working towards best practices. BMC Med Ethics, 14(1), 5. http://doi.org/10.1186/1472-6939-14-5
- Eder, M. L., Yamokoski, A. D., Wittmann, P. W., & Kodish, E. D. (2007). Improving informed consent: Suggestions from parents of children with leukemia. Pediatrics, 119(4), e849–e859. https://doi.org/10.1542/peds.2006-2208
- Fernan, C., Schuldt, J. P., & Niederdeppe, J. (2018). Health halo effects from product titles and nutrient content claims in the context of “protein” bars. Health Commun, 33(12), 1425–1433. https://doi.org/10.1080/10410236.2017.1358240
- Gage, E. A., & Panagakis, C. (2012). The devil you know: Parents seeking information online for paediatric cancer. Sociology of Health and Illness, 34(3), 444–458. https://doi.org/10.1111/j.1467-9566.2011.01386.x
- Gage-Bouchard, E. A., LaValley, S., Mollica, M., & Beaupin, L. K. (2017). Cancer communication on Social media: Examining how cancer caregivers Use facebook for cancer-related communication. Cancer Nursing, 40(4), 332–338. https://doi.org/10.1097/NCC.0000000000000418
- Gilbert, D. T., Krulloch, D. S., & Malone, P. S. (1990). Unbelieving the unbelievable: Some problems in the rejection of false information. Journal of Personality and Social Psychology, 59(4), 601–613. https://doi.org/10.1037/0022-3514.59.4.601
- Gilovich, T., Griffin, D. W., & Kahneman, D. (2002). Heuristics and biases: The psychology of intuitive judgment. Cambridge University Press.
- Godskesen, T. E., Fernow, J., & Eriksson, S. (2018). Quality of online information about phase I clinical cancer trials in Sweden, Denmark and Norway. European Journal of Cancer Care, 27(6), e12937. https://doi.org/10.1111/ecc.12937
- Haslam, K., Doucette, H., Hachey, S., MacCallum, T., Zwicker, D., Smith-Brilliant, M., & Gilbert, R. (2019). Youtube videos as health decision aids for the public: An integrative review. Canadian Journal of Dental Hygiene: JCHD, 53(1), 53–66.
- Hazen, R. A., Zyzanski, S., Baker, J. N., Drotar, D., & Kodish, E. (2015). Communication about the risks and benefits of phase I pediatric oncology trials. Contemporary Clinical Trials, 41, 139–145. https://doi.org/10.1016/j.cct.2015.01.015
- HHS.gov. (2018). Attachment C-New “Key Information” Informed Consent Requirements. U.S. Department of Health & Human Services. Retrieved July 09, from https://www.hhs.gov/ohrp/sachrp-committee/recommendations/attachment-c-november-13-2018/index.html
- Hillyer, G. C., Beauchemin, M., Garcia, P., Kelsen, M., Brogan, F. L., Schwartz, G. K., & Basch, C. H. (2020). Readability of Cancer Clinical Trials Websites. Cancer Control, 27(1), 107327481990112. https://doi.org/10.1177/1073274819901125
- Hillyer, G. C., MacLean, S. A., Beauchemin, M., Basch, C. H., Schmitt, K. M., Segall, L., Kelsen, M., Brogan, F. L., & Schwartz, G. K. (2018). YouTube videos as a source of information about clinical trials: Observational study. JMIR Cancer, 4(1), e10060. https://doi.org/10.2196/10060
- Howie, L. J., & Peppercorn, J. M. (2014). The ethics of clinical trials for cancer therapy. North Carolina Medical Journal, 75(4), 270–273. https://doi.org/10.18043/ncm.75.4.270
- Kahneman, D. (2013). Thinking, fast and slow (1st pbk. ed.). Farrar, Straus and Giroux.
- Khatri, P., Singh, S. R., Belani, N. K., Yeong, Y. L., Lohan, R., Lim, Y. W., & Teo, W. Z. (2020). YouTube as source of information on 2019 novel coronavirus outbreak: A cross sectional study of English and mandarin content. Travel Medicine and Infectious Disease, 35, 101636. https://doi.org/10.1016/j.tmaid.2020.101636
- Kim, A., Fox, E., Warren, K., Blaney, S. M., Berg, S. L., Adamson, P. C., Libucha, M., Byrley, E., Balis, F. M., & Widemann, B. C. (2008). Characteristics and outcome of pediatric patients enrolled in phase I oncology trials. The Oncologist, 13(6), 679–689. https://doi.org/10.1634/theoncologist.2008-0046
- Kocyigit, B. F., Nacitarhan, V., Koca, T. T., & Berk, E. (2019). Youtube as a source of patient information for ankylosing spondylitis exercises. Clinical Rheumatology, 38(6), 1747–1751. https://doi.org/10.1007/s10067-018-04413-0
- Lagan, B. M., Sinclair, M., & Kernohan, W. G. (2010). Internet use in pregnancy informs women's decision making: A web-based survey. Birth, 37(2), 106–115. https://doi.org/10.1111/j.1523-536X.2010.00390.x
- Lindemann, H., McLaughlin, J., & Verkerk, M. (2019). What about the family? Practices of responsibility in care. Oxford University Press.
- Madathil, K. C., Rivera-Rodriguez, A. J., Greenstein, J. S., & Gramopadhye, A. K. (2015). Healthcare information on YouTube: A systematic review. Health Informatics Journal, 21(3), 173–194. https://doi.org/10.1177/1460458213512220
- Matar, A., Hoglund, A. T., Segerdahl, P., & Kihlbom, U. (2020). Autonomous decisions by couples in reproductive care. BMC Medical Ethics, 21(1), 30. https://doi.org/10.1186/s12910-020-00470-w
- Meyer, G. (2008). Internet user preferences in relation to cognitive and affective styles. Thesis. (Publication Number AAI1453906) Iowa State University]. Iowa. https://lib.dr.iastate.edu/rtd/15368/
- Monaco, V., & Krills, S. K. (2003). Online information about cancer clinical trials: Evaluating the Web sites of comprehensive cancer centers. AMIA Annu Symp Proc, 470–475. https://www.ncbi.nlm.nih.gov/pubmed/14728217
- Morgenstern, D. A., Hargrave, D., Marshall, L. V., Gatz, S. A., Barone, G., Crowe, T., Pritchard-Jones, K., Zacharoulis, S., Lancaster, D. L., Vaidya, S. J., Chisholm, J. C., Pearson, A. D., & Moreno, L. (2014). Toxicity and outcome of children and adolescents participating in phase I/II trials of novel anticancer drugs: the Royal Marsden experience. Journal of Pediatric Hematology/Oncology, 36(3), 218–223. https://doi.org/10.1097/mph.0000000000000003
- Mussweiler, T., & Strack, F. (2000). The use of category and exemplar knowledge in the solution of anchoring tasks. Journal of Personality and Social Psychology, 78(6), 1038–1052. https://doi.org/10.1037//0022-3514.78.6.1038
- Patel, C. O., Garg, V., & Khan, S. A. (2010). What do patients search for when seeking clinical trial information online? AMIA Annu Symp Proc, 2010, 597–601.
- Phillips, C. A., Hunt, A., Salvesen-Quinn, M., Guerra, J., Schapira, M. M., Bailey, L. C., & Merchant, R. M. (2019). Health-related Google searches performed by parents of pediatric oncology patients. Pediatric Blood & Cancer, 66(8), e27795. https://doi.org/10.1002/pbc.27795
- Pritchard-Jones, K., & Hargrave, D. (2014). Declining childhood and adolescent cancer mortality: Great progress but still much to be done. Cancer, 120(16), 2388–2391. https://doi.org/10.1002/cncr.28745
- Radonjic, A., Fat Hing, N. N., Harlock, J., & Naji, F. (2020). Youtube as a source of patient information for abdominal aortic aneurysms. Journal of Vascular Surgery, 71(2), 637–644. https://doi.org/10.1016/j.jvs.2019.08.230
- Ranade, A. S., Belthur, M. V., Oka, G. A., & Malone, J. D. (2020). Youtube as an information source for clubfoot: A quality analysis of video content. Journal of Pediatric Orthopaedics B, 29(4), 375–378. https://doi.org/10.1097/BPB.0000000000000694
- Saletta, F., Seng, M. S., & Lau, L. M. (2014). Advances in paediatric cancer treatment. Transl Pediatr, 3(2), 156–182. https://doi.org/10.3978/j.issn.2224-4336.2014.02.01
- Strychowsky, J. E., Nayan, S., Farrokhyar, F., & MacLean, J. (2013). Youtube: A good source of information on pediatric tonsillectomy? International Journal of Pediatric Otorhinolaryngology, 77(6), 972–975. https://doi.org/10.1016/j.ijporl.2013.03.023
- Swartling, U., Helgesson, G., Hansson, M. G., & Ludvigsson, J. (2009). Split views among parents regarding children's right to decide about participation in research: A questionnaire survey. Journal of Medical Ethics, 35(7), 450–455. https://doi.org/10.1136/jme.2008.027383
- TechSmith. (2021). Video statistics, habits, and trends you need to know [new research]. https://www.techsmith.com/blog/video-statistics/
- Unguru, Y., Sill, A. M., & Kamani, N. (2010). The experiences of children enrolled in pediatric oncology research: Implications for assent. Pediatrics, 125(4), e876–e883. https://doi.org/10.1542/peds.2008-3429