Abstract
Objectives: Atrial fibrillation (AF) affects an estimated 1.5 million individuals in Japan, increasing their stroke risk and imposing considerable costs on the Japanese healthcare system. To reduce stroke incidence, guidelines recommend using anticoagulants in moderate-to-high risk non-valvular AF (NVAF) patients; however, many patients receive no treatment, aspirin only, or remain poorly-controlled on vitamin K antagonists (VKAs) due to high VKA discontinuation rates and non-adherence to guidelines. A prevalence-based Markov model was developed to estimate the clinical and budgetary impact of treating these patients with XareltoTM (rivaroxaban, Bayer AG) in Japan.
Methods: Population, baseline risk of events, and associated management costs were estimated using data from Japanese publications where available. Treatment efficacy and safety were derived from published data and the J-ROCKET AF trial. Drug and physician visit costs were based on data from the Ministry of Health, Labor, and Welfare, the J-ROCKET AF trial, and Japanese clinical guidelines.
Results: This model demonstrates that increased use of rivaroxaban in inadequately-managed NVAF patients could avoid 456 081 non-fatal ischemic strokes (IS) and 76 975 cardiovascular deaths over 10 years in Japan. This clinical benefit offsets the increased incidence of myocardial infarctions and anticoagulant-related bleeding. Decreased event costs could lead to a ¥188.4 billion decrease in net spending over the analysis time horizon.
Conclusions: Introducing rivaroxaban may decrease the burden of NVAF in Japanese society. From a clinical perspective, the reduction in IS and embolic events outweighs the increased risk of anticoagulant-related bleeding; from an economic perspective, reduced event costs offset drug and physician visit costs, resulting in cost savings.
Introduction
Atrial fibrillation (AF) is the most common sustained abnormality of heart rhythmCitation1. Approximately 1.5 million individuals were estimated to have AF in Japan in 2010, based on an extrapolation of AF prevalence reported in a community-based registryCitation2,Citation3. This number is expected to increase further due to the aging of the populationCitation1,Citation4,Citation5. AF significantly increases the risk of stroke 5-foldCitation6. Stroke represents a major burden for Japanese society; one in five persons 65 years of age or older who require long-term care have suffered a strokeCitation7. In 2015, it is estimated that there are 2.8 million prevalent cases of stroke and that 300,000 new patients will suffer from stroke in JapanCitation8.
AF-related stroke is a type of cardioembolic stroke, which represents 27.0% of all ischemic strokes (IS)Citation9 and imposes both greater mortality and disability than non-cardioembolic strokesCitation10–12. A prospective study in Japan showed that 13% of patients with cardioembolic stroke died during hospitalizationCitation13. Furthermore, a cohort study reported that 12.5% of patients with cardioembolic stroke died within 1 year following hospital dischargeCitation14. Cardioembolic stroke is also reported to impose the longest hospitalization, lowest levels of activities of daily livingFootnote* (ADLs) both at admission and discharge, and highest medical cost compared to other types of strokeCitation15. Given that in 2014 the Japanese healthcare system spent ¥1777 billion ($15 billion)Citation16 managing cerebrovascular disease, there is clearly a need for improved control of conditions that contribute to, and exacerbate the humanistic and economic burden of cerebrovascular disease.
Non-valvular AF (NVAF) guidelines recommend anticoagulants in NVAF patients with moderate-to-high risk of stroke, as defined by risk scores such as the CHADS2 or CHADS2-VAScCitation17,Citation18. Until recently, vitamin K agonists (VKAs) were the only anticoagulants used to manage AF, but they have a narrow therapeutic rangeCitation19 and interact with a variety of foodsCitation20 and commonly-used drugsCitation21, making it difficult to maintain the international normalized ratio (INR) within the required rangeCitation22,Citation23. Burdensome INR monitoringCitation24 and lifestyle changes required for VKA therapy often lead to poor persistenceCitation25. Only half of NVAF patients are receiving VKAs, as demonstrated in several studies in JapanCitation2,Citation26,Citation27. Even patients receiving VKAs may not be adequately managed due to the difficulty of remaining in the target INR range. A prior study has shown that almost half of those receiving VKAs remained poorly controlledCitation28, highlighting the need for novel approaches of anti-coagulation.
The Japanese Circulation Society (JCS) updated the guidelines for pharmacotherapy of NVAF in January 2014Citation18, promoting the use of anti-coagulants in moderate-to-high risk patients identified using the CHADS2 score (i.e. CHADS2 ≥1). Xarelto (rivaroxaban)Footnote* is a Factor Xa inhibitor that prevents thrombin formationCitation29. It has been studied in more than 14 000 NVAF patients in the ROCKET AF trialCitation30 and 1280 Japanese NVAF patients in the dose-adjusted J-ROCKET AFCitation31 trial, and was included in the JCS guideline updateCitation18.
Assuming new oral anti-coagulants would improve adherence to treatment guidelines, we assessed the clinical and economic impact to the Japanese healthcare system of treating moderate-to-high risk patients currently inadequately-managed (i.e. those receiving no treatment, aspirin only, or poorly-controlled on VKA) with rivaroxaban, over 10 years.
Methods
Model design
A prevalence-based simulation was conducted from the perspective of the Japanese healthcare payers over a 10-year time horizon, taking into account drug acquisition, physician visit, and event costs associated with the treatment patterns before the introduction of novel oral anticoagulants (NOAC), and a revised treatment pattern in which rivaroxaban was used to simulate perfect adherence to treatment guidelines This perspective encompasses patient co-payments—in Japan, patients pay a proportion of direct medical costs, which varies based on their age and income level. The structure of our model is based on a cost-effectiveness model evaluating rivaroxaban for the prevention of stroke and systemic embolism (SE) in patients with AF. The initial model had been reviewed by the UK’s National Institute for Health and Care Excellence (NICE), and used to generate outcomes and cost estimates for Spain, Greece, Slovakia, and the UKCitation32–36. Our budget impact simulation was developed on the basis of this model by not factoring in treatment discontinuation, limiting the time horizon of the model, evaluating fewer comparators, tallying direct costs only, and considering outcomes, rather than quality-adjusted life years (QALYs), as a measure of treatment efficacy.
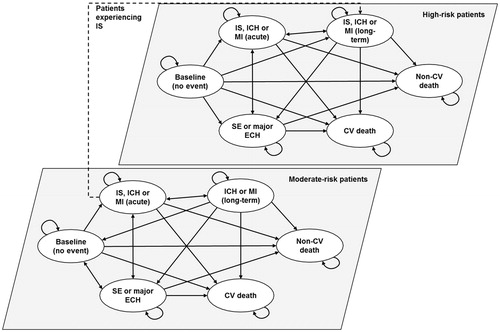
A Markov chain was used to model cardiovascular (CV) events and associated costs in the eligible patient population. IS, intracranial hemorrhage (ICH), or myocardial infarction (MI) resulted in long-term care, whereas SE and major extracranial hemorrhage (ECH) did not lead to long-term care (). Moderate-risk patients—CHADS2 score of 1—experiencing a non-fatal IS moved to the high-risk cohort, defined as a CHADS2 score of 2 or higher. High-risk patients had the same baseline and recurrent risk of IS, which is based on the risk for patients with CHADS2 score of 2 or higher. The size of the eligible patient population due to disease incidence and all-cause and CV mortality was reflected in the number of patients who incurred treatment, physician visit, and event costs. The risk of all-cause mortality was uniformly applied to patients in all states. Patients transitioned in the model in 1-year cycles. A half-cycle correction was applied to account for the timing of events as per the International Society for Pharmacoeconomics and Outcomes Research guidelines recommendationsCitation37.
Model inputs
Population inputs
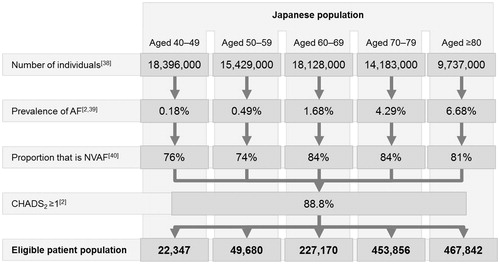
The number of patients initially eligible to receive rivaroxaban was based on the number of individuals aged 40 years and above estimated to reside in Japan in 2014 and taken from population projections from the Japanese National Institute of Population and Social Security ResearchCitation38 (). This source was selected as a representative estimate of the population size during the model’s start year. Within each age category, individual annual population growth estimates were applied year-on-year.
Table 1. Population, population growth, prevalence of NVAF and non-CV mortality, by ageCitation2,Citation38–41.
The age-specific prevalence of AF was based on data from the Fushimi registryCitation2,Citation39 (), a community-based survey of AF patients, which reported 3183 enrollees as of June 2012. The age-specific proportion of NVAF was based on another study evaluating the prevalence of AFCitation40.
Among patients with NVAF, only those with moderate- or high-risk, defined as CHADS2 = 1 and CHADS2 ≥2, respectively, were considered as per guideline recommendationsCitation17,Citation18. The proportion of patients with moderate (26%) and high risk (63%) is based on the Fushimi registryCitation2. Only patients aged 40 years and above were considered in the model; the proportion of the population younger than 40 years were assumed to be of limited interest due to their inherently lower risk of AF and lower CHADS2 score.
The algorithm defining the eligible patient population is outlined in .
Figure 2. Eligible patient population. AF, Atrial fibrillation; CHADS2, Congestive heart failure, Hypertension, Age (≥75 years), Diabetes Mellitus, Prior Stroke or TIA; NVAF, Non-valvular atrial fibrillation.
All-cause mortality was based on the Handbook of Health and Welfare Statistics published in 2013 by the Japanese Ministry of Health, Labour and WelfareCitation41. To avoid double-counting, CV mortality (categories Hi05 and Hi06) was subtracted from all-cause mortality.
Market share
In the base-case scenario, 54%, 3%, and 43% of NVAF patients with moderate risk and 46%, 4%, and 50% of patients with high-risk were assumed to receive no treatment, aspirin only, and VKA, respectively, based on treatment patterns in 273 Japanese acute care hospitalsCitation26 (). Patients currently receiving VKA were assumed to be either well- or poorly-controlled, based on a multi-center study, in which 49% of moderate- and 32% of high-risk patients receiving VKA were poorly controlledCitation28. To model adherence to treatment guidelines in the revised treatment pattern, all patients with moderate-to-high risk of stroke, except those who were well controlled on VKA, were switched to rivaroxaban.
Table 2. Treatment patternsCitation26–28.
Baseline risk of events
The model estimates the number of major and minor IS, SE, MI, ICH, and major ECH (). Major and minor strokes were defined as having a National Institutes of Health Stroke Scale (NIHSS) score above 16 and up to 15, respectivelyCitation14. Major ECH was defined as clinically overt bleeding that was associated with a fall in hemoglobin ≥20 g/L, a transfusion of ≥2 units of packed red blood cells or whole blood, involved a critical site, or had a fatal outcomeCitation31. Minor bleeding events were not considered in this model.
Table 3. Baseline risk of events, by ageCitation14,Citation31,Citation42–44.
Table 4. CV mortality associated with eventsCitation14,Citation31,Citation45–47.
The baseline risk of IS, SE, MI, ICH, and ECH in untreated patients with high-risk of stroke was calculated by adjusting data from the J-ROCKET AF trialCitation31, with results from a network meta-analysis (NMA) including 32 clinical trials (Data on file, Oxford Outcomes). The event rates in untreated patients were obtained on the basis of event rates in patients receiving warfarin or rivaroxaban. The J-ROCKET AF trial was conducted in patients whose CHADS2 score was ≥2; therefore, the risk of IS in patients with moderate risk of stroke was calculated by dividing the rate of IS in high-risk patients by 6.77, based on the relative risk of stroke in moderate- and high-risk patients identified in a prior Japanese studyCitation42. The baseline rates of IS, MI, and ICH were adjusted by age using data from a Japanese multi-center population-based cohort studyCitation43. Due to limitations in data acquisition, it was assumed that patients who were 80 years of age or older had similar baseline risks of events to those aged between 70–79 years of age—this assumption is conservative in that the true rate of events will likely be higher in the older patient sub-group.
The recurrent rate of IS in moderate risk patients is the same as the baseline risk of IS in high-risk patients as those patients move to the high-risk cohort. It is assumed that the recurrent risk of IS among high-risk patients is the same as the baseline risk. IS and ICH rates were adjusted by age group using age-specific cumulative event rates for all strokes from the same studyCitation43; IS rates ranged from 0.2–3.2% and 1.1–21.7% in the moderate- and high-risk groups, respectively. ICH rates ranged from 0.1–0.6% and 5.5–8.8% in the moderate- and high-risk groups, respectively. MI rates were also adjusted by age group based on data from the same sourceCitation43, ranging from 0.1–4.1% (MI rates did not vary by moderate and high-risk patient groups as the model structure is based on CHADS2 score). In the absence of relevant data, major ECH and SE rates were not adjusted by age group.
High-risk patients are assumed to have increased risk of ICH and major ECH considering the overlap between the factors affecting HAS-BLED risk score and the CHADS2 score. However, as the model is based on CHADS2 score, only the incidence of ischemic strokes was assumed to result in transition of patients from the moderate-to-high risk patient sub-groups in the model (). In absence of relevant Japanese data, the baseline risk of major ECH among high-risk patients was calculated by extrapolating data from an American studyCitation44.
CV mortality was estimated based on the proportion of the above-mentioned fatal eventsCitation14,Citation31,Citation45–47 (). The breakdown of major and minor IS—41% and 59%, respectively—and the mortality associated with those events was based on data from a Japanese registryCitation14. SE were assumed to be non-fatal, in line with an economic analysis of the best treatment pathways for patients with heart diseaseCitation45. The mortality rate of MI was based on the results from a cross-sectional study in OkinawaCitation46. The mortality of ICH and ECH were based on the Hisayama study and the J-ROCKET AF trial, respectivelyCitation31,Citation47.
Treatment efficacy and safety
Treatment efficacy and safety data were based on the results from a NMA (including the J-ROCKET trial) comparing event rates with aspirin, VKA, and rivaroxaban to no treatment (Data on file, Oxford Outcomes; ). It was assumed that increases and reductions in event rates associated with different treatments were the same across the risk groups, in line with findings from the ROCKET AF trialCitation30.
Table 5. Relative risk (RR) of events (including 95% CI) compared to no treatment.
Costs
All costs are evaluated and presented in 2014 Japanese Yen (¥)Citation48; no discounting was applied in the base case, as per ISPOR guidelines on budget impact analysesCitation49; however, discounting of costs was included within sensitivity analyses. The daily cost of treating patients with aspirin, VKA, or rivaroxaban was based on the daily recommended dose as per the J-ROCKET AF trial protocol (Data on File, Bayer), the J-RHYTHM registryCitation50, and post-marketing surveillance of rivaroxabanCitation51, respectively, as well as on the 2014 revision of medical fees by the Ministry of Health, Labour, and WelfareCitation52 (). It was assumed that 23.3% and 76.7% of patients receiving rivaroxaban received 10 mg and 15 mg doses, respectively, based on a post-marketing surveillance studyCitation51. Patients receiving no treatment were not associated with drug costs.
Table 6. Drug costsCitation50–52.
The annual physician visit cost for well- and poorly-controlled VKA patients were based on the cost of a follow-up consultation including prothrombin-time check and diagnosis fee (¥2150), as outlined in the Medical Fee Ministerial Notification No.57 of the Ministry of Health, Labour, and WelfareCitation53 and the average number of visits per year. Following recommendations from guidelinesCitation18, well-controlled patients were estimated to have an average of 12 monitoring visits, while poorly-controlled patients had 15 such visits per year based on the assumption that poorly-controlled patients were managed similarly to VKA-naïve patients—four visits during the month of drug initiation, followed by one visit per subsequent month (Data on file, Bayer). As a result, well- and poorly-controlled patients commanded an annual monitoring cost of ¥25 800 and ¥32 250, respectively. The annual physician-visit cost of patients receiving aspirin or rivaroxaban was assumed to be the same (¥8640), assuming one physician visit per month based on the cost of follow-up visits (¥720) outlined by the Ministry of Health, Labour, and WelfareCitation53.
The first year cost of a major IS, MI, or ICH was based on 3 months of acute care, followed by 9 months of long-term care; the cost of event management in subsequent years was based on 12 months of long-term care (). The cost of minor IS, SE, major ECH, and CV deaths included 3 months of acute care. The acute costs of managing minor and major IS, SE, MI, or ICH were based on a prior study of Diagnosis Procedure CombinationFootnote* (DPC) dataCitation26. The cost of managing an acute, major ECH and the cost of 1 year of long-term care was based on a Japanese pharmacoeconomic studyCitation54. Long-term care costs for IS and ICH were based on costs of managing patients based on their modified Rankin Scale (mRS) score, as reported by Hattori et al.Citation55, weighted by the proportion of patients with each mRS score as identified in the Stroke Data Bank 2009Citation56. Long-term care costs for MI were based on costs reported in a Japanese retrospective studyCitation57. All costs were inflated to 2014 Japanese Yen using the Japanese consumer price indexCitation48.
Table 7. First and subsequent year costsCitation26,Citation48,Citation54–57.
Sensitivity analyses
One-way sensitivity analyses were performed by modifying the following inputs by ±10%: proportion of patients with moderate or high-risk of stroke; baseline risk of IS, MI, and ICH; cost per dose of aspirin and VKA; annual physician visit cost of VKAs (well- and poorly-managed), aspirin, and rivaroxaban; and the acute and long-term care costs of major IS, MI, and ICH. The relative risk of IS due to VKA and rivaroxaban was varied based on the 95% confidence interval (CI) identified in the NMA; relative risks for other events were not amended as none were statistically significant for rivaroxaban. Lower and upper values for the cost per dose of rivaroxaban were obtained by assuming that all patients received low- or high-dose of rivaroxaban—costing ¥383.00 and ¥545.60—respectively.
In addition, results for the following scenario analyses were calculated: using pooled data from the ROCKET AFCitation30 and J-ROCKET AFCitation31 trials as part of the NMA; decreasing and increasing all acute and long-term care costs of events by 10%; treating only high-risk (rather than moderate-to-high risk) patients with rivaroxaban; discounting costs by 2% and 4% in line with Japanese guidelines for health economic evaluationCitation58.
Results
Base case scenario
The model followed a population of 1.37 million Japanese patients with NVAF, with 353,345 at moderate (CHADS2 = 1) and 867,550 at high (CHADS2 ≥ 2) risk of stroke, respectively, over a period of 10 years. Of those, 589,879 received no treatment, 45,302 were treated with aspirin alone, and 585,713 were treated with VKAs, of whom 198,877 were poorly managed.
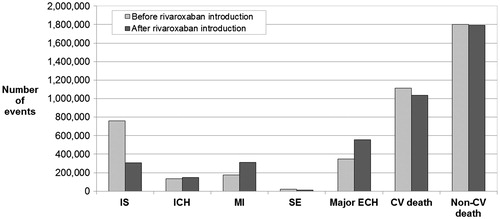
Treating inadequately-managed Japanese NVAF patients with rivaroxaban led to a 60% decrease in the number of non-fatal IS, with 456 081 fewer patients experiencing a non-fatal IS (). Treatment with rivaroxaban also resulted in 6622 (34%) fewer non-fatal SE. However, the revised treatment pattern was associated with additional events, notably 15,638 non-fatal ICH, 132,535 non-fatal MI, and 207,523 non-fatal major ECH. Applying event-specific mortality rates to overall events showed that 76,975 CV deaths were avoided by adequately treating NVAF patients, representing a 7% reduction in the number of CV deaths.
Figure 3. Breakdown of events experienced over the analysis time horizon. CV, cardiovascular; ECH, extracranial hemorrhage; ICH, intracranial hemorrhage; IS, ischemic stroke; MI, myocardial infarction; SE, systemic embolism.
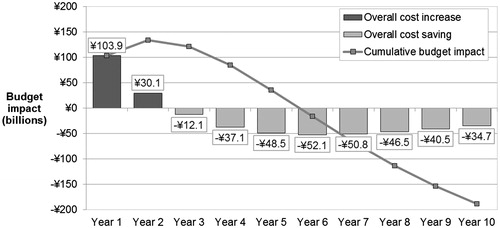
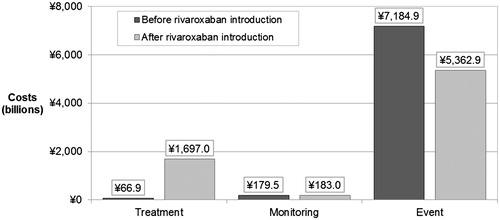
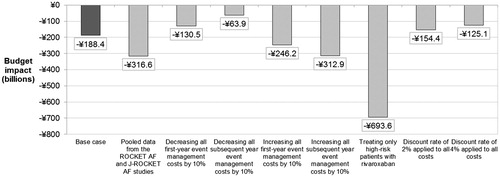
Treating inadequately-managed patients with rivaroxaban led to a net spending of ¥7242.8 billion (US $60.1 billion) on drug acquisition, physician visits, and event management over the analysis time horizon. This represented a decrease in costs of ¥188.4 billion (US $1.6 billion) compared to net spending under the treatment pattern before the introduction of NOAC (¥7431.2 billion; US $61.7 billion). Although treating patients with rivaroxaban in early years led to an increase in costs (mainly attributable to higher drug acquisition costs), decreased event costs in the long-term led to cost savings at 3 years that were maintained over the model time horizon (). The reduction in event management costs was driven primarily by the avoidance of major IS, and resulted in a considerable reduction in costs of care in the first and subsequent years. Annual cost savings began to present in the third year of the analysis, and a net cumulative saving occurred from year 6 onward (at 5 years, the cumulative budget impact was 1.22%). Annual savings reduced over time due to discounting and reduction in the size of the surviving eligible patient population ().
Increases in treatment and physician visit costs were completely offset by a 25% reduction in event management costs (¥1822.0 billion; US $15.1 billion), as shown in .
Sensitivity analyses
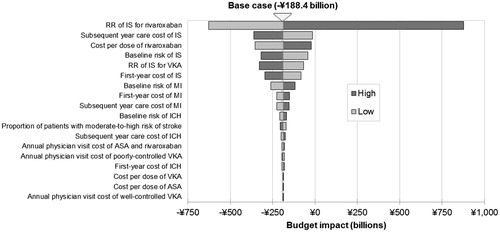
The reduction in the risk of IS associated with rivaroxaban was the most influential factor affecting the budget impact of all model inputs (). The relative risk of IS for rivaroxaban was the only input value that changed the direction of overall results to an overall cost increase (using the upper bound value). All other inputs maintained the cost savings registered in the base case scenario. The cost per dose of rivaroxaban and cost of IS per subsequent year were the next most impactful inputs, whereas the cost per dose of aspirin and VKA were among the least impactful input values in the model.
Scenario analyses
All scenario analyses consistently yielded cost savings; the lowest cost saving arose from decreasing all long-term event management costs by 10%, leading to a saving of ¥130.5 billion (US $1.1billion). Indeed, cost savings increased compared to the baseline scenario when only high-risk patients (CHADS2 ≥ 2) received rivaroxaban (¥693.6 billion; US $5.8 billion). This is consistent with these patients’ higher risk of stroke and the benefit of rivaroxaban in preventing IS and the associated costs. When relative risk data were based on pooled data from the global ROCKET-AF and J-ROCKET AF trials, cost savings were increased compared to the base case (¥316.6 billion; US $2.6 billion, ).
Discussion
Using a prevalence-based, deterministic budget impact model, we found that treating inadequately-managed Japanese NVAF patients with rivaroxaban could have a positive clinical and economic impact over the analysis time horizon. Indeed, the revised treatment pattern resulted in a 60% decrease in non-fatal IS, corresponding to a reduction of 186 993 major and 269 088 minor non-fatal IS events. It was also associated with a 34% decrease in non-fatal SE. A 76% increase in non-fatal MI was observed; this was due to an increased relative risk reported in the J-ROCKET-AF studyCitation31, and thus the NMA upon which risk reductions in CV events in our analysis were based. It should be noted, though, that the increased MI rates from this study contrast with results from the ROCKET-AF studyCitation30, in which a non-statistically significant trend towards reduced MI compared to VKA was observedCitation30. An increase in non-fatal ICH and non-fatal major ECH was also observed. Overall, a 7% decrease in the number of CV deaths was observed. These results apply to a modelled population of 1.37 million patients with NVAF over 10 years.
The observed increase in the incidence of non-fatal ICH and non-fatal major ECH is mostly due to the increased use of rivaroxaban in patients currently receiving no treatment, and should be considered in the context of decreased ischemic events and CV mortality. Additionally, clinical trials evaluating novel oral anti-coagulants compared to VKAs have tended to show a reduction in ICHCitation59, as confirmed by results from both J-ROCKET AF and ROCKET AF trialsCitation30,Citation31.
Following Japanese NVAF treatment guidelinesCitation18 also had a positive economic impact, with a decrease in net spending on drug acquisition, physician visit, and event management costs of ¥188.4 billion (US $1.6 billion) over the analysis time horizon. These savings were driven by reductions in event management costs (¥1822.0 billion; US $15.1 billion), due in part to the avoidance of major ischemic events and the associated long-term care costs. This suggests that adequately managing NVAF patients may have a positive impact not only on the clinical outcomes of moderate-to-high risk NVAF patients, but also on the costs of managing this condition over patients’ lifetime.
A range of one-way sensitivity and scenario analyses showed that the model was particularly sensitive to inputs affecting the number and costs of IS, which are the most prevalent and costly events in the model, and, therefore, a main driver of costs. The costs of long-term care for IS were particularly impactful, confirming the fact that decreased events associated with rivaroxaban drive the cost savings identified in the model, while physician visit costs and the cost per dose of aspirin and VKA have a limited impact on results. The cost of rivaroxaban was the third most impactful input in the model. Although using the upper limits from the 95% CI of the relative risks reported in the NMA suggested substantial additional costs instead of the savings shown in the base case scenario, it should be noted that these relative risks ranged from 0.44–122.59 across all events, and that only two were significant compared to no treatment (namely, the relative risk of IS associated with VKA and rivaroxaban). Another scenario analysis showed that savings were maintained when only high-risk patients (rather than those with moderate-to-high risk of stroke) received rivaroxaban, illustrating the need for adequate management of patients who otherwise remain at significant risk of life-altering yet preventable events.
We could find no budget impact analyses directly comparable to our own (especially considering the Japanese setting) within the literature. There are, however, numerous economic analyses assessing the cost-effectiveness of rivaroxaban specific to stroke-prevention in AF across a range of countries and settings. Wang et al.Citation60 conducted an analysis from the perspective of the Singaporean healthcare system, concluding that rivaroxaban offered a cost-effective treatment option compared to warfarin, and dominated both high- and low-dose treatment with dabigatran. The cost-effectiveness of rivaroxaban compared to warfarin was reflected in analyses from other country settings. A US-based analysis from a payer/Medicare perspectiveCitation61 found rivaroxaban to be highly cost-effective compared to warfarin, as did analyses from the German Statutory Health Insurance perspectiveCitation62 and the Belgian healthcare payer (National Institute for Health and Disability Insurance, and patient) perspectiveCitation63. Morais et al.Citation64 conducted an analysis from a Portuguese societal perspective, yielding similar conclusions compared to both warfarin and a mix of comparators based on real-world Portuguese prescribing patterns. Kourlaba et al.Citation65 concluded rivaroxaban to be a dominant alternative (both cost saving and improving quality-of-life), from a Greek third-party payer perspective. These studies report rivaroxaban to be cost-effective across a broad range of settings, which, while difficult to place in context of our own analysis (which does not consider quality-of-life), does support the concept of economic offsets achieved through reductions in the rate of CV events through the use of rivaroxaban. However, it is also important to note that the results in our analysis are specific to Japan, and cannot be directly generalized to other countries.
Limitations
Our analysis has several limitations. First, our model assumes that well- and poorly-controlled VKA patients are equally at risk of stroke. This assumption may be conservative, as suggested by the GARFIELD-AF registry dataCitation66, and thereby under-estimate benefits arising from the increased use of rivaroxaban.
Second, this model does not take medication discontinuation and adherence into account. However, as bleeding rates are reasonably similar in VKA and rivaroxabanCitation31, it is unlikely that this omission favors rivaroxaban. In fact, economic benefits of rivaroxaban compared to VKA may even be under-estimated due to discontinuation rates and adherence not being modelled, since rivaroxaban’s simple, one-tablet, once-daily dosing and no need for routine monitoring may lead to fewer discontinuations, as suggested by real-world evidenceCitation67, and better adherence than with VKACitation24,Citation25,Citation68.
Third, the treatment pattern of patients with different stroke risks was based on NVAF patients in 273 Japanese acute care hospitalsCitation26. The use of VKA tends to be higher in hospitalized patients than in the general NVAF population. The proportion of well- and poorly-managed patients, however, was based on patients from six institutions and one clinic across five prefectures in JapanCitation28. As a result, our model may over-estimate the percentage of treated patients and under-estimate the proportion of VKA patients who are poorly-managed, as the data may reflect a higher-quality of management than could be expected for the general population. Overall, treatment patterns may vary amongst hospitals, although evidence suggests an over-arching trend towards inadequate treatmentCitation2,Citation26,Citation69,Citation70.
Fourth, the prevalence of AF was based on the community-based Fushimi AF registry, which may not represent the prevalence of AF in Japan. However, the age distribution in the Fushimi area was similar to that of the general population in Japan.
Finally, our analysis required some structural assumptions which must be noted, all of which were applied uniformly to all patients, regardless of the treatment they received, and were, therefore, unlikely to favor any specific treatment. First, no increase in the risk of recurrent event for MI, ICH, SE, major ECH, and IS (beside moderate risk patient transitioning to the high-risk cohort following an IS) was applied. Indeed, the model uses a Markov-based approach which does not track individual patients’ history of events. Second, in the absence of relevant data, it does not differentiate between moderate- and high-risk patients’ baseline risk of experiencing a SE and MI. Also, changes in patient age (i.e. movement of patients to the next age category, and, thus, to a higher baseline risk of CV events) was not simulated over the model’s 10-year time horizon; this assumption is in fact conservative in nature, as the risk of CV events in some patients will be under-estimated. Finally, due to the inherent lack of model ‘memory’ attributable to the Markov-based approach in general, patients who experience an event followed by long-term rehabilitation, followed by an event with no long-term rehabilitation, will see their rehabilitation cost-effectively cancelled (as the model is unable to return them to their previous state automatically); again, this assumption conservatively under-estimates the true costs associated with CV events. Last, this is a simulation to test the potential effect of introducing rivaroxaban in the Japanese healthcare system in light of the current unmet medical need. Although it is unlikely that all inadequately-managed NVAF patients will receive rivaroxaban, it does not critically influence the conclusion of the analysis, which emphasizes the importance of stroke prevention in AF.
Conclusion
Over one million Japanese individuals are estimated to have NVAFCitation5,Citation27, the majority of which are currently receiving no treatment, aspirin onlyCitation26, or remain poorly-controlled despite receiving VKACitation28. Optimal treatment for these patients, many of whom have a CHADS2 score pertaining to an increased risk of preventable strokeCitation27, represents an issue that Japanese guidelines seek to address by recommending the adequate use of anti-coagulation therapy in NVAF patientsCitation18.
Using the example of rivaroxaban, our model demonstrates that adequate treatment of NVAF patients could avoid a large number of non-fatal IS and CV deaths, the clinical benefit of which may offset concomitant increases in the incidence of non-fatal ICH, MI, and major ECH. Increases in treatment and physician visit costs through the use of rivaroxaban may be offset by savings associated with decreased event management and long-term care costs, such that treating inadequately-managed patients with rivaroxaban could be associated with an overall decrease in costs over the analysis time horizon. In view of the positive clinical and economic impact demonstrated in this model, novel oral anti-coagulants should be considered as a viable option by payers and clinicians in the management of moderate-to-high risk NVAF patients, especially among inadequately-managed patients.
Transparency
Declaration of funding
This study was funded by Bayer Pharma AG.
Declaration of financial/other relationships
SI, KO, SM, and YK are paid consultants to Bayer Yakuhin, Ltd. CM was an employee of Covance at the time of the analysis. LR is an employee of Covance. EWF and BR are employees of Bayer Yakuhin, Ltd. TE and JBB are employees of Bayer Pharma AG. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Notes
*ADLs consist of self-care tasks one normally performs in daily living, such as feeding oneself, bathing, and dressing, and are often used as part of functional assessments in the elderly population.
*Xarelto is a registered trademark of Bayer Pharmaceuticals, Inc., Berlin, Germany
*DPC is a fixed reimbursement system per hospitalization day based on diagnostic categories.
References
- Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N-9N
- Akao M, Chun YH, Wada H, et al. Current status of clinical background of patients with atrial fibrillation in a community-based survey: the Fushimi AF Registry. J Cardiol 2013;61:260-6
- National Institute of Population and Social Security Research. Japanese population in 2010 by age and gender. http://www.ipss.go.jp/. Accessed March 2015
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53
- Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol 2009;137:102-7
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8
- Astellas Pharma, Inc. Almanac 2013/Medical Care and Pharmaceutical Industry. http://www.astellas.com/jp/corporate/information/data.html. Accessed January 2014
- Ministry of Health, Labour and Welfare; Ministry of Education, Culture, Sports, Science and Technology. The Stroke Project. http://www.stroke-project.com/data_pref.php. Accessed April 2015
- Takahashi S. Nonvalvular atrial fibrillation and stroke. Japan Med Assoc J 2006;49:119-24
- Iqbal MB, Taneja AK, Lip GY, et al. Recent developments in atrial fibrillation. BMJ 2005;330:238-43
- Jorgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996;27:1765-9
- Lamassa M, di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke 2001;32:392-8
- Yoneda Y, Okuda S, Hamada R, et al. Hospital cost of ischemic stroke and intracerebral hemorrhage in Japanese stroke centers. Health Policy 2005;73:202-11
- Kimura K, Minematsu K, Yamaguchi T; Japan Multicenter Stroke Investigators' Collaboration (J-MUSIC). Atrial fibrillation as a predictive factor for severe stroke and early death in 15831 patients with acute ischemic stroke. J Neurol Neurosurg Psychiatry 2005;76:679-83
- Sakai H, Muramatsu K, Kobori S, et al. How does the accuracy of ICD coding influence for resource consumption under the case mix based evaluation. Asian Pac J Dis Manag 2011;5:19-22
- Ministry of Health, Labour and Welfare. Handbook of Health and Welfare Statistics 2014. Table 5-29: medical care expenditure of medical care by inpatient - outpatient, age group and category of disease. http://www.mhlw.go.jp/english/database/db-hh/5-1.html. Accessed October 2015
- Camm AJ, Lip GY, de Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385-413
- JCS Joint Working Group. Guidelines for pharmacotherapy for atrial fibrillation. 2013. http://www.j-circ.or.jp/guideline/pdf/JCS2013_inoue_h.pdf. Accessed February 2014
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001;119:8S-21S
- Warfarin. Summary of Product Characteristics, Teva, 2014
- Lu Y, Won KA, Nelson BJ, et al. Characteristics of the amiodarone-warfarin interaction during long-term follow-up. Am J Health Syst Pharm 2008;65:947-52
- Turpie AG. Warfarin replacements: mechanisms underlying emerging agents. Can J Cardiol 2008;24(Suppl C):56-60C
- McBride D, Brüggenjürgen B, Roll S, et al. Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. J Thromb Thrombolysis 2007;24:65-72
- Dantas GC, Thompson BV, Manson JA, et al. Patients’ perspectives on taking warfarin: qualitative study in family practice. BMC Fam Pract 2004;5:1
- Maeda K, Sakai T, Hira K, et al. Physicians’ attitudes toward anticoagulant therapy in patients with chronic atrial fibrillation. Intern Med 2004;43:553-60
- Ikai H, Briere JB, Imanaka Y. Prevention of cardiovascular events related to non-valvular atrial fibrillation (NVAF) in acute care hospitals. JCMI 2009;29(Suppl):1-4
- Koretsune Y. Atrial fibrillation and the GARFIELD registry in Japan. Presented at the Japanese Society of Electrocardiology 29th Annual Congress, Oct 13 2012, Chiba, Japan
- Okumura K, Komatsu T, Yamashita T, et al. Time in the therapeutic range during warfarin therapy in Japanese patients with non-valvular atrial fibrillation - a multicenter study of its status and influential factors. Circ J 2011;75:2087-94
- Rivaroxaban (Xarelto) Summary of Product Characteristics. Bayer, Leverkusen, 2013
- Patel M, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91
- Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation. Circ J 2012;76:2104-11
- National Institute for Health and Care Excellence (NICE). Technology appraisal guidance 256. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. 2012
- Restovic G, Carcedo D, McLeod EJ, et al. Cost-effectiveness of rivaroxaban versus acenocumarol in the stroke prevention in patients with non-valvular atrial fibrillation in the Spanish setting. Value Health 2012;15:A375
- Kourlaba G, Maniadakis N, Andrikopoulos G, et al. Economic evaluation of rivaroxaban in stroke prevention among patients with atrial fibrillation in Greece. Value Health 2012;15:A371
- Psenkova M, Lukac M, Mackovicova S, et al. Prevention of stroke in patients with atrial fibrillation: cost-utility analysis of rivaroxaban versus warfarin in Slovakia. Value Health 2012;15:A375
- Asukai Y, Duran A, Lloyd A, et al. Cost-effectiveness of rivaroxaban for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors - a UK perspective. Value Health 2012;15:A371
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices - overview: a report of the ISPOR–SMDM modeling good research practices task force-1. Value Health 2012;15:796-803
- National Institute of Population and Social Security Research. Population projections for Japan 2011-2060. www.ipss.go.jp. Accessed September 2014
- City of Kyoto. Results of National Population Census 2010. http://www.city.kyoto.lg.jp/fushimi/page/0000013497.html. Accessed April 2015
- Tomita F, Kohya T, Sakurai M, et al. Prevalence and clinical characteristics of patients with atrial fibrillation: analysis of 20,000 cases in Japan. Jpn Circ J 2000;64:653-8
- Ministry of Health, Labour and Welfare. Handbook of Health and Welfare Statistics 2013. http://www.mhlw.go.jp/english/database/db-hh/1-2.html. Accessed October 2014
- Komatsu T, Sato Y, Ozawa M, et al. Comparison between CHADS2 and CHA2DS2-VASc score for risk stratification of ischemic stroke in Japanese patients with non-valvular paroxysmal atrial fibrillation not receiving anticoagulant therapy. Int Heart J 2014;55:119-25
- Ishikawa S, Kayaba K, Gotoh T, et al. Incidence of total stroke, stroke subtypes, and myocardial infarction in the Japanese population: the JMS Cohort Study. J Epidemiol 2008;18:144-50
- Hylek EM, Evans-Molina C, Shea C, et al. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689-96
- Eckman MH, Levine HJ, Pauker SG. Decision analytic and cost-effectiveness issues concerning anticoagulant prophylaxis in heart disease. Chest 1992;102:538S-49S
- Fukiyama K, Kimura Y, Wakugami K, et al. Incidence and long-term prognosis of initial stroke and acute myocardial infarction in Okinawa, Japan. Hypertens Res 2000;23:127-35.
- Ueda K, Hasuo Y, Kiyohara Y, et al. Intracerebral hemorrhage in a Japanese community, Hisayama: incidence, changing pattern during long-term follow-up, and related factors. Stroke 1988;19:48-52
- Statistics Bureau, Consumer price index 1970–2013. Available at: www.stat.go.jp. Accessed December 2014
- Sullivan SD, Mauskopf JA, Augustovski FA, et al. Budget Impact Analysis - Principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014;17:5–14
- Atarashi H, Inoue H, Okumura K, et al. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation: a report from the J-RHYTHM Registry. Circ J 2011;75:1328-33
- Ogawa S, Ikeda T, Kitazono T, et al. Present profiles of novel anticoagulant use in Japanese patients with atrial fibrillation: insights from the Rivaroxaban Postmarketing Surveillance Registry. J Stroke Cerebrovasc Dis 2014;23:2520-6
- Ministry of Health, Labour and Welfare. Revision of medical fees in 2014. www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000032996.html. Accessed March 2015
- Medical Fee Ministerial Notification No.76 of the Ministry of Health, Labour and Welfare on March 5th, 2012. http://www.mhlw.go.jp. Accessed November 2012
- Hori M, Koretsune Y, Yasaka M, et al. Pharmacoeconomic analysis of dabigatran etexilate for prevention of stroke in patients with non-valvular atrial fibrillation. Pharma Medica 2011;29:151-64
- Hattori N, Hirayama T, Katayama Y. Medical care for chronic-phase stroke in Japan. Neurol Med Chir (Tokyo) 2012;52:175-80
- Kobayashi S, ed. Japanese Stroke Data Bank 2009. Tokyo: Nakayama Shoten, 2009 [In Japanese]
- Tanihata S, Nishigaki K, Kawasaki M, et al. Outcomes of patients with stable low-risk coronary artery disease receiving medical- and PCI- preceding therapies in Japan: J-SAP Study 1-1. Circ J 2006;70:365-9
- Tokyo: Research group on economic evaluation for Japanese public medical benefits. Guideline for economic evaluation of healthcare technologies in Japan. 2013. http://hta.umin.jp/guideline_e.pdf. Accessed May 2016
- Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013;44:1891-6
- Wang Y, Xie F, Kong MC, et al. Cost-effectiveness of dabigatran and rivaroxaban compared with warfarin for stroke prevention in patients with atrial fibrillation. Cardiovasc Drugs Ther 2014;28:575-85
- Lee S, Anglade MW, Pham D, et al. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol 2012;110:845-51
- Mensch A, Stock S, Stollenwerk B, et al. Cost effectiveness of rivaroxaban for stroke prevention in German patients with atrial fibrillation. Pharmacoeconomics 2015;33:271-83
- Kleintjens J, Li X, Simoens S, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics 2013;31:909-18
- Morais J, Aguiar C, McLeod E, et al. Cost-effectiveness of rivaroxaban for stroke prevention in atrial fibrillation in the Portuguese setting. Rev Port Cardiol 2014;33:535-44
- Kourlaba G, Maniadakis N, Andrikopoulos G, et al. Economic evaluation of rivaroxaban in stroke prevention for patients with atrial fibrillation in Greece. Cost Eff Resour Alloc 2014;12:5
- ten Cate H, Haas S, Accetta G, et al. Quality of vitamin K antagonist control and 1-year outcomes: a global perspective from the Garfield-AF registry. Int Soc Thromb Haemostas 2015;13(2 Suppl):130
- Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban versus warfarin among patients with non-valvular atrial fibrillation. Curr Med Res Opin 2014;30:2461-9
- Evers T, O’Neil WM, Ogilvie IM, et al. Stroke prevention in patients with atrial fibrillation: inappropriate anticoagulation and poor INR control. Presented at the ISPOR 14th Annual European Congress, 5-8 Nov 2011, Madrid, Spain
- Shimizu H, Kawarai T, Saji N, et al. Re-evaluation of clinical feature and risk factors of acute ischemic stroke in Japanese longevity society. Kobe J Med Sci 2009;55:E132-9
- Ono A, Fujita T. Stroke prevention in patients with atrial fibrillation. J Clin Neurosci 2003;10:71-3