Abstract
Objective: Philadelphia chromosome negative [Ph(−)] relapsed or refractory (R/R) B-precursor acute lymphoblastic leukemia (ALL) is an extremely rare condition requiring intensive treatment. This retrospective chart review aimed to quantify hospitalizations and reimbursement in this patient population in France.
Methods: Patients aged ≥18 years and with at least one hospitalization for Ph(−) R/R B-precursor ALL were included in the study. They were relapsed with first remission lasting <12 months, relapsed after first salvage therapy, relapsed any time after hematopoietic stem cell transplant (HSCT), or were refractory to initial or salvage therapy. Data were collected from the index date (first diagnosis of R/R ALL) until death or loss to follow-up. The chemotherapy period was defined as the first chemotherapy date after the index date to the earliest of death, loss to follow-up, last chemotherapy dose plus 30 days, or initiation of HSCT. The primary outcome was the percentage of time hospitalized during the chemotherapy period.
Results: Thirty-three patients were included, with a mean age of 49 years. The mean proportion of time spent in the hospital during the chemotherapy period was 46% (95% CI =34–57%). Patients had a mean of 2.2 (SD =1.5) inpatient hospitalizations and the mean length of stay per hospitalization was 16.8 (SD =14.8) days. During the chemotherapy period, the mean amount reimbursed per hospitalization was €31 067 (SD = €4850) and the total hospitalization reimbursement per patient was €68 344. From the index date to death, excluding HSCT, the total reimbursement per patient was €108 873.
Limitations: The sample size was small, although this was expected given the rarity of the patient population.
Conclusions: Adults with Ph(−) R/R B-precursor ALL had repeated and prolonged hospitalizations during salvage chemotherapy. Approximately half the follow-up period was spent in the hospital, and this time was associated with high economic burden in France.
Introduction
Acute lymphoblastic leukemia (ALL) is a rare hematologic malignancy with an estimated annual incidence rate in adults of approximately 1.4 per 100 000Citation1,Citation2. In ALL, the aggressive production of abnormal lymphoblasts suppresses and crowds out the production of normal blood cells in the bone marrow, causing hematological deficiency—specifically anemia, immune system impairment, and thrombocytopeniaCitation3. In most patients with ALL, the disease is diagnosed within a few weeks after onset of symptoms, and the symptoms are severe enough to require urgent medical attention. Diagnosis invariably leads to immediate hospital admission for symptom management and treatment initiation, without which most patients with ALL would die within days to weeksCitation3.
Philadelphia chromosome negative [Ph(−)] B-cell precursor ALL is a sub-population of ALL, which historically has had no targeted treatment options after relapsing or becoming refractory (R/R) to treatment. The estimated annual incidence rate of adults with Ph(−) R/R B-cell precursor ALL is 0.2 per 100 000 person-years, corresponding to 120–130 new patients per year in FranceCitation4. Until the recent approval of blinatumomabCitation5,Citation6, treatment options were limited to salvage chemotherapiesCitation7. The selection of salvage chemotherapy is challenging, given that many of the options include classes of drugs the patient has already been exposed to and failedCitation8–10. In addition, there is no standard of care for adults with Ph(−) R/R B-cell precursor ALL. A French study of 421 adult ALL patients who relapsed after participation in the LALA-94 trial reported that a variety of multi-agent salvage chemotherapy regimens were administered following the relapse, including the same chemotherapies that were used for initial induction therapy, but no clear choice of salvage chemotherapy was apparent and outcomes were universally poorCitation11. Furthermore, salvage chemotherapies are associated with severe toxicities, including hematological adverse events, infections, and neurologic toxicitiesCitation12–15. With salvage chemotherapy, treatment-related mortality is reported to range from 11–23%Citation8,Citation16, and it is widely recognized that most patients require extensive inpatient hospitalization for treatment administration and toxicity management.
Although clinicians treating adults with Ph(−) R/R B-cell precursor ALL recognize that hospitalization is required to manage these patients, the extent of the burden has not previously been measured or reported in the literature. The primary objective of this study was to quantify proportion of time spent in the hospital by adults with Ph(−) R/R B-cell precursor ALL treated with salvage chemotherapy. Secondary objectives to further determine the burden of hospitalization were to identify the main reasons for hospitalization, determine the frequency of hospitalizations, and estimate the costs associated with hospitalization stays.
Methods
Study design and patient selection
This was a retrospective chart review of adults with Ph(−) R/R B-cell precursor ALL who were treated in French hospitals. Hospitals were selected to include those with inpatient diagnostic and treatment facilities for patients with ALL, a network of hematologists/oncologists who treat R/R ALL patients, and that had been operational for at least 2 years.
The study period was from 2003–2014. Patient records or charts were screened from October 2013 going backwards until at least 30 eligible patients were identified. Eligible patients were adults aged 18 years or older, with Ph(−) B-cell precursor ALL who were relapsed with first remission lasting less than 12 months, or relapsed after first salvage therapy, or relapsed any time after HSCT, or refractory to primary induction or salvage therapy. Patients were to have been hospitalized for at least one episode of R/R ALL with an electronic medical record or chart available for review and data collection. Patients enrolled in either blinatumomab- or inotuzumab ozogamicin-related clinical trials were excluded.
The protocol was approved by French national regulatory authorities, CCTIRS (Comité Consultatif sur le traitement de l’information en matière de recherche dans le domaine de la santé) reference number 14.803, and CNIL (Commission Nationale de l’Informatique et des Libertés) reference number DR-2015-71.
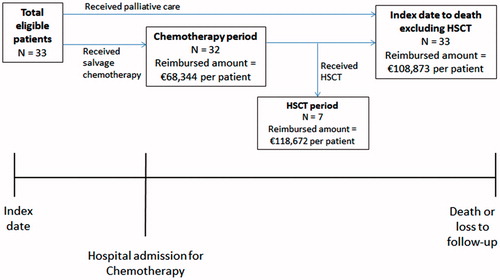
Patient data were collected from the ‘index date’ until the patient died or was lost to follow-up, and outcomes were evaluated during pre-specified ‘time periods’ (). The index date was the first time an ALL patient was recorded as refractory or relapsed, as per inclusion criteria. The ‘chemotherapy period’ was pre-specified to represent the time during which the most intensive chemotherapy is assumed to be administered and before patients receive a transplant. The chemotherapy period was defined as the first chemotherapy date after the index date to the earliest of last chemotherapy dose plus 30 days, initiation of HSCT, death, or loss to follow-up. For patients who received HSCT after the index date, the ‘HSCT period’ was defined as the time from starting HSCT to the earliest of death, loss to follow-up, or relapse of ALL. Patients with no recorded death were lost to follow-up and considered alive at the end of the study.
Figure 1. Study schema. The index date was the first time in the medical records the patient was diagnosed with ALL that was refractory or had relapsed according to the inclusion criteria (with a first remission duration ≤12 months, within 12 months of HSCT, or in second or greater salvage therapy). The chemotherapy period was the time from first hospital admission for chemotherapy to the earliest of last dose of chemotherapy +30 days, start of HSCT, after 105 days, death, or loss to follow-up. The HSCT period was the time from start of HSCT to earliest of relapse, death, or loss to follow-up.
Data collection
Collected data included dates of initial diagnosis, chemotherapy administration, first, second and most recent relapse, HSCT, and death; age at diagnosis and relapse; length of inpatient hospital stays during salvage therapy; and reasons for hospitalization.
Outcomes of interest
The primary outcome was the percentage of time spent in the hospital during the chemotherapy period. In addition, the number and types of hospital admissions, length of hospital stay(s) and reasons for hospitalization were summarized, and reimbursement amounts of hospitalizations were estimated.
Statistical analysis
All patients who met the inclusion criteria were included in the analyses. For the primary outcome, the percentage of time spent in the hospital during the chemotherapy period was calculated as the number of days in the hospital divided by the total number of days during the chemotherapy period.
Other outcomes related to the number of hospital admissions and length of hospital stays were calculated for the chemotherapy period. The same analyses were calculated from the index date until death or loss to follow-up, including and excluding the HSCT period.
Reimbursement amounts were estimated using a weighted average of the amount reimbursed by the French Public Insurance for any hospital stay given a diagnosis-related group (DRG) code of adult patients with ALL. Hospital stays for ALL were classified into one of eight categories (chemotherapy, transfusions, fever, transfusion plus fever, infections, sepsis, sepsis plus fever, no comorbidity) and DRG codes were assigned depending on the complexity and severity of the hospitalization. Severity levels range from 1–4, with reimbursement increasing as the severity level increases. When multiple categories of diagnoses are present for a given hospital stay, the DRG code with the highest reimbursement is assigned to the stay. To obtain the weighted average of reimbursement amount per hospitalization, the relative proportion of all hospital stays in 2013 for ALL was multiplied by the respective mean amount reimbursed in 2013 for each severity of hospital stay, and the weighted values for each severity level were added together. A retrospective analysis of the French National Hospital database (PMSI) was conducted to determine the number of hospital stays and reimbursement amount assigned to each stay according to severity level during 2013 (Supplementary Table 1). Descriptive statistics were calculated for all outcomes. Means (standard deviation [SD]) or medians (interquartile range and range) were provided for continuous variables. Incidence rates and proportions were provided for categorical variables.
Results
Study population
Thirty-three patients from four sites in France met the inclusion criteria and were included in the analyses. The median age of the patients was 54 years (range =19–75 years), and most (61%) were male (). Over half of the patients (52%) were relapsed, with a first remission duration of less than 1 year. Approximately one-fifth of patients received a HSCT following salvage chemotherapy (21%) and salvage chemotherapy was palliative in one patient. By the end of the observation period 29 patients (88%) had died; of these, 23 patients had not received a transplant. Four patients were alive and lost to follow-up.
Table 1. Patient characteristics and treatment received during salvage.
Hospitalizations and reimbursement during the chemotherapy period
Thirty-two patients received salvage chemotherapy and were included in the chemotherapy period. The mean (SD) duration of the chemotherapy period was 87 (35) days. During the chemotherapy period patients spent almost half of their time in the hospital (46%, 95% CI =34–57%). The 32 patients experienced 71 inpatient hospitalizations, 70 day-hospital stays, and six outpatient visits (). The mean number of inpatient hospitalizations was 2.2 (SD =1.5) per patient, with a mean length of stay of 16.8 days (SD =14.8) per hospitalization.
Table 2. Hospitalizations in patients with Ph-negative B-cell precursor R/R ALL.
The mean reimbursement per hospitalization was €31 067 (SD =4850) for an inpatient stay, €674 (SD =0) for a day stay, and €394 (SD =0) for an outpatient visit (). The calculated total hospitalization reimbursement per patient during the chemotherapy period was €68 344.
Table 3. Reimbursement in patients with Ph-negative B-cell precursor R/R ALL.
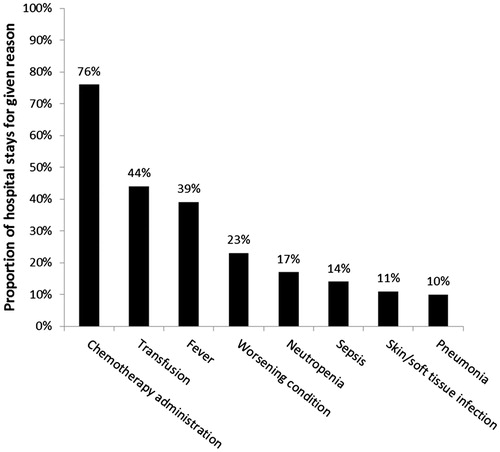
Most patients were hospitalized for more than one reason during each admission. The most common reason was to administer chemotherapy, cited in 76% of hospital admissions (). The next most common reasons were blood transfusion (44%) and fever (39%).
Total hospitalizations and reimbursement from R/R ALL diagnosis to death
Total hospitalizations and reimbursement excluding the HSCT period
As the total reimbursement from R/R ALL diagnosis to death is likely to be substantially impacted by HSCT, the reimbursed amounts are reported here, excluding those related to HSCT. Excluding hospital visits for HSCT, there were 121 inpatient hospitalizations, 141 day-hospital stays, and 23 outpatient visits (). A mean of 3.7 (SD =3.1) inpatient hospitalizations per patient was observed when the HSCT period was excluded, and the mean length of stay was 13.7 (SD =13.5) days.
The mean reimbursement per hospitalization was €28 832 (SD =8867) for an inpatient stay, €674 (SD =0) for a day stay, and €394 (SD =0) for an outpatient visit. The calculated total hospitalization reimbursement per patient excluding the HSCT period was €108 873 ().
HSCT period only
Hospitalizations and reimbursement were evaluated during the HSCT period for the seven patients who received a transplant after the index date. The mean time from the index date to HSCT was 116 (SD 43) days. During the HSCT period, these seven patients experienced 18 inpatient hospitalizations, 20-day hospital stays and 79 outpatient visits (). Per patient, the mean number of inpatient hospitalizations was 2.6 (SD =2.2), with a mean length of stay of 33.9 (SD =38.0) days.
The mean reimbursement per hospitalization was €43 672 (SD =33 241) for an inpatient stay, and the day-stay and outpatient reimbursements were the same as for other study periods (). The calculated total reimbursement per patient in the HSCT period was €118 672.
Discussion
Very little data exist on the burden of treating adults with Ph(−) R/R B-cell precursor ALL using salvage chemotherapy in France. Using a hospital retrospective chart review approach, we found that adults with Ph(−) R/R B-cell precursor ALL currently experience significant hospitalization burden. Once diagnosed with R/R disease, patients spent almost half (46%) of their time in the hospital during the chemotherapy period.
Adults with Ph(−) R/R B-cell precursor ALL are extremely ill. These patients receive intensive chemotherapy regimens, which are commonly associated with significant toxicity that requires inpatient managementCitation8,Citation16. As a result, patients experienced prolonged periods of hospitalization during the chemotherapy period (mean length of hospital stay was 16.8 days), with patients requiring multiple hospital admissions (mean number of inpatient hospitalizations per patient was 2.2). A high economic burden was observed as a consequence of the duration and frequency of hospitalizations: the reimbursement per patient during the chemotherapy period was €68 344 and the reimbursement from the time a patient was diagnosed with Ph(−) R/R ALL until death was €108 873. Both of these values exclude any reimbursement associated with stem cell transplant, which is also known—and confirmed in this study—to be a very expensive procedureCitation17,Citation18.
The results from this French study are consistent with findings from a similar analysis conducted in the US using a US commercial claims databaseCitation19. The US study found the mean length of hospital stay among 205 patients was 13.1 days, and patients spent 56.2% of their time in hospital while receiving treatment with chemotherapy. The mean reimbursed amount per hospitalization was $89 663. Other studies conducted in Italy and Germany also showed comparable results in terms of length of inpatient hospital stay (20–25 days) and proportion of time spent in the hospital during the chemotherapy period (56–58%)Citation20,Citation21.
One limitation of this study is the small sample size, although this was expected given the rarity of the patient population—the estimated annual incidence rate of adult R/R Ph(−) B-precursor ALL is 0.2 per 100 000 person-years, corresponding to 120–130 new patients per year in FranceCitation4. Within this population, our inclusion criteria selected an even smaller sub-set of poor-prognosis patients, including restrictions to those with early first relapse, relapse after first salvage, and relapse after HSCT. Although the sample size was small, the variation in the reimbursement amounts for the hospitalizations was relatively small (SD = €4850 for inpatient stays during the chemotherapy period), giving increased confidence in the reimbursement estimates. The mean patient age was older than seen in other observational studies in patients with R/R ALLCitation11,Citation16,Citation22,Citation23. Older patients may have more hospitalization due to poorer health or less hospitalization due to administration of less toxic chemotherapy. However, we did not explore age-stratified hospitalizations due to the small patient numbers. Another limitation is that the number of outpatient visits may be under-estimated if patients are seeing physicians outside of the hospital setting, although, since ALL is a severe disease that is treated mainly in the inpatient setting, this would not increase significantly the overall estimates.
In conclusion, this study showed that, currently in France, adults with Ph(−) R/R B-cell precursor ALL have repeated and prolonged hospitalizations. Almost half of the treatment period is spent in hospital, and this time is associated with extremely high economic costs. This study highlights the hospital burden of treating adults with Ph(−) R/R B-cell precursor ALL using salvage chemotherapies, and the need for more effective, tolerable treatments.
Transparency
Declaration of funding
This study was funded by Amgen.
Declaration of financial/other relationships
HD is a consultant and received research support and honoraria from Amgen; MM received research support and honoraria from Amgen; JR is an employee of RJM who received funding from Amgen to conduct this study; EN and BB are employees and stockholders of Amgen; AB was an employee of Amgen at the time the study was conducted. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary_Table_1.docx
Download MS Word (13.6 KB)Acknowledgments
The DRG analysis was provided by Gilbert Caranhac, an employee of HoxCom Analytiques who was sub-contracted by RJM. Medical writing assistance was provided by James O’Kelly, an employee of Amgen.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute. http://seer.cancer.gov/csr/1975_2011/. based on November 2013 SEER data submission, posted to the SEER web site, April 2014
- Forman D, Bray F, Brewster DH, et al. Cancer incidence in five continents, Vol X. IARC Scientific Publication No. 164. Lyon: International Agency for Research on Cancer. http://ci5.iarc.fr/Default.aspx.
- Bassan R, Gatta G, Tondini C, Willemze R. Adult acute lymphoblastic leukemia. Crit Rev Oncol Hematol 2004;50:223–61
- Katz AJ, Chia VM, Schoonen WM, et al. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control 2015;26:1627–42
- Food and Drug Administration (FDA). Blincyto drug approval package. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125557Orig1s000TOC.cfm. Last accessed February 2016
- Committee for Medicinal Products for Human Use (CHMP). Blinatumomab EPAR. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003731/WC500198227.pdf.
- Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukemia. Nat Rev Drug Discov 2007;6:149–65
- Kantarjian HM, Thomas D, Ravandi F, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer 2010;116:5568–74
- Gökbuget N. Recommendations of the European Working Group for Adult ALL, 1st edn. 2011
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol 2011;29:532–43
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007;21:1907–14
- Camera A, Annino L, Chiurazzi F, et al. GIMEMA ALL - Rescue 97: a salvage strategy for primary refractory or relapsed adult acute lymphoblastic leukemia. Haematologica 2004;89:145–53
- Di Bona E, Pogliani E, Rossi G, et al. Transplant-finalized salvage of adult acute lymphoblastic leukemia: results of a mitoxantrone- and methotrexate-based regimen in 36 patients. Leuk Lymphoma 2005;46:879–84
- Giebel S, Krawczyk-Kulis M, Adamczyk-Cioch M, et al. Fludarabine, cytarabine, and mitoxantrone (FLAM) for the treatment of relapsed and refractory adult acute lymphoblastic leukemia. A phase study by the Polish Adult Leukemia Group (PALG). Ann Hematol 2006;85:717–22
- Advani AS, Gundacker HM, Sala-Torra O, et al. Southwest Oncology Group Study S0530: a phase 2 trial of clofarabine and cytarabine for relapsed or refractory acute lymphocytic leukemia. Br J Haematol 2010;151:430–34
- O’Brien S, Thomas D, Ravandi F. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer 2008;113:3186–91
- Saito AM, Cutler C, Zahrieh D, et al. Cost of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant 2008;14:197–207
- Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood 2012;23:1545–51
- Barlev A, Lin VW, Song X. Burden of hospitalization in relapsed acute lymphoblastic leukemia. Curr Med Res Opin 2016:1–16. [Epub ahead of print]
- Pagano L, Oberti M, Esposito B, et al. Retrospective chart review of hospitalizations during chemotherapy for adult patients with Ph-negative B-precursor relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) in Italy. Value Health 2015;18:A443
- Kreuzer K, Stuhlmann R, Lebioda A, et al. Hospitalizations among adult patients with Ph-negative B-precursor relapsed or refractory (R/R) acute lymphoblastic leukemia (ALL) receiving chemotherapy in Germany: a retrospective chart review. Value Health 2015;18:A443
- Fielding AK, Richards SM, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007;109:944–50
- Oriol A, Vives S, Hernandez-Rivas JM, Chopra R. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica 2010;95:589–96