Abstract
Objective: This study compared the cost-effectiveness of chronic hepatitis C virus (HCV) genotype 1b (GT1b) therapy ombitasvir/paritaprevir/ritonavir (OBV/PTV/r) vs daclatasvir + asunaprevir (DCV/ASV) and no treatment in patients without cirrhosis. Cost-effectiveness analyses (CEAs) that compared OBV/PTV/r against DCV/ASV and sofosbuvir/ledipasvir (SOF/LDV) in Y93H mutation-negative, GT1b patients with and without cirrhosis were also included.
Methods: A health state transition model was developed to capture the natural history of HCV. A CEA over a lifetime horizon was performed from the perspective of the public healthcare payer in Japan. Costs, health utilities, and rates of disease progression were derived from published studies. Sustained virologic response (SVR) rates of OBV/PTV/r and DCV/ASV were extracted from Japanese clinical trials. Analyses were performed for treatment-naïve and -experienced patients. Alternative scenarios and input parameter uncertainty on the results were tested.
Results: OBV/PTV/r exhibited superior clinical outcomes vs comparators. For OBV/PTV/r, DCV/ASV, and no treatment, the lifetime risk of decompensated cirrhosis in treatment-naïve patients without cirrhosis was 0.4%, 1.4%, and 9.2%, and hepatocellular carcinoma was 6.5%, 11.4%, and 49.9%, respectively. Quality-adjusted life years (QALYs) were higher in treatment-naïve and -experienced patients without cirrhosis treated with OBV/PTV/r (16.41 and 16.22) vs DCV/ASV (15.83 and 15.66) or no treatment (11.34 and 11.23). In treatment-naïve and -experienced patients without cirrhosis, the incremental cost-effectiveness ratios (ICERs) of OBV/PTV/r vs DCV/ASV were JPY 1,684,751/QALY and JPY 1,836,596/QALY, respectively; OBV/PTV/r was dominant compared with no treatment. In scenario analysis, including GT1b patients with and without cirrhosis who were Y93H mutation-negative, the ICER of OBV/PTV/r vs DCV/ASV was below the Japanese willingness-to-pay threshold of JPY 5 million/QALY, while the ICER of SOF/LDV vs OBV/PTV/r was above this threshold; thus, OBV/PTV/r was cost-effective.
Conclusion: OBV/PTV/r appears to be a cost-effective treatment for chronic HCV GT1b infection against DCV/ASV. OBV/PTV/r dominates no treatment in patients without cirrhosis.
Introduction
An estimated 160–170 million people worldwide (2.35% of the world population) have a chronic hepatitis C virus (HCV) infection, and ∼2 million of these individuals reside in JapanCitation1–3. HCV genotype 1b (GT1b) is the most prevalent sub-type in Japan (65%), followed by GT2 (34%)Citation4. The spread of disease began in the 1930s through contaminated syringes (injection drug use) and blood transfusionsCitation1,Citation3.
Approximately 80% of the cases of HCV progress to chronic infectionCitation5. The primary clinical concern with chronic HCV infection is the occurrence of fibrosis and the progression from non-cirrhosis to cirrhosis in the liverCitation6. Of these patients, some develop long-term stable cirrhosis (compensated cirrhosis), whereas others develop decompensated cirrhosis (DCC) and/or hepatocellular carcinoma (HCC), which may eventually lead to liver failure, the need for liver transplantation, and ultimately deathCitation5. The prevalence of HCC has increased in Japan over the past 50 years, and more than 30,000 patients die of HCC every yearCitation3,Citation7. In Japan, direct medical costs associated with the treatment of patients with chronic HCV were estimated at JPY 256 billion per year (at the time of publication, $1 USD = JPY 118.72)Citation8,Citation9.
Sustained virologic response (SVR) is a marker for viral eradication in HCV infection. A study of long-term outcomes for the treatment of patients with HCV infection using interferon-based treatments showed that, in 97% of patients who achieved SVR, the response was durable and was associated with a favorable long-term prognosis, a lack of evidence of progressive liver disease, and reduced mortalityCitation10,Citation11.
Until recently, treatment of patients with HCV infection relied on pegylated interferon-α + ribavirin (PR); however, SVR rates remained suboptimal at <50%, and the tolerability of treatment with PR was poorCitation12–15. SVR rates increased (∼70%) in treatment-naïve patients with HCV GT1b infection with newer triple-therapy regimens consisting of a protease inhibitor (e.g. telaprevir) + PRCitation16–18. However, the protease inhibitor further exacerbated the tolerability issues of PR treatmentCitation19.
Since the introduction of direct-acting antivirals (DAAs), the management of chronic HCV has dramatically improvedCitation20. In July 2014, the first interferon-free, all-oral, twice-daily, 24-week DAA regimen comprising daclatasvir (DCV) and asunaprevir (ASV) was approved for the treatment of interferon-ineligible or interferon-experienced patients with HCV GT1 infection in JapanCitation15. In 2015, two all-oral, once-daily, 12-week regimens, namely sofosbuvir/ledipasvir (SOF/LDV) and ombitasvir/paritaprevir/ritonavir (OBV/PTV/r), were approved to treat treatment-naïve and treatment-experienced GT1 patientsCitation15.
A recent cost-effectiveness analysis (CEA) study in Japan indicated that DCV/ASV provided better outcomes and cost-savings vs standard of care for patients with HCV GT1b infection who failed prior therapy (i.e. vs telaprevir + PR, and PR) or were interferon-ineligible/intolerant (vs no treatment)Citation21. The cost-effectiveness of OBV/PTV/r has not been assessed in treatment-naïve or treatment-experienced HCV GT1b infected patients without cirrhosis in Japan.
The purpose of this study was to compare SVR rates, direct medical costs, liver outcomes, and quality-adjusted life years (QALYs) of mono-infected HCV patients treated with OBV/PTV/r vs DCV/ASV and no treatment, in patient segments without cirrhosis. Analogous data for DCV/ASV were not available in patients with cirrhosis to make a cost-effectiveness comparison to OBV/PTV/rCitation1,Citation3.
The presence of a Y93H mutation in patients with HCV GT1b infection is an important predictor of virologic failureCitation22. According to Kumada et al.Citation23, 14% of Japanese patients with HCV GT1b infection have the Y93H mutation. Furthermore, the recent Japan Society of Hepatology clinical guidelines recommended that patients are tested for the presence of this mutation before treatment with DCV/ASV or OBV/PTV/rCitation15. DCV/ASV and OBV/PTV/r are generally used in patients without the Y93H mutation; thus, in a scenario analysis, we compared OBV/PTV/r vs DCV/ASV, sofosbuvir and ledipasvir (SOF/LDV), and no treatment across all patients with HCV GT1b infection without the Y93H mutation (not stratified by treatment history or cirrhosis status).
Methods
We developed an HCV model from the Japanese public healthcare payer perspectiveCitation24, based on previously published health state transition models with a lifetime horizon and annual cyclesCitation25,Citation26.
Natural history of HCV
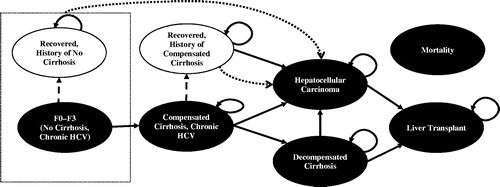
The health state transition model structure, based primarily on Ishida et al.Citation26 (), was used to model the lifetime disease progression of treatment-naïve and treatment-experienced patients with HCV GT1b infection. The model consisted of five disease progression states, including chronic HCV, without cirrhosis; chronic HCV, compensated cirrhosis; DCC; HCC; and liver transplantation. Two SVR recovered states (i.e. recovered, without history of cirrhosis; and recovered, history of compensated cirrhosis) and an absorbing mortality state (i.e. liver-related mortality or non-liver mortality) were also included in the model.
Figure 1. Natural history model schematic. CC, compensated cirrhosis; DCC, decompensated cirrhosis; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virologic response. Patients with HCV infection initiated treatment in the first cycle. Health states are depicted by ellipses, while arrows represent permissible transitions between health states. Hashed arrows depict the possibility of achieving SVR. Dotted arrows depict progress to HCC from any recovered states, including without history of cirrhosis and history of compensated cirrhosis. Background mortality is possible from any health state. Liver-related mortality is possible from DCC, HCC, and liver transplantation.
The transition probabilities were based on previously published clinical trials and economic evaluations of patients with HCV infection in Japan; model inputs for the base case, including transition probabilities, are shown in Citation17,Citation23,Citation26–31.
Table 1. Model inputs for the base case, deterministic, and probabilistic sensitivity analyses.
In the model, patients with HCV infection initiated treatment in the first cycle. Patients who did not achieve an SVR were at risk for progressive liver disease, and faced the same risks of disease progression as untreated patients. These patients transitioned to more advanced liver disease states, including HCC, DCC, and liver transplantation. Patients who achieved SVR moved to recovered states. These patients still faced an excess risk of developing HCC, albeit at a lower rate than patients who did not achieve an SVR. Advanced liver disease states (i.e. DCC, HCC and liver transplantation) carried excess liver-related mortality risksCitation25,Citation26,Citation32. Patients also faced background age- and gender-related mortality risks in any cycleCitation33.
Patient baseline characteristics
Data from treatment-naïve and treatment-experienced patients with HCV GT1b infection were analyzed. Based on the GIFT-I (Japan) clinical trial, the mean age at baseline for treatment-naïve and treatment-experienced patients was 61.2 years, as reported in Kumada et al.Citation23. Fifty-five per cent of treatment-naïve patients were male, as were 64% of treatment-experienced patientsCitation31.
Clinical inputs
The clinical inputs for treatment efficacy (SVR at post-treatment week 12) and trial-based, expected treatment duration were derived from Japan-based phase III trials in patients with HCV GT1b infection (). Inputs for treatment with OBV/PTV/r were extracted from the GIFT-I studyCitation23. Efficacy data for DCV/ASV were extracted from the AI447026 trialCitation34. The model assumed that patients receiving no treatment had a 0% SVR rate. In addition, discontinuation rates from trials were extracted, which in turn were used to calculate the expected treatment duration for each treatment.
In a scenario analysis examining patients with and without cirrhosis without the Y93H mutation, SVR data for OBV/PTV/r, DCV/ASV, and SOF/LDV were extracted from the Pharmaceuticals and Medical Devices Agency website ()Citation35. SVR data for patients without the Y93H mutation were not stratified by treatment history and cirrhosis status. For completeness, the category no treatment was included in the analysis. Further, we assumed that discontinuation rates did not differ by the presence or absence of the Y93H mutation. Thus, data were taken from the respective Japanese pivotal trials, namely Kumada et al.Citation23,Citation34 and Mizokami et al.Citation36.
Treatment-related adverse events were not included in the model, given that the adverse event rates observed in the OBV/PTV/r and DCV/ASV trials were low and the costs associated with these adverse events were assumed negligible.
Health utilities
Disease-state health utility () reflects the expected annual health-related quality-of-life in patients with chronic HCV over the complete disease course. Our base case disease-state utility values were derived from Ishida et al.Citation26. The health state utility without cirrhosis was imputed from Ishida et al.Citation26 using the weights for mild and moderate fibrosis from Yoshida et al.Citation31. We assumed that patients with no HCV infection had the same health utilities as those who recovered without developing cirrhosis.
Treatment-related health utility reflects the change in health-related quality-of-life resulting from treatment-related adverse events and inconvenience. Treatment-related health utility data for OBV/PTV/r were derived from the Euro-Qol-5 Dimensions-5 Levels (EQ-5D-5L) questionnaires included in the GIFT-I clinical study reportCitation37. As there were no EQ-5D data for DCV, ASV, or the combination DCV/ASV, treatment-related health utility data for DCV/ASV were assumed to be the same as that of OBV/PTV/r, but weighted appropriately by treatment duration.
Treatment-related health utility decreased over the duration of therapy for all treatments evaluated. The decrements in utility were annualized using expected treatment duration calculated from trial data, and were applied to baseline utilities in the first cycle in which treatment was received. Annualized changes by patient segments are presented in Citation26.
Costs
Drug costs were obtained from the Japanese National Health Insurance drug price list as of April 2016Citation26,Citation38. The daily regimen costs of OBV/PTV/r, DCV/ASV, and SOF/LDV were JPY 46,115, JPY 13,598, and JPY 54,797, respectively. Total treatment cost is a function of regimen daily cost and expected treatment duration. In turn, the expected treatment duration was obtained from trial publications, and was based on the labeled regimen. Health state costs were derived from Japanese sources, including Ishida et al.Citation26 and McEwan et al.Citation17. Only direct medical costs were considered. Cost data and their sources are provided in Citation17,Citation26,Citation28,Citation38.
Analyses
Costs and outcomes were discounted at 2% per yearCitation39. We reported lifetime direct medical costs, liver outcomes, QALYs, as well as incremental cost-effectiveness ratios (ICERs).
Deterministic sensitivity analyses (DSA) and probabilistic sensitivity analyses (PSA) were performed in patient segments comparing OBV/PTV/r with DCV/ASV and no treatment. We tested treatment and non-treatment-specific variables, including SVR rates, baseline patient characteristics, transitional probabilities related to disease progression, health state costs and utilities, discount rates, and time horizon. SVR rates were varied using ±1.96-times the standard deviation of base values in the DSA, and using a beta distribution in the PSA, characterized by the trial segment sample size and percentage with SVR. For each input parameter tested in the model, presents the input parameter value range tested in the DSA and the statistical moments and probability distributions used in the PSA. PSA diagrams were derived for treatment-naïve and treatment-experienced patients for all treatment regimens. In the scenario analysis, CEAs in patients with and without cirrhosis without the Y93H mutation were conducted. Similarly, we conducted DSA and PSA in the scenario analysis by varying input parameter values using the same ranges to compare OBV/PTV/r, DCV/ASV, and SOF/LDV. For the DSA, the 95% confidence interval of SVR rates were calculated using an exact binomial distribution (the Clopper-Pearson method). For the PSA, SVR rates were estimated using a beta distribution. In instances where the SVR rates were 100%, we applied the methodology proposed by Briggs et al.Citation40 to assess uncertainty around the parameter estimate. Key model outputs, including clinical outcomes, total costs, QALYs, and ICERs, were computed for each patient segment (stratified by treatment experience). Results were compared to the willingness-to-pay threshold of JPY 5 million/QALY, which is a generally accepted threshold in JapanCitation41.
Results
Liver disease outcomes
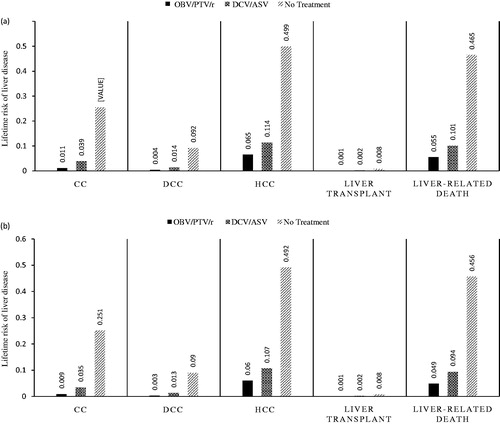
The percentages of treatment-naïve and treatment-experienced patients without cirrhosis who reached compensated cirrhosis, DCC, HCC, liver transplantation, and liver-related death are shown in . As expected, patients in the no treatment category were most likely to experience a liver event. Across patient populations, OBV/PTV/r was associated with significant improvements in health outcomes, including the lowest rates of long-term progressive liver disease complications such as compensated cirrhosis, DCC, HCC, liver transplantation, and liver-related mortality. For instance, in treatment naïve patients without cirrhosis, the lifetime risk of DCC was 0.4%, 1.4%, and 9.2% with OBV/PTV/r, DCV/ASV, and no treatment, respectively. The lifetime risk of HCC was 6.5%, 11.4%, and 49.9%; liver transplantation was 0.1%, 0.2%, and 0.8%; and liver-related mortality was 5.5%, 10.1%, and 46.5% with OBV/PTV/r, DCV/ASV, and no treatment, respectively.
Cost-effectiveness
In treatment-naïve patients without cirrhosis, treatment with OBV/PTV/r was cost-effective compared with DCV/ASV treatment: it conferred higher QALYs (16.41 vs 15.83) at higher costs (JPY 5,410,171 vs JPY 4,439,917); accordingly, its ICER of JPY 1,684,751/QALY was below the willingness-to-pay threshold of JPY 5 million/QALY (). In treatment-experienced patients without cirrhosis, treatment with OBV/PTV/r was also cost-effective vs DCV/ASV, assuming a JPY 5 million/QALY willingness-to-pay threshold, with an ICER of JPY 1,836,596/QALY (QALYs: 16.22 vs 15.66; costs: JPY 5,322,416 vs JPY 4,297,182, respectively). In patients without cirrhosis, regardless of treatment history, treatment with OBV/PTV/r dominated no treatment (higher QALYs at lower costs).
Table 2. Total costs, LYs, QALYs, ICERs, and effectiveness across patient segments.
Sensitivity analysis
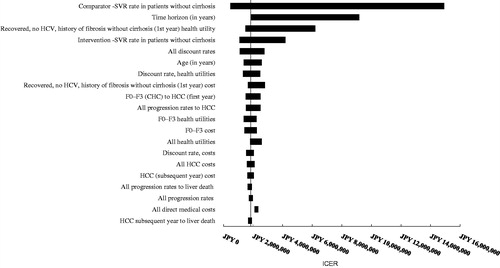
Deterministic sensitivity analyses were performed for both patient segments comparing OBV/PTV/r with DCV/ASV (treatment-naïve, ; treatment-experienced, ). In treatment-naïve patients without cirrhosis, three parameters exceeded the JPY 5 million/QALY threshold: time horizon (JPY 9,036,236), comparator SVR rate (JPY 7,806,335), and health utility in patients who reached SVR (JPY 5,693,408). In treatment-experienced patients without cirrhosis, three parameters exceeded the JPY 5 million/QALY threshold: comparator SVR rate (JPY 14,925,112), time horizon (JPY 9,175,581), and health utility in patients who reached SVR (JPY 6,202,278). Time horizon was an influential parameter in the model, as it affected the number of patients reaching advanced liver disease states that were associated with higher costs.
Figure 3. OBV/PTV/r vs DCV/ASV in HCV GT1b treatment-naïve patients without cirrhosis (base case: JPY 1,684,751). CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; DCV/ASV, daclatasvir/asunaprevir; GT1b, genotype 1b; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; SVR, sustained virologic response.
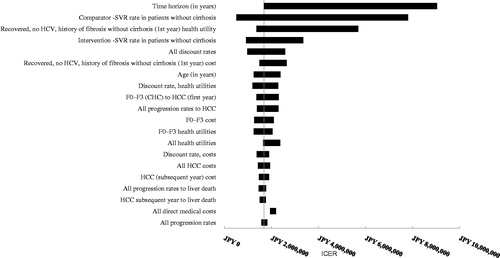
Figure 4. OBV/PTV/r vs DCV/ASV in HCV GT1b treatment-experienced patients without cirrhosis (base case: JPY 1,836,596). CHC, chronic hepatitis C; HCC, hepatocellular carcinoma; DCV/ASV, daclatasvir/asunaprevir; GT1b, genotype 1b; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; SVR, sustained virologic response.
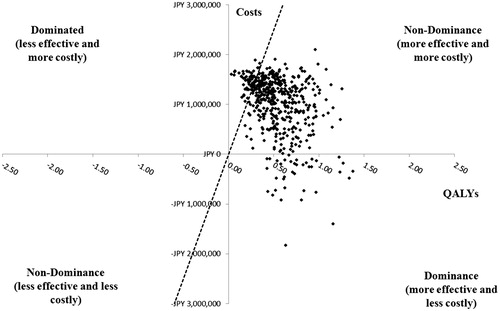
A PSA was performed on each of the patient segments without cirrhosis for OBV/PTV/r vs DCV/ASV (treatment-naïve patients, ; treatment-experienced patients, ). For each PSA, 500 Monte Carlo simulations were conducted. At the willingness-to-pay threshold of JPY 5 million/QALY, OBV/PTV/r was the cost-effective option in treatment-naïve patients in 86.0% of model simulations; in treatment-experienced patients, OBV/PTV/r was the cost-effective option in 88.2% of model simulations.
Figure 5. Probabilistic sensitivity analysis in treatment-naïve HCV GT1b patients without cirrhosis. GT1b, genotype 1b; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; WTP, willingness-to-pay. PSA estimated on 500 simulations. OBV/PTV/r is the optimal therapy in at least 86.0% of the simulations, assuming payers have a WTP threshold of JPY 5 million/QALY. The dashed line indicates the JPY 5 million WTP threshold; the data points to right of this line yield ICERs below the WTP threshold.
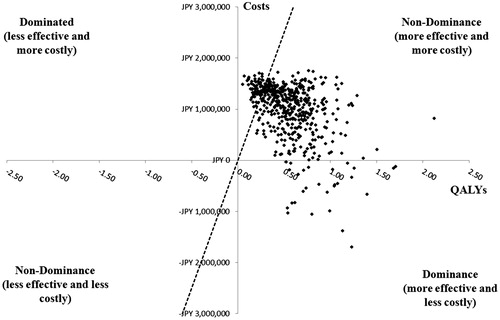
Figure 6. Probabilistic sensitivity analysis in treatment-experienced patients with HCV GT1b infection without cirrhosis. GT1b, genotype 1b; HCV, hepatitis C virus; ICER, incremental cost-effectiveness ratio; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; WTP, willingness-to-pay. PSA estimated on 500 simulations. OBV/PTV/r is the optimal therapy in at least 88.2% of the simulations, assuming payers have a WTP threshold of JPY 5 million/QALY. The dashed line indicates the JPY 5 million WTP threshold; the data points to right of this line yield ICERs below the WTP threshold.
Scenario analysis
In patients without the Y93H mutation, treatment with OBV/PTV/r dominated no treatment (). Treatment with OBV/PTV/r was also cost-effective vs DCV/ASV, with an ICER of JPY 2,889,945/QALY (below the JPY 5 million/QALY willingness-to-pay threshold). Comparatively, SOF/LDV was not a cost-effective alternative vs OBV/PTV/r, with an ICER more than 2.5-times the willingness-to-pay per QALY threshold (JPY 13,296,227/QALY).
Table 3. Total costs, QALYs, and ICER results for patients without the Y93H mutation.
Deterministic sensitivity analyses were performed for all patients comparing OBV/PTV/r and SOF/LDV. The single most disease-influential model parameter was SVR rate in all patients (with and without cirrhosis), which was expected, given that the SVR rates for OBV/PTV/r and SOF/LDV were similar. These results were robust in the PSA, where, in 97.2% of simulations, treatment with OBV/PTV/r was the optimal choice (vs SOF/LDV) when assuming a willingness-to-pay threshold of JPY 5 million/QALY. In fact, in 25.6% of the simulations, treatment with OBV/PTV/r was dominant (less costly and more efficacious).
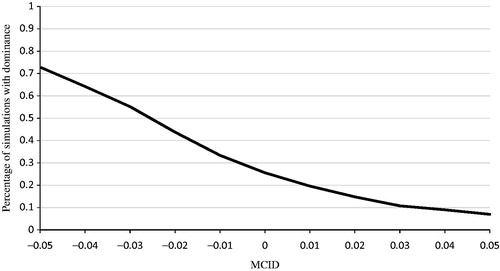
In the primary virologic failure population, the 95% confidence interval of SVR for OBV/PTV/r (99%; 95% confidence interval = 97–100%; n = 304) overlapped with that of SOF/LDV (100%; 95% confidence interval = 97–100%; n = 127) in patients without the Y93H mutation. Therefore, we conducted a minimally clinical important difference analysis (MCID) to determine whether the effectiveness of OBV/PTV/r was non-inferior to SOF/LDV. For this analysis, non-inferior effectiveness was defined as at least a ±0.05 difference in QALYs between OBV/PTV/r and SOF/LDV, compared with the more limited range of MCID ≥0 used previously. Using this definition, OBV/PTV/r dominated SOF/LDV in 7.0–73.0% of simulations, as the definition of MCID in QALYs varied from 0.05 to –0.05 ().
Figure 7. Probabilistic sensitivity analysis: results using MCID of ±0.05 in QALYs between OBV/PTV/r and SOF/LDV. MCID, minimally clinical important difference; OBV/PTV/r, ombitasvir/paritaprevir/ritonavir; PSA, probabilistic sensitivity analysis; QALY, quality-adjusted life year; SOF/LDV, sofosbuvir/ledipasvir. PSA estimated on 500 simulations. OBV/PTV/r is a dominant strategy (i.e. less costly and more effective) vs SOF/LDV in 25.6% of simulations (i.e. MCID ≥0). In addition, when we varied the MCID in QALYs from 0.05 to –0.05, OBV/PTV/r dominates SOF/LDV in 7.0–73.0% of simulations.
Discussion
In treatment-naïve patients without cirrhosis, treatment with OBV/PTV/r was associated with significant improvements in health outcomes, including the lowest rates of long-term progressive liver disease complications (e.g. DCC, HCC, liver transplantation). Treatment with OBV/PTV/r was cost-effective vs DCV/ASV across patient groups (including patients without the Y93H mutation), with ICERs below the willingness-to-pay threshold of JPY 5 million/QALY. OBV/PTV/r dominated no treatment in all patient segments studied. Finally, in a majority of simulations, OBV/PTV/r dominated SOF/LDV, with an MCID in QALYs of –0.05.
This is the first study to assess the cost-effectiveness of OBV/PTV/r in treatment-naïve and treatment-experienced patients with HCV GT1b infection without cirrhosis in Japan, and, as such, it should be of interest to payers who make decisions regarding formulary and coverage for patients with chronic HCV infection for two reasons. First, the budget impact of HCV treatment is high. In 2015, ∼ 70,000 patients were treated with interferon-free therapy in JapanCitation42. Second, CEAs to inform Japanese National Health Insurance pricing are being considered in Japan; the current analysis helps inform this decision. Of note, the current Japanese model allowed patients without cirrhosis (with and without SVR) to transition to HCC. This unique feature was included in the model based on expert feedback, and aligned to previous Japanese studiesCitation17,Citation26,Citation43. By allowing a transition to HCC from a health state without cirrhosis, patients in the current model had a higher probability of transitioning to HCC compared with other published HCV models, including the UK-based, Hartwell et al.Citation25 model. In turn, patients in the Japanese model had a higher likelihood of reaching HCC than DCC.
Patients with cirrhosis (n = 42) in the GIFT-I study were not stratified by treatment history or interferon eligibility/tolerance; thus, there were insufficient data to weight the SVR in patients with cirrhosis appropriately to make a cost-effectiveness comparison to DCV/ASV in patients with cirrhosis. In addition, the relatively small sample size in the GIFT-I study implied that any imputation to stratify compensated cirrhosis patients would lead to even smaller sub-group sample sizes and greater uncertainty in our estimates. Thus, a comparison between OBV/PTV/r and DCV/ASV in patients with cirrhosis was not feasible and was, therefore, excluded in our analyses.
Recent guidelines from the Japan Society of Hepatology recommended that testing for the presence of the Y93H mutation be conducted because the mutation affects the efficacy of treatment with DCV/ASV and to some extent that of OBV/PRV/r. If so, this test could improve resource utilization by predicting virologic responseCitation15. In patients with HCV GT1b infection without the Y93H mutation, efficacy data that included patients with cirrhosis were available, albeit not stratified by cirrhosis status and treatment history. Thus, in scenario analysis, we compared treatment with OBV/PTV/r vs DCV/ASV, SOF/LDV, and no treatment, which filled the analytical gap of excluding patients with compensated cirrhosis in the core analysis. Our results reinforced the hypothesis that OBV/PTV/r was cost-effective vs DCV/ASV across all patients without the Y93H mutation (i.e. treatment-naïve and treatment-experienced, with and without cirrhosis). In addition, vs SOF/LDV, OBV/PTV/r was also the optimal choice for payers because of the fully overlapped 95% CIs of SVR and a more than JPY 2 million lower treatment cost, leading to an ICER above the willingness-to-pay threshold of JPY 5 million/QALY.
The model presented in this article has several strengths. The model schematic is in line with Hartwell et al.Citation25, which has been widely adapted; it is also aligned with Ishida et al.Citation26. The model used Japanese-based input parameters to model disease progression and health state utilities. In addition, the model contained two recovered health states representing virologic cure of chronic HCV infection, which were differentiated by the patients’ stage of liver disease prior to treatment (i.e. no cirrhosis vs compensated cirrhosis); this stratification facilitated modeling of the subsequent risk of progressive liver disease important for recovered patients with a history of compensated cirrhosis. Finally, the model included SVR data extracted directly from published clinical trials: Kumada et al.Citation23 for OBV/PTV, and Kumada et al.Citation34 for DCV/ASV.
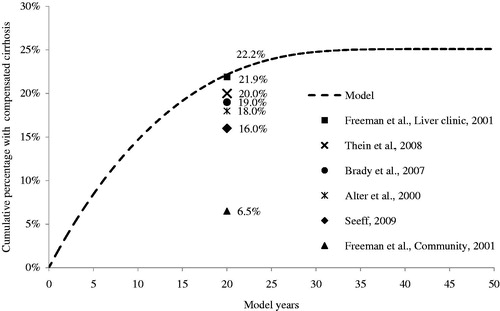
We validated our model externally; assuming 100% of patients entered the model with mild disease, the model estimated that 22.2% of patients would have a history of compensated cirrhosis 20 years post-infection. The compensated cirrhosis rate estimated over 50 years appear in , including cirrhosis estimates from Freeman et al.Citation44, Thein et al.Citation45, Alter and Seeff et al.Citation46, SeeffCitation47, and Brady et al.Citation32. Our 20-year compensated cirrhosis rate was concordant with rates from other HCV studies. Adaptation of the current model to the US demonstrated favorable cost-effectiveness and long-term liver morbidity outcomes for the AbbVie Regimen in GT1 patientsCitation48,Citation49. In addition, based on adaptations of the current model, Canada and the UK recommended reimbursement of the AbbVie Regimen to patients with GT1 HCV infection in 2015, thus affirming the quality of the model.
The model has several limitations. It did not include treatment-related adverse events, primarily because of the lack of reliable adverse event costs data. However, given that our analysis compared OBV/PTV/r with DCV/ASV and SOF/LDV, and all these regimens had low adverse event rates, the overall adverse event costs would not be an influential and differentiating feature of the analysis. Second, there was limited information on the baseline characteristics of patients with chronic HCV infection in Japan; in particular, we found no recently published information on the baseline characteristics for patients in the sub-groups used in the model. As such, we used baseline characteristics from patients with HCV GT1b infection without cirrhosis who were enrolled in the Japanese-specific GIFT-I clinical trial.
To determine whether these and other limitations affected our findings, we conducted DSA and PSA where inputs were varied across a range of plausible values. For these analyses, treatment history, background mortality rate, and regimen costs and duration did not vary. Patient segments were defined by their treatment histories; background death rates were based on large national samples with little measurement error; drug costs were endogenous; last, no data were identified for varying regimen duration, which came from trials. The DSA and PSA confirmed the robustness of our findings in our base case and scenario analysis. In DSA, time horizon and SVR rates were the most influential parameters: time horizon affected the number of patients reaching advanced liver disease states that were associated with higher costs, and, therefore, was a pivotal parameter in the model. The SVR differences between the intervention and comparator were not large; QALY differences were, in turn, small. Thus, the denominator of the cost-effectiveness ratio was small, and changes in SVR had a relatively large impact on the upper and lower bounds of the ICERs. Nevertheless, caution is advised when generalizing these results, as they may differ by settings that affect treatment outcomes, such as individual patient comorbidities and characteristics, treatment administration, and adherence.
The HCV market in Japan is a dynamic one, where new drugs quickly become widely prescribed, and the price of a drug is not a primary consideration when selecting treatment partially due to the co-payment subsidy scheme exclusively available for hepatitis treatment. According to manufacturers’ estimates, 20,000 and 32,700 GT1 patients in Japan were treated with DCV/ASV and SOF/LDV, respectively, within the 6-month period after their respective launchesCitation50,Citation51. An estimated 2,930 patients were treated with OBV/PTV/r within the 5-month period after its launchCitation52. As a result, the Central Social Insurance Medical Council (also known as Chuikyo), an advisory board of the National Health Insurance for the Minister of Health, Labor and Welfare (MHLW), has become concerned with the impact of HCV drugs on overall healthcare budget as well as their cost per patient, and a new rule for repricing “huge-selling” drugs was introduced in April 2016. Drugs with recorded annual sales from 100–150 billion yen and above 150% or more than initial forecasts had prices cut by 25%, and those with sales of more than 150 billion yen and 130% or more than initially forecast had prices cut by as much as 50%. When applied to the HCV regimens included in this manuscript, the repricing rule introduced price cuts of 13.2%, 14.0%, 14.0%, and 31.7% for ASV, DCV, OBV/PTV/r, and SOF/LDV, respectively, and these lower prices are reflected in the analysesCitation38,Citation53. Also in April 2016, MHLW introduced a pilot scheme to assess the cost-effectiveness of selected drugs. The aim of the assessment was to inform price revisions of the selected drugs in 2018. All three regimens analyzed in this paper have been selected for the pilot assessment. Although it is not yet clear how the revised prices in 2018 would further affect results, our analysis offers important insight on the cost-effectiveness of novel DAA treatments for patients with HCV GT1b infection for the public healthcare payer in Japan.
Conclusion
Treatment with OBV/PTV/r is a cost-effective option, assuming a JPY 5 million/QALY willingness-to-pay threshold, when compared to DCV/ASV in patients with HCV GT1b infection without cirrhosis. In patients with HCV GT1b infection with or without cirrhosis who do not have the Y93H mutation, OBV/PTV/r remains a cost-effective treatment option vs treatment with DCV/ASV. Treatment with SOF/LDV is not cost-effective, as it has the same clinical effectiveness but higher cost compared with OBV/PTV/r. Compared with no treatment, OBV/PTV/r dominates in all patient segments. Thus, OBV/PTV/r is the optimal choice in all patient segments analysed. The results indicate the value of extending treatment to HCV infected patients throughout Japan. These results should inform decisions on treatment strategy, where the benefits conferred to patients and cost-effectiveness of treatment are of primary importance.
Transparency
Declaration of funding
Funding was provided by AbbVie Global to Medicus Economics to develop the cost-effectiveness model, and to DNT Research and Consulting to provide technical writing assistance.
Declaration of financial/other relationships
SJ and SV are employees of Medicus Economics LLC, a consulting company that conducts economic evaluations in a variety of therapeutic areas for pharmaceutical companies. DM is a contractor to Medicus Economics. K. Yasui and K. Yamazaki are employees of AbbVie GK and may own AbbVie stock. CY is an employee of AbbVie Pte. Ltd and may own AbbVie stock. JCS is an employee of AbbVie Inc and may own AbbVie stock. AbbVie is the manufacturer of ombitasvir/paritaprevir/ritonavir. AI received a grant from AbbVie GK for conduct of the current study. In addition, AI has received grants and personal fees from Pfizer Japan Inc., Gilead Science K.K., and Milliman Inc. He has received grants from CSL Behring Japan Inc., TOWA Pharmaceuticals Co. Ltd., Terumo corporation, and Fuji Film K.K. He has also received personal fees from Novartis Pharma K.K., AbbVie GK, CRECON Research and Consulting Inc., Sony Inc., Kanter Health Inc., Astellas Pharma K.K., Chugai Pharmaceutical Co. Ltd., Intuitive Surgical Inc., Eli Lilly Japan K.K., Health and Global Policy Institute, American Medical Devices and Diagnostics Manufacturers’ Association, Bristol-Myers Squibb K.K., Creative Ceuticals K.K., and Abbott Japan K.K., outside the submitted work.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors acknowledge the technical writing assistance of Nicole Tunstall, an employee of DNT Research and Consulting, who was contracted by Medicus Economics, in the preparation of this manuscript.
References
- European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392-420
- Higuchi M, Tanaka E, Kiyosawa K. Epidemiology and clinical aspects on hepatitis C. Jpn J Infect Dis 2002;55:69-77
- Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology 2010;53:39-43
- Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61:S45-S57
- Liang TJ, Rehermann B, Seeff LB, et al. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med 2000;132:296-305
- Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36:S35-S46
- Ikai I, Arii S, Okazaki M, et al. Report of the 17th nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res 2007;37:676-91
- Liu GG, DiBonaventura M, Yuan Y, et al. The burden of illness for patients with viral hepatitis C: evidence from a national survey in Japan. Value Health 2012;15:S65-S71
- Toyokawai S, Kobayashi Y, Ohmori M. Refined method for estimating medical expenditures for liver disease using the patient survey and claim data in Japan. Nihon Koshu Eisei Zasshi 2005;52:957-61
- Koh C, Heller T, Haynes-Williams V, et al. Long-term outcome of chronic hepatitis C after sustained virological response to interferon-based therapy. Aliment Pharmacol Ther 2013;37:887-94
- Backus LI, Boothroyd DB, Phillips BR, et al. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol 2011;9:509-16 e1
- Kwo P, Lacerda M. Next generation direct-acting antiviral agents for hepatitis C treatment. N A J Med Sci 2014;7:23-32
- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350-9
- Desmond CP, Roberts SK, Dudley F, et al. Sustained virological response rates and durability of the response to interferon-based therapies in hepatitis C patients treated in the clinical setting. J Viral Hepat 2006;13:311-15
- Asahina Y, Izumi N, Hiromitsu K, et al. JSH Guidelines for the Management of Hepatitis C Virus Infection: a 2016 update for genotype 1 and 2. Hepatol Res 2016;46:129-65
- National Institute of Health and Care Excellence. Telaprevir for the treatment of genotype 1 chronic hepatitis C. London: National Institute of Health and Care Excellence; 2012. http://www.nice.org.uk/guidance/ta252. Accessed December 23, 2015
- McEwan P, Ward T, Webster S, et al. Estimating the long-term clinical and economic outcomes of daclatasvir plus asunaprevir in difficult-to-treat Japanese patients chronically infected with Hepatitis C Genotype 1b. Value Health Regional Issues 2014;3:136-45
- National Institute of Health and Care Excellence. Simeprevir in combination with peginterferon alfa and ribavirin for treating genotypes 1 and 4 chronic hepatitis C. London: National Institute of Health and Care Excellence; 2015. http://www.nice.org.uk/guidance/ta331. Accessed December 23, 2015
- Park C, Jiang S, Lawson KA. Efficacy and safety of telaprevir and boceprevir in patients with hepatitis C genotype 1: a meta-analysis. J Clin Pharm Ther 2014;39:14-24
- Asahina Y, Hayashi N, Izumi N, et al. JSH Guidelines for the management of hepatitis c virus infection: a 2014 update for Genotype 1. Hepatol Res 2014;44:59-70
- McEwan P, Ward T, Webster S, et al. Estimating the cost-effectiveness of daclatasvir plus asunaprevir in difficult to treat Japanese patients chronically infected with hepatitis C genotype 1b. Hepatol Res 2016;46:423-33
- Yoshimi S, Ochi H, Murakami E, et al. Rapid, sensitive, and accurate evaluation of drug resistant mutant (NS5A-Y93H) strain frequency in genotype 1b HCV by invader assay. PLoS One 2015;10:e0130022
- Kumada H, Chayama K, Rodrigues L Jr, et al. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology 2015;62:1037-46
- Fukuda T, Shiroiwa T, Ikeda S, et al. Guideline for economic evaluation of healthcare technologies in Japan. J Natl Inst Public Health, 62:2013 (Japanese only)
- Hartwell D, Jones J, Baxter L, et al. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 2011;15:i-xii, 1–210
- Ishida H, Yotsuyanagi H. Examination of the cost-effectiveness of the standard of care for chronic HCV treatment. Research on health-economics of various initiatives related to viral liver diseases, Ministry of Health, Labour and Welfare, Japan (PI: Hirao T) 2014;127-192 (Japanese Only). http://mhlw-grants.niph.go.jp/niph/search/Download.do?nendo=2013&jigyoId=135013&bunkenNo=201333004A&pdf=201333004A0005.pdf. Accessed January 6, 2016
- Maruoka D, Imazeki F, Arai M, Kanda T, Fujiwara K, Yokosuka O. Long–term cohort study of chronic hepatitis C according to interferon efficacy. J Gastroenterol Hepatol. 2012;27:291-9.
- Ishida K, Imai H, Ogasawara K, et al. Cost-utility of living donor liver transplantation in a single Japanese center. Hepatogastroenterology 2006;53:588-91
- Suga M, et al. Development of the fundamental model for HCV. Research on health-economics of various initiatives related to viral liver diseases, Ministry of Health, Labour and Welfare, Japan (PI: Hirao T), 2014;76-95 (Japanese Only). http://mhlw-grants.niph.go.jp/niph/search/Download.do?nendo=2013&jigyoId=135013&bunkenNo=201333004A&pdf=201333004A0004.pdf. Accessed January 6, 2016
- Tanaka J, Kumada H, Ikeda K, et al. Natural histories of hepatitis C virus infection in men and women simulated by the Markov model. J Med Virol 2003;70:378-86
- Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med 1999;131:174-81
- Brady B, Sieber U, Sroczynski G, et al. Pegylated Interferon combined with ribavirin for chronic hepatitis C virus infection: an economic evaluation [Technology Report No. 82]. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health (CADTH); 2007
- Ministry of Health Labour and Welfare. Abridged Life Tables for Japan 2013. Statistics and Information Department. Minister's Secretariat. Government of Japan: Japan, 2016. http://www.mhlw.go.jp/english/database/db–hw/lifetb13/index.html. Accessed January 6, 2016
- Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology 2014;59:2083-91
- BMS KK, AbbVie GK, Gilead Sciences KK, Interview Form available from PMDA website (in Japanese only). Daklinza Interview Form: http://www.info.pmda.go.jp/go/interview/1/670605_6250040F1020_1_005_1F. Viekirax Interview Form: http://www.info.pmda.go.jp/go/interview/1/112130_62501A2F1027_1_1F. Harvoni Interview Form: http://www.info.pmda.go.jp/go/interview/1/230867_62501A1F1022_1_001_1F.pdf. 2015. Japan. Accessed January 6, 2016
- Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015;15:645-53
- AbbVie GK. Data on file. GIFT-I Clinical study report. Japan. 2015
- Ministry of Health Labour and Welfare. Japanese National Health Insurance drug price list, effective April 2016. Japan: Ministry of Health Labour and Welfare; 2016. http://www.mhlw.go.jp/topics/2014/03/tp0305–01.html (Japanese only). Accessed March 4, 2016
- Fukuda T, Shiroiwa T, Ikeda S, et al. Guideline for economic evaluation of healthcare technologies in Japan. J Natl Inst Public Health 2013;62:625-40
- Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making 2003;23:341-50
- Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness–to–pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ 2010;19:422-37
- AbbVie GK Data on file. Japan. 2015
- Kuwabara H, Westerhout K, Treur M, et al. Cost-effectiveness analysis of simeprevir in combination with peginterferon and ribavirin for treatment-naive chronic hepatitis C genotype 1 patients in Japan. J Med Econ 2015;18:502-11
- Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809-16
- Thein HH, Yi Q, Dore GJ, et al. Estimation of stagespecific fibrosis progression rates in chronic hepatitis C virus infection: a metaanalysis and metaregression. Hepatology 2008;48:418-31
- Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis 2000;20:17-35
- Seeff LB. The history of the “natural history” of hepatitis C (1968–2009). Liver Int 2009;29(1 Suppl):89-99
- Johnson S, Parise H, Virabhak S, et al. Percent of subjects experiencing liver morbidity over a lifetime horizon with Abbvie 3D (ABT-450/Ritonavir/Ombitasvir And Dasabuvir) versus no treatment. Presented at: European Association for the Study of the Liver (EASL) 50th Annual Meeting, Vienna, Austria, April, 2015
- Saab S, Parisé H, Virabhak S, et al. Cost-effectiveness of currently recommended direct-acting antiviral treatments in patients infected with genotypes 1 or 4 hepatitis C virus in the US. J Med Econ 2016:1-11, doi:10.1080/13696998.2016.1176030
- Bristol-Myers Squibb. Report of early post marketing phase vigilance of daklinza/sunvepra. Japan :Bristol-Myers Squibb; 2015. https://file.bmshealthcare.jp/renewal/pdf/phdasu/development/05.pdf (Japanese only). Accessed May 31, 2016
- Gilead Sciences Ltd. Report of early post marketing phase vigilance of harvoni. Japan: Gilead Sciences Ltd; 2016. https://www.harvoni.jp/∼/media/files/japan/japan-gilead/pdf/20160513/hvn_post_marketing_surveillance_final_report.pdf?la=ja-jp (Japanese only). Accessed May 31, 2016
- AbbVie GK. Report of early post marketing phase vigilance of viekirax. Japan. 2016. https://abbvie-channel.com/contents/contents/download.aspx?file=pdf/viekirax/report05.pdf (Japanese only). Accessed May 31, 2016
- Fujiwara Y. Evolution of frameworks for expediting access to new drugs in Japan. Nat Rev Drug Discov 2016;15:293-4