Abstract
Aim: To describe treatment pattern, complications, and direct medical costs associated with ankylosing spondylitis (AS) in Chinese urban patients.
Methods: The 2013 China Health Insurance Research Association (CHIRA) urban insurance claims database was used to identify patients with AS. The identified patients were stratified by AS treatments for the comparisons of well established AS-related complications and direct medical costs. Conventional regression analyses adjusted the collected patient baseline characteristics to confirm the impact of treatments on complications and direct medical costs.
Results: Of the identified 1299 patients with AS, 18.0% received non-steroidal anti-inflammatory drugs (NSAID), 11.2% received immunosuppressant, 48.2% received NSAID plus immunosuppressant, 4.6% received biologic agents, and 17.9% received medications without indication for AS. Biologic group was associated with the lowest proportion of AS-related complications (8.3%) that was confirmed by multiple logistic regression analysis (odds ratio = 0.200, p = .017). The biologic group was also associated with highest direct medical costs (median: RMB = 14,539) that were confirmed by the multiple generalized linear model (coefficient = 1.644, p < .001).
Conclusions: Biologics were not commonly used for AS in Chinese patients likely due to their high cost. Future studies are needed to confirm the potential long-term clinical benefits associated biologic treatment for AS.
Introduction
The prevalence of ankylosing spondylitis (AS) in China is 0.3%Citation1. The main clinical symptoms associated with AS are long-term pain and stiffness of spineCitation2. The persistent AS could lead to bone spur with ligament and bamboo-like spine that significantly limit patient physical function, impair patient quality-of-life, and reduce patient productivityCitation3. Thus, the primary treatment goal for AS is to control the AS-related symptoms and delay the disease progressionCitation4.
Biologic agents targeting tumor necrosis factor-α (TNF-α), the chimeric monoclonal IgG1 antibody infliximab, and the recombinant 75-kD TNF receptor IgG1 fusion protein etanercept, have substantially changed the AS treatment landscape in high-income countriesCitation5,Citation6, because of their superior treatment effects over conventional AS treatments, such as non-steroidal anti-inflammatory drugs (NSAID) and immunosuppressantCitation7,Citation8. Biologics have been used for AS in China for over 10 years. However, the impact of biologic agents on the treatment pattern and disease burden in Chinese patients with AS hasn’t been well studied due to limited data availabilities and access. Since 2013, the China Health Insurance Research Association (CHIRA) started to use a longitudinal approach to create a national database consisting of claims records of randomly selected urban patients across China. Thus, this database enables studies describing the treatment pattern, estimating the prevalence of disease-related complications, and measuring direct medical costs associated with Chinese patients living in urban areas.
Patients and methods
The 2013 CHIRA claims database randomly sampled 6.25 million claimants of urban workers and residents public insurance plans in 25 provinces and four central cities of ChinaCitation9. As of 2013, the urban workers and residents insurance plans covered over 90% of the urban population in ChinaCitation10. Because the 2013 CHIRA claims database only contained the 1-year claims records associated with selected claimants in 2013, our study was designed as a cross-sectional study using the 2013 CHIRA claims database to describe treatments for AS, AS-related complications, and direct medical costs in Chinese urban patients with AS in a 1-year period from January 1, 2013 to December 31, 2013.
Data sources
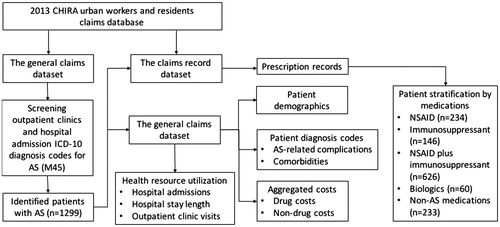
The 2013 CHIRA database consisted of two datasets, the general dataset and the claims record dataset. The general dataset contained patient demographics, insurance plan, hospital admissions, the Tenth revision of the international classification of disease (ICD-10) diagnosis codes associated with hospital admissions and outpatient clinic visits. The general dataset also included aggregated direct medical costs classified as drug costs and non-drug costs. The claims record dataset contained each claims record associated with in-patient care and out-patient care. Each claims record contained health resource name, transaction date, health resource unit cost, and purchased health resource units. The two datasets can be linked through the unique patient identification number.
Patient identification and data extraction
The ICD-10 diagnose code for AS (M45) was used as the keyword to search AS patients in the general dataset. The extracted information associated with identified patients with AS included patient demographics, insurance plans, patient residence city, hospital admissions, diagnosis codes, aggregated drug costs, and aggregated non-drug costs. Even though the current Chinese public health insurance plans, including urban workers and residents plans, don’t make full reimbursement of utilized health resources, the utilized health resources were recorded in the insurance claims database, irrespective of their reimbursement status. The identified patients were further linked with the claims record dataset to extract their prescribed medications. The process of patient identification and data extraction from the 2013 CHIRA claims database is illustrated in .
Outcome measures
Our study collected the following outcomes. First, the identified diagnosis codes associated with hospitalizations and outpatient clinic visits were used to assess the AS-related complications, which included iridocyclitis (ICD-10 code: H20), coxarthropathy (ICD-10 code: M12.05), lung diseases including sarcoidosis (ICD-10 code: D86) and bronchiectasis (ICD-10 code: J47), and chronic renal disease (ICD-10 code: N18)Citation11. Second, we classified the prescribed medications as medications without indication for AS (non-AS treatment), non-steroidal anti-inflammatory drugs (NSAIDs), immunosuppressant, and biologics according to AS treatment guidelinesCitation4. Third, the health resources utilization were aggregated to calculate the number of hospital admissions, the length of hospital stay, and the number of outpatient clinic visits in 2013. Finally, the direct medical costs associated with the included patients with AS were aggregated as the sum of drug costs and non-drug costs, respectively. All costs in this study were presented with 2013 Chinese currency Renminbi (RMB), which could be exchanged for US dollar at the rate of 1:0.16.
Statistical data analysis
The treatment pattern was described using the distribution of the medications that were classified as non-AS treatment, NSAID, immunosuppressant, NSAID plus immunosuppressant, and biologics. The classified medications were further used to stratify the included patients for unadjusted comparisons of patient baseline characteristics, the prevalence of AS-related complications, health resources utilization, and direct medical costs. The statistical methods used for the unadjusted comparisons between non-AS treatment group and the other four AS treatment groups included Student t-test for continuous variables/outcomes, Fisher’s exact test for dichotomous variables/outcomes, Poisson distribution test for count outcomes including hospital admissions and out-patient visits, and Wilcoxon rank sum test for cost outcomes. Multiple logistic regression analysis and generalized linear regression analyses with adjustment for patient baseline characteristics and the classified treatments were conducted to confirm the statistical significance detected in the unadjusted comparisons. The statistical significance defined in this study was two-sided p-value less than 0.05. SAS 9.2 was used to perform the statistical data analyses.
Results
The search of the general dataset of the 2013 CHIRA database identified 1,299 patients with AS diagnosis code. The prescribed medications in these identified patients included NSAID plus immunosuppressant (48.2%), NSAID (18.0%), non-AS treatment (17.9%), immunosuppressant (11.2%), and biologics (4.6%).
Treatment pattern and patient baseline characteristics
Among the prescribed medications in the identified 1,299 AS patients, diclofenac was the most frequently used NSAID (29.1%), salazosulfapyridine was the most commonly used immunosuppressant (30.2%), and the recombinant human tumor necrosis factor-Fc (Etanercept) was the most often used biologic agent (3.6%). When compared to the non-AS treatment group (patients receiving medications without indication for AS), the NSAID group was associated with significantly higher proportions of patients living in the east of China (73.1% vs 64.4%, p = .046), patients visiting tier III hospitals (86.8% vs 72.5%, p < .001), and patients with comorbidities for sacroiliac disease (18.4% vs 7.3%, p < .001), lung diseases (18.4% vs 6.0%, p < .001), hypertension (14.5% vs 3.4%, p < .001), gastritis (12.4% vs 5.6%, p = .014), osteoporosis (6.0% vs 1.7%, p = .028), and osteoarthritis (8.1% vs 0.4%, p < .001). The immunosuppressant group and the non-AS treatment group had highly comparable patient baseline characteristics except the prevalence of osteoarthritis (4.8% vs 0.4%, p = .006). The NSAID plus immunosuppressant group and the NSAID group had similar patient baseline characteristics. Finally, the biologic group differed from the non-AS treatment group by including more patients living in central cities (38.3% vs 17.2%, p = .001), more patients visiting higher rank tertiary hospitals (visiting tier III hospitals: 95.0% vs 72.5%, p < .001), and more patients with rheumatoid arthritis (15.0% vs 0.4%, p < .001). The patient baseline characteristics associated with the five treatment groups are summarized in .
Table 1. Comparisons of patient baseline characteristics associated with the identified patients with AS between the non-AS treatment group and the other four AS-related treatment groups.
AS-related complications
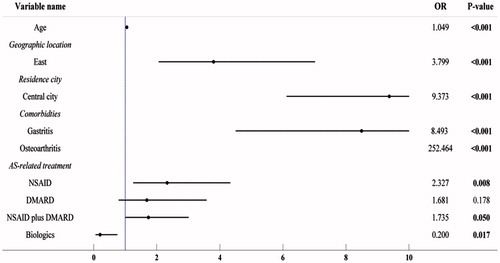
Of the stratified five treatment groups, the biologic group was associated with the lowest proportion prevalence of any AS-related complications (8.3%). Further adjustment of collected patient baseline characteristics through multiple logistic regression analysis still observed statistical significance for the lowest odds ratio (OR) (0.200, p = .017) associated with biologics for any AS-related complications among the five classified treatments. Additionally, the multiple logistic regression analysis observed significantly higher risk of any AS-related complications associated with NSAID (OR = 2.327, p = .008), NSAID plus immunosuppressant (OR = 1.735, p = .05), age (OR = 1.049, p < .001), patients living in the east of China (OR = 3.799, p < .001) and central cities (OR = 9.373, p < .001), and the comorbidities with gastritis (OR = 8.493, p < .001) and osteoarthritis (OR = 252.464, p < .001) ().
Health resources utilization and direct medical costs
When using the non-AS treatment group as the reference, NSAID, NSAID plus immunosuppressant, and biologic treatment groups were associated with significantly more utilization of health resources. For instance, significantly more outpatient clinic visits were observed in the NSAID group (19.8 visits vs 8.0 visits, p < .001) and NSAID plus immunosuppressant group (20.5 visits vs 8.0 visits, p < .001). Significantly more hospital admissions and longer length of hospital stay were observed in the NSAID plus immunosuppressant group (hospital admissions: 1.1 vs 0.6, p < .001; hospital stay length: 13.9 days vs 8.3 days, p < .001) and the biologic group (hospital admissions: 2.7 vs 0.6, p < .001; hospital stay length: 21.5 days vs 8.3 days, p < .001), respectively. The direct medical costs of five treatment groups ranged from RMB2,702 for the non-AS treatment group to RMB 14,539 for the biologic treatment group (). Multiple generalized linear regression analyses with full adjustment of collected patient baseline characteristics confirmed that the association between biologics and direct medical costs was ranked as the highest among the five treatments (coefficient = 1.644, p < .001) ().
Table 2. Health resources utilization and direct medical costs associated with the defined treatment groups from the identified patients with AS.
Table 3. The summary of the results from the multiple generalized linear regression analyses assessing the impact of AS-related treatments on direct medical costs in the identified 1299 patients with AS.
Discussion
The 2013 CHIRA database includes the reimbursement records of over 6 million urban workers and residents across China and enables us to identify a relatively large cohort of AS patients. The treatment pattern of AS suggested that the introduction of biologic agents for AS had little impact on the AS treatment landscapes in China and the current mainstream therapy for AS is the combination of NSAID and immunosuppressant, which was also reported by a treatment pattern survey study for rheumatoid arthritis in ChinaCitation12. Our study found the lowest prevalence of AS-related complications in patients receiving biologics. Even though the impact of biologics on the clinical symptoms and disease activity of AS has been well established, the long-term clinical benefits, such as the reduction of complications, are seldom reported because of slow disease progression. Thus, this study finding could be used as preliminary evidence encouraging future studies to confirm the potential long-term clinical benefits associated with biologics for AS. As expected, biologic treatment associated with much higher drug costs than conventional treatment because of high acquisition costs. However, the higher non-drug costs and more hospital admissions related to biologics were inconsistent with the observed impact of biologics on health resources utilization associated with AS in high-income countriesCitation13,Citation14.
The treatment pattern for AS in our study cohort indicated a significant barrier for patient access to biologic agents in China. Except the barrier associated with higher acquisition costs of biologics, there could be some other factors discouraging the utilization of biologics for AS in China. First, AS is a chronic disease that could last for decades without a noticeable short-term survival impact on patients. The medical needs of AS patients are not well recognized and the new treatments for AS are unlikely to be taken into account as the reimbursement priority by the Chinese public payers. Second, there’s insufficient robust local evidence confirming the real world effectiveness of biologics. Third, the typical management settings for AS are community hospitals, where the knowledge translation for new treatment usually takes a much longer timeCitation15. Thus, patients in our study received biologics treatment mainly through Tier III hospitals, the highest rank hospitals in China. Finally, the hospital-based reimbursement policy in China could be another significant barrier to the access to biologics treatment, as patients might reduce their willingness to use biologics because of the time-consuming process of hospitalizationCitation16.
Our study observed a significantly higher number of hospital admissions and longer length of hospital stay in patients receiving biologic treatment, which was against our hypothesis that biologics could reduce hospitalizations by improving clinical symptoms and reducing disease activity. According to the discussion with the clinical rheumatologists about this finding, patients usually receive biologic treatment in hospital settings under the current reimbursement policy which only covers hospital drugs. The present hospital-based reimbursement policy could increase hospitalizations in patients receiving biologics treatment and offset the potential cost savings associated with treatment effects of biologic therapies. Thus, our study could be a case study to demonstrate the confounding effects related to reimbursement policy for health resources utilization. The confounding effects associated with the reimbursement policy in patients receiving biologics are difficult to be adjusted in our study.
The observed lowest prevalence of AS-related complications in patients receiving biologic treatment should not be over-interpreted due to the limitations associated with our data source, which doesn’t include sufficient information to verify the identified AS-related complications and the onset of biologic treatments. However, the cumulated clinical evidence in other studies suggest that biologic treatments could improve long-term health outcomes in patients with AS. For example, one study compared the radiographic progression of AS patients treated with infliximab vs historical controls never treated with biologics over 8 years and observed a significantly lower number of new syndesmophytes in patients receiving infliximab (1.0 ± 0.6 vs 2.7 ± 0.8, p = .007)Citation17. Another registry study following up 1,436 patients receiving biologics TNF-α inhibitor observed better survival in those patients without treatment switchingCitation18. Because the lack of response was the primary reason for treatment switching, the sustainable response to biologics could reduce the risk of AS-related complications that have a substantial impact on patient survival. Thus, our finding of the potential association between biologics and reduced risk of AS-related complications could be used as preliminary evidence encouraging future studies to confirm the long-term clinical benefits associated with biologics for AS in Chinese patients and generate more robust evidence to support the use of biologics for AS.
As the only national representative database for urban workers and residents, the 2013 CHIRA database identified a considerable sample size to give a reliable description of treatment pattern, health resources utilization, and direct medical costs for AS in China. However, cautions to the primary limitations associated with the database are still needed when interpreting our study findings. First, this database collected very minimum clinical information related to hospitalization. The collected information from this data source was unable to track previous treatment history, estimate the actual duration of biologic therapies, and assess disease activity. Thus, the adjustments in our regression analyses are highly limited, and the unknown confounding effects could substantially bias our study results. Second, the observation time in this data source was only 1 year, which was unlikely to be long enough to observe the impact of AS-related treatments on long-term health outcomes and health resources utilization. Third, the 2013 CHIRA database didn’t collect utilization information of medications that were purchased outside hospital settings. This missing information could under-estimate direct medical costs and bias the comparisons of health resources utilization and direct medical costs in our study. Because high cost medications are often paid from patient out-of-pocket costs that couldn’t be fully captured by the claims databaseCitation19, the utilization of biologics in our study was likely to be under-estimated. Finally, the database doesn’t include rural patients. Thus, the evidence generated from this study has limited generalizability when used to support reimbursement decision-making across China.
Our study suggested that the use of biologics for AS in Chinese patients is uncommon. The study showed the lowest prevalence of AS-related complications associated with biologics treatment, suggesting possible long-term clinical benefits related to biologic treatments. Future studies are needed to confirm the reduced AS-related complications in patients receiving biologic therapies. The current hospital-based reimbursement policy in China could confound hospital admissions and length of stay in patients receiving biologic treatments and offset the potential cost savings associated with clinical benefits of biologics treatment.
Transparency
Declaration of funding
This manuscript was funded by Xian Janssen.
Declaration of financial/other relationships
QL and SG are employees of Beijing North Medical & Health Economics Research Center, a research organization receiving industry funds/grants for claims data analysis. YC and WC are the employees of Normin Health, a consulting firm receiving industry funds for health economics and outcomes research. YY is an employee of Xian Janssen. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Zeng SY, Gong Y, Zhang YP, et al. Changes in the prevalence of rheumatic diseases in Shantou, China, in the past three decades: a COPCORD Study. PLoS One 2015;10:e0138492
- Jiménez-Balderas FJ, Mintz G. Ankylosing spondylitis: clinical course in women and men. J Rheumatol 1993;20:2069-72
- Boonen A, van der Linden S M. The burden of ankylosing spondylitis. J Rheumatol 2006;78:4-11
- Ward MM. Update on the American College of Rheumatology/Spondyloarthritis Research and Treatment Network/Spondylitis Association of America axial spondyloarhtritis treatment guidelines project. Clin Rheumatol 2014;33:739-40
- Huscher D, Rudwaleit M, Thiele K, et al. SAT0447 Changes in treatment and outcomes of ankylosing spondylitis in routine care in germany 2000 to 2010. Ann Rheum Dis 2013;71(3 Suppl):623
- Howe A, Eyck LT, Dufour R, et al. Treatment patterns and annual drug costs of biologic therapies across indications from the Humana commercial database. J Manage Care Pharm 2014;20:1236-44
- van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582-91
- Gorman JD, Sack KE, Davis JC, Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med 2002;346:1349-56
- Yang Y, Du F, Ye W, et al. Inpatient cost of treating osteoporotic fractures in mainland China: a descriptive analysis. ClinicoEcon Outcomes Res: CEOR 2015;7:205
- Eggleston K. Health Care for 1.3 Billion: An Overview of China?s Health System (January 9, 2012). Stanford Asia Health Policy Program Working Paper No. 28
- Braun JV, Van den Berg R, Baraliakos X, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 2011;70:896-904
- Wang XR, Su Y, An Y, et al. [Survey of tumor necrosis factor inhibitors application in patients with rheumatoid arthritis in China]. J Peking Uni Health Sci 2012;44:182-7
- Kvamme MK, Lie E, Kvien TK, et al. Two-year direct and indirect costs for patients with inflammatory rheumatic joint diseases: data from real-life follow-up of patients in the NOR-DMARD registry. Rheumatology 2012;51:1618-27
- Ward MM. Functional disability predicts total costs in patients with ankylosing spondylitis. Arthritis Rheum 2002;46:223-31
- Zeng ZC, Zhao D, Wang WH, et al. The routes of obtaining medical knowledge and resolving clinical issues in Beijing community physicians. Cardio Pulm Vasc Dis J (Chinese) 2004;23:193-5
- Pan YH, Song J. Risk factors affecting treatment compliance in patients with ankylosing spondylitis. Nurs Care J (Chinese) 2012;2:103-4
- Baraliakos X, Haibel H, Listing J, et al. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis 2014;73:710-5
- Glintborg B, Østergaard M, Krogh NS, et al. Clinical response, drug survival and predictors thereof in 432 ankylosing spondylitis patients after switching tumour necrosis factor α inhibitor therapy: results from the Danish nationwide DANBIO registry. Ann Rheum Dis 2013;72:1149-55
- Chen Y, Schweitzer SO. Issues in drug pricing, reimbursement, and access in China with references to other Asia‐Pacific Region. Value Health 2008;11(s1):S124-S9