Abstract
Objective: Enzalutamide (ENZA) and abiraterone acetate plus prednisone (AA) are approved second-generation hormone therapies for chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC). This study compared ENZA with AA in chemotherapy-naïve mCRPC by calculating the number needed to treat (NNT) and associated incremental costs to achieve one additional chemotherapy-naïve patient with mCRPC free of radiographic progression, chemotherapy, or death over a 1-year time horizon.
Methods: Clinical outcomes were obtained from the PREVAIL and COU-AA-302 trials. Three outcomes were evaluated: radiographic progression-free survival, time to cytotoxic chemotherapy initiation, and overall survival at 1 year. NNT was calculated as the reciprocal of the outcome event rate difference for ENZA compared with AA. The incremental costs to achieve one additional outcome at 1 year were calculated as the difference in cost per treated patient multiplied by the NNT. Per-treated-patient costs were considered from a US payer perspective and included medications, monitoring, adverse events, post-progression treatments, and end-of-life care.
Results: Within a 1-year time horizon, the total cost per treated patient for ENZA was $2,666 less than AA. Compared with AA, treating 14 patients with ENZA resulted in one additional patient free of progression or death over 1 year; treating 26 patients with ENZA resulted in one additional patient with chemotherapy delayed over 1 year; and treating 91 patients with ENZA resulted in one additional patient free of death over 1 year. Therefore, ENZA is cost-effective compared with AA for all three outcomes evaluated, and the modeled results suggest ENZA is associated with potentially improved clinical outcomes in delaying chemotherapy initiation and disease progression for chemotherapy-naïve patients. The results are robust in sensitivity analyses, where the effect of changes in key model inputs and assumptions were tested.
Conclusion: The results modeled in the present study suggest ENZA is cost-effective compared with AA for treating chemotherapy-naïve patients with mCRPC.
Introduction
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related death among men in the USCitation1. Castration-resistant prostate cancer (CRPC) is an advanced form of prostate cancer characterized by disease progression following surgical or pharmacological (e.g. androgen deprivation) castration, a treatment modality aimed at suppressing androgen production that contributes to stimulating growth of prostate cancer cells. CRPC is estimated to account for 10–20% of prostate cancer cases, with over 70% of these cases defined as metastatic CRPC (mCRPC)Citation2. The prognosis for patients with mCRPC is generally poor, although the introduction of several novel oral oncolytic agents in recent years has increased the overall survival of these patientsCitation2–4.
Among therapies that have recently gained regulatory approval for the treatment of chemotherapy-naïve mCRPC, enzalutamide (ENZA) and abiraterone acetate plus prednisone (AA) are second-generation hormonal agents that received Category 1 recommendation in the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of mCRPCCitation5. The median survival was 35.3 months with ENZACitation6 and 34.7 months with AACitation7 in their respective pivotal clinical trials.
There are currently no published head-to-head clinical trials comparing ENZA with AA for the treatment of chemotherapy-naïve patients with mCRPC. Therefore, indirect comparisons are useful to evaluate the relative efficacy and cost-effectiveness of the two oral oncolytic agents. The efficacy of ENZA and AA, observed in their respective randomized controlled trials with similar study populations, suggests that ENZA is associated with potentially improved clinical outcomes with respect to delaying chemotherapy initiation and delaying disease progression throughout the chemotherapy-naïve settingsCitation3,Citation4. One prior study compared the drug cost per median overall survival month of ENZA to AA in chemotherapy-naïve patients with mCRPC from a US payer perspectiveCitation8. Even though a sensitivity analysis was conducted to include monitoring costs, the study did not consider adverse event-related costs, costs associated with subsequent therapies or incremental costs of disease progression or death.
To evaluate the relative value of these two treatment options from a US third-party payer perspective, the current analysis was established to compare ENZA with AA indirectly with respect to cost-effectiveness by calculating the number needed to treat (NNT) and the associated incremental cost per outcome. The NNT methodology was introduced in 1988 as a way to quantify the clinical usefulness of different treatmentsCitation9. NNT is calculated as the inverse of the event rate difference between two treatment alternatives, with the outcome representing the number of patients who require treatment to prevent one additional event compared to treatment with an alternative therapy. From the NNT calculation, the associated incremental cost per additional outcome can be estimated and interpreted as the cost-effectiveness of alternative treatmentsCitation10–16. The use of NNT in health economic evaluations is demonstrated among clinicians and formulary decision-makers. Additionally, there are many literature-based examples of NNT and incremental cost-per-additional-outcome applications in oncologyCitation10–16; however, none have been published for mCRPC treatments to date.
The current study compared ENZA with AA using the NNT method with the following outcomes: (1) achieving one additional patient free of radiographic progression or death; (2) achieving one additional patient with chemotherapy delayed; and (3) achieving one additional patient with death avoided, with a 1-year time horizon. The corresponding associated incremental cost-effectiveness values (i.e. the incremental cost per additional outcome) among chemotherapy-naïve patients with mCRPC were also calculated from a US third-party payer perspective. The cost components considered in this study included medications, monitoring, adverse events, post-progression treatments, progression, and end-of-life care.
Methods
In the current study comparing ENZA with AA, NNT and incremental cost per additional patient with clinically meaningful outcome were estimated. The study outcomes were representative of an average patient with mCRPC treated with ENZA or AA and were evaluated over the 1-year time horizon following the initiation of ENZA or AA. A 1-year time horizon was selected after consideration of the annual formulary review process and budget time horizons of US payers.
Input data: efficacy in the base case
Data on the clinical efficacy of ENZA and AA were obtained from the PREVAIL and COU-AA-302 trials, respectively ()Citation3,Citation6,Citation7,Citation17. Both trials included asymptomatic or mildly symptomatic patients with mCRPC without prior chemotherapy. Patient age, Gleason score, pain level, and laboratory measures including alkaline phosphatase and lactate dehydrogenase at baseline were similar between the two trials. In the PREVAIL trial, 11.2% of patients had visceral disease at screening, whereas the COU-AA-302 trial excluded patients with visceral disease. Given most patient characteristics were comparable at baseline except for visceral disease, the efficacy outcomes were directly used. The difference in the proportion of visceral disease may lead to a conservative efficacy estimate for ENZA in the current study. The outcomes evaluated in the study included radiographic progression-free survival (rPFS), time to initiation of chemotherapy, and overall survival. These three outcomes were considered clinically meaningful in the current study and were measured in both the PREVAIL and COU-AA-302 trials; specifically, rPFS and overall survival were the co-primary end-points. In addition, time to initiation of chemotherapy was evaluated and considered a valuable outcome for patients and caregiversCitation18. The observed 1-year rates of rPFS, chemotherapy delay, and overall survival were obtained from the respective Kaplan-Meier curves using pixel analysis without adjustment.
Table 1. Input data for drug costs, monitoring, and efficacy.
Input data: cost and healthcare resource use in the base case
Unit costs of ENZA, AA, and prednisone were obtained from Red Book® Online (2016 wholesale acquisition cost [WAC], accessed on March 15, 2016; )Citation19. Dosing schedules of ENZA, AA, and prednisone were based on product labels in the USCitation6,Citation7. Furthermore, mean treatment duration within 1 year was estimated from the trial dataCitation6,Citation7, and assumptions for real-world compliance rates were obtained from the Astellas Clinformatics™ Data Mart database (OptumInsight, Eden Prairie, MN) by utilizing the medication possession ratio methodology. Concomitant use of prednisone is required as a labeled indication for patients using AA; similarly, the proportion of patients using ENZA with concomitant prednisone use was obtained from the product labelCitation6,Citation7.
Frequency of monitoring tests within 1 year after treatment initiation was based on each product label, and unit costs for monitoring tests were obtained from the Centers for Medicare & Medicaid Services ()Citation20,Citation21. Grade 3/4 adverse events reported in ≥1% of patients from the PREVAIL or COU-AA-302 trials and any events noted in warnings in the product labels were considered in the model ()Citation3,Citation6,Citation7,Citation22. The unit costs of treating each adverse event were obtained from the literatureCitation20,Citation23–30. The model also assumes that the adverse events, which may be etiologically related (e.g. increased alanine aminotransferase and increased aspartate aminotransferase, edema, and cardiac events/acute renal failure), are experienced by different patients, or at different times if incurred by the same patient.
Table 2. Input data for adverse events.
Upon disease progression, patients with mCRPC were assumed to incur one hospitalization and receive post-progression treatments. Unit costs for progression-related hospitalization were obtained from Healthcare Cost and Utilization Project data, based on admission costs of prostate cancer patients ()Citation24. Dosing schedules of post-progression treatments were obtained from product labels, and unit costs were obtained from Red Book® Online®Citation6,Citation7,Citation19,Citation31–33. The model assumed that only deceased patients within the study time horizon would incur end-of-life costs. Subsequently, to remain consistent with the model time horizon, only patients who progressed or were deceased within the first year following therapy initiation were considered to incur progression, end-of-life, and post-progression treatment costs. Aside from drug costs based on 2016 WAC values in US dollars, all other costs were inflated to 2015 US dollars using the medical component of the Consumer Price Index in the USCitation34.
Table 3. Input data for post-progression and end-of-life costs.
Calculation: costs
Total average costs included pre-progression, post-progression, and end-of-life costs. Pre-progression costs included drug costs, monitoring costs, and adverse-event-related costs, whereas post-progression costs included costs for post-progression treatments and hospitalizations. In addition, end-of-life care was comprised of costs for hospitalization and hospice service. For all cost components, only costs incurred within the 1-year period following the initiation of ENZA or AA were considered.
Drug costs for ENZA and AA within 1 year of therapy initiation were calculated, based on the dosing schedule, unit price, proportion of patients with concomitant prednisone use, compliance rate, and mean treatment duration within the year for each cohort. The mean treatment duration within 1 year was estimated using the area-under-the-curve method, based on the median treatment duration (17.5 months for ENZA and 13.8 months for AA) and a constant hazard rate of discontinuation assumptionCitation6,Citation7. Specifically, the probability of treatment discontinuation within a half-month cycle was derived for ENZA and AA, respectively, based on the median treatment durations and assuming a constant hazard rate for discontinuation. A time-to-discontinuation curve was constructed to model the probability of being on treatment for each therapy, and the mean treatment duration during the 1-year period after treatment initiation was estimated by the area-under-the-curve method based on the curve.
Monitoring cost within 1 year was estimated based on the monitoring schedule and unit costs. The AA product label specified that patients are monitored for blood pressure, serum potassium, symptoms of fluid retention, and hepatotoxicity at least once per monthCitation7. Therefore, patients incurred one physician visit, one test for basic metabolic panel, and one test for hepatic function panel per month. No specific monitoring schedule was required for ENZA, according to the product labelCitation6. Patients treated with ENZA were assumed to have the same frequency of physician visits as those treated with AA.
Adverse event-related cost within 1 year was calculated from event rates and associated unit costs. The 1-year adverse event rates were prorated from the reported rates in the trials to reflect gradual incidence of Grade 3/4 adverse events for chemotherapy-naïve patients with mCRPC in the PREVAIL trialCitation3,Citation6,Citation7,Citation22. Furthermore, the 1-year adverse event rates were calculated as the overall event rates observed in the PREVAIL trial and the COU-AA-302 trial divided by the corresponding overall median treatment duration and multiplied by 12 months.
Post-progression costs within 1 year were calculated as the sum of post-progression treatment cost and hospitalization cost related to progression. The model assumed that only patients with disease progression would receive post-progression treatments and progression-related hospitalization. Subsequent treatments reported in the ENZA and AA trials were considered (including sipuleucel-T, docetaxel, and cabazitaxel)Citation3,Citation22. Re-treatment with the initial product was not allowed in the model (e.g. patients initially treated with ENZA would not be re-treated with ENZA post-progression). Specifically, it was assumed that the proportion of patients re-treated with ENZA in the PREVAIL trial for ENZA would receive AA as the post-progression treatment in the real world, with ENZA for AA patients. The proportion of patients receiving each type of post-progression treatment was estimated based on the clinical trial reports. Sipuleucel-T was a one-time personalized therapy, and the full treatment course was considered among recipients. Costs of the other (recurring) post-progression treatments were calculated as the monthly drug cost multiplied by the eligible treatment duration. The eligible treatment duration was the difference of mean duration of overall survival and mean duration of rPFS within the 1-year period (i.e. the time patients were alive, but after progression during the 1-year period following initial treatment with ENZA or AA). Average post-progression treatment costs within 1 year were estimated as the weighted average cost of all post-progression treatments considered in the model.
End-of-life cost was calculated as the sum of end-of-life hospitalization cost and hospice cost; only deceased patients within 1 year incurred end-of-life costs. End-of-life hospitalization cost was calculated as the unit cost of hospitalization multiplied by the proportion of deceased patients. Additionally, hospice care cost was calculated as the proportion of deceased patients multiplied by the proportion of patients receiving hospice before death and multiplied by the average length of stay and daily cost.
Calculation: NNT and incremental cost per additional patient outcome
The NNT and cost per additional patient with clinically meaningful outcome comparing ENZA with AA were calculated for each outcome, respectively (). NNT was calculated as the reciprocal of the event rate difference of clinically meaningful outcomes between ENZA and AA from published clinical trials of the same population. An incremental cost per additional patient with clinically meaningful outcome was calculated as the difference in total costs (ENZA vs AA) multiplied by the corresponding NNT. In situations where the difference in total cost is negative and the NNT is positive, the incremental cost per additional patient with clinically meaningful outcome was denoted as “dominant” to comply with the standard reporting approach for cost-effectiveness analysesCitation35.
Sensitivity analysis
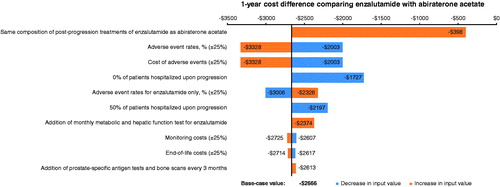
One-way sensitivity analyses were conducted to examine the effect of change in one key model input or assumption on model results, while others are held at the base-case value. The following was tested in the one-way sensitivity analyses: (1) varying the proportion of patients hospitalized upon disease progression from 100% in the base case to 50% and 0%, (2) changing post-progression treatment composition from values observed in the respective clinical trial in the base case to be the same between ENZA and AA, (3) varying rates of adverse events by ±25% of base-case values for both arms and by ±25% for ENZA only, (4) varying costs of adverse events by ±25% of base-case values, (5) varying monitoring costs by ±25% of base-case values, (6) adding monthly metabolic panel and hepatic function tests for ENZA, (7) adding prostate-specific antigen tests and bone scans every 3 months, and (8) varying end-of-life costs by ±25% of base-case values. The real-world concomitant use of corticosteroid with ENZA may also be different from the proportion observed in the clinical trial. However, due to the low cost of corticosteroid therapies, the impact on study results was expected to be minimal and, therefore, was not considered in the sensitivity analysis.
Results
Base-case results
Within a 1-year time horizon, the total estimated cost per treated mCRPC patient was $94,440 for ENZA and $97,105 for AA (). As a net impact, the 1-year total cost was $2666 lower for an average patient treated with ENZA compared with AA. While the acquisition costs for ENZA were considered higher than those for AA (difference of $6880, which is partly due to longer treatment duration), ENZA was associated with lower costs for monitoring (difference of −$236), adverse events (difference of −$2650), post-progression treatments (difference of −$6465), and end-of-life care (difference of −$194).
Table 4. Total cost per treated patient within a 1-year time horizon.
The NNT for rPFS was 14 when comparing ENZA with AA; thus, treating 14 mCRPC patients with ENZA resulted in one additional patient with rPFS at 1 year vs treating with AA. Additionally, the NNT for chemotherapy delayed was 26 when comparing ENZA with AA; thus, treating 26 mCRPC patients with ENZA resulted in one additional patient with chemotherapy delayed at 1 year vs treating with AA. Lastly, the NNT for overall survival was 91 when comparing ENZA with AA; thus, treating 91 mCRPC patients with ENZA resulted in one additional patient alive at 1 year vs treating with AA (). ENZA was cost-effective compared to AA for all three outcomes; specifically, the modeled results suggest ENZA was associated with lower costs and favorable clinical outcomes (). Treatment of chemotherapy-naïve patients with mCRPC with ENZA vs AA would result in more mCRPC patients with potentially improved outcomes at a lower overall cost.
Table 5. Clinical outcomes and associated costs comparing ENZA with AA within a 1-year time horizon.
One-way sensitivity analysis results
In the one-way sensitivity analyses (), ENZA is consistently associated with cost savings compared with AA under all tested scenarios. The cost difference between ENZA and AA was most sensitive to post-progression treatment composition—when ENZA has the same treatment composition as AA, the cost savings were reduced to $398 from $2666 in the base case. In other sensitivity analyses, the cost savings comparing ENZA to AA ranged from $1727–$3328. In terms of the incremental cost per additional patients with outcome, ENZA was cost-effective compared with AA in all sensitivity analyses (specifically, potential for improved outcomes at a lower overall cost).
Discussion
This study used clinical trial data supplemented with references in the literature to calculate NNT (and associated incremental costs) comparing two novel oral oncolytic agents (ENZA and AA) for treating chemotherapy-naïve patients with mCRPC. The results suggest ENZA compared with AA can potentially prolong survival, decrease the risk of radiographic progression, and delay chemotherapy in chemotherapy-naïve patients with mCRPC. Results from the incremental costs per additional patient with clinically meaningful outcome analysis conclude that the clinical benefits of ENZA were achieved at a lower cost compared with AA within a 1-year time horizon from a US payer perspective. Extensive sensitivity analyses have been conducted for the key inputs and assumptions used in the model, including post-progression treatment, adverse event rates and costs, disease monitoring, and end-of-life costs. The study results are robust in all sensitivity analyses.
NNT and incremental cost per additional patient with clinically meaningful outcome (overall survival, rPFS, and chemotherapy delayed) were selected as the evaluation approach in the current study because these outcomes are easily interpretable and relevant to payer decision-making. This approach allows the comparison of alternative treatments with respect to efficacy and costs. To the knowledge of the authors, no publication has applied this approach for evaluating treatments in mCRPC, although this approach has been used for evaluating other oncology treatmentsCitation11,Citation14–16. For example, a published meta-analysis concluded that adding long-term hormone therapy resulted in an NNT of 14 to achieve one additional locally advanced prostate cancer patient with clinical progression-free survival compared with radiotherapy aloneCitation11. However, there is no published threshold for NNT, and determining whether an NNT value is clinically meaningful depends on the disease and patient expectations. In the current study, the NNT to achieve one additional rPFS for ENZA vs AA was 14, and the clinical benefit was achieved with cost savings. Similarly, when chemotherapy delayed or death avoided was evaluated, treating mCRPC patients with ENZA was associated with more patients experiencing potentially improved outcomes at a lower cost compared to treating patients with AA.
A previous study compared ENZA and AA for the treatment of chemotherapy-naïve mCRPC in the US utilizing a different methodology; it concluded the cost per median overall survival month was lower for AA than for ENZACitation8. However, the study focused on drug acquisition costs and incorporated 2015 WAC values, without considering costs of adverse events and costs of downstream events associated with different clinical outcomes such as post-progression treatments and end-of-life careCitation8. Similarly, another previous study used administrative claims data and demonstrated that AA was associated with lower monthly pharmacy costs, but similarly did not consider other costs associated with these treatmentsCitation36.
The current study was designed to compare ENZA and AA from a US third-party payer perspective and considered cost components beyond drug acquisition cost, specifically aligned with a 1-year budget decision cycle. Subsequently, although the estimated acquisition cost of ENZA was higher than AA, the clinical benefits of ENZA offset the higher acquisition cost. Additionally, treatment with AA requires monitoring of mineralocorticoid excess, adrenocortical insufficiency, and hepatotoxicity at least monthly; however, there is no requirement for regular monitoring with ENZA specified in the product label. The difference in toxicity profiles was also associated with a higher cost of adverse events for AA compared with ENZA.
Based on our assumptions, patients treated with ENZA experienced less disease progression and spent more time free of progression compared with those treated with AA; therefore, they were associated with lower post-progression treatment costs. In addition, the greater 1-year overall survival rate of patients treated with ENZA compared with those treated with AA resulted in lower end-of-life costs for ENZA. Lastly, the higher acquisition cost of ENZA in the model was partly due to the longer mean treatment duration of ENZA compared with AA (9.6 vs 9.0 months), which may be associated with improved clinical outcomes of ENZA (e.g. longer treatment duration and less discontinuation). When evaluating treatment options, it is important to consider aspects beyond the drug acquisition cost and examine the complete measure of overall costs in addition to the clinical benefits.
There are known limitations of the current study. First, the model used results reported in two randomized clinical trials to estimate the safety and efficacy profiles of ENZA and AA. Safety and efficacy outcomes from clinical trials may not be representative of the experience of the general patient population; therefore, the generalizability to real-world clinical practice may be limited. In addition, the current study did not test uncertainties associated with the efficacy of ENZA and AA. Future US real-world studies are needed to further differentiate these two treatment options in terms of benefits, risks, and associated costs. Second, the current economic model assumed that patients would have one hospitalization upon disease progression and would receive one of the following post-progression treatments: sipuleucel-T, docetaxel, ENZA, AA, or cabazitaxel, and the proportions of patients receiving each treatment are the same as reported in the respective clinical trials. Patients with mCRPC in the real world may receive treatments that are not considered in this study (e.g. radium-233) or may have different probabilities of receiving each treatment from those observed in the respective clinical trials, or even receive multiple sequential treatments, after progression. It is also possible that some patients with mCRPC will not be hospitalized upon disease progression. To evaluate the impact of these assumptions and inputs, different probabilities of hospitalization after progression and different composition of post-progression treatments were tested in these sensitivity analyses, and the results are robust. Third, while the time horizon is 1 year in the current study, some patients would incur additional costs beyond 1 year. Future studies would be beneficial to evaluate costs incurred by these patients beyond the time horizon evaluated in this study.
In conclusion, the current study suggests that ENZA is cost-effective compared with AA for treating chemotherapy-naïve patients with mCRPC. ENZA, compared with AA, potentially decreases the risk of radiographic progression and death and delays chemotherapy in chemotherapy-naïve patients with mCRPC based on results from randomized controlled trials with similar study populations. Additionally, ENZA is associated with a lower cost, compared with AA, due to reduced post-progression, end-of-life, monitoring, and adverse event costs.
Transparency
Declaration of funding
This research was sponsored by Astellas Pharma, Inc. and Medivation, Inc., the co-developers of enzalutamide.
Declaration of financial/other relationships
MM, CNB, BJP, BB, and SF are employees of Astellas. HY, JG, YS, and EW have served as consultants to Astellas. AB is an employee of Medivation. MB was an employee of Medivation at the time this work was initiated. Astellas Pharma, Inc. and Medivation, Inc. participated in the interpretation of data and the review and approval of the publication. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank Jing Zhao from Analysis Group for a significant contribution toward analytical support and Ana Bozas from Analysis Group for medical writing assistance. Editorial assistance, funded by both sponsor companies, was provided by Charlene Rivera, PhD, Stephanie Rippon, and Lauren Smith from Complete HealthVizion.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29
- Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011;65:1180-92
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48
- National Comprehensive Cancer Network, Pennsylvania, USA 2014. NCCN Guideline Prostate Cancer. 2014. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed August 10, 2016
- Food and Drug Administration Maryland, USA, 2016. Xtandi [package insert]. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/203415s007lbl.pdf. Accessed October 21, 2015
- Food and Drug Administration, Maryland, USA 2016. Zytiga [package insert]. 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/202379s016lbl.pdf. Accessed December 10, 2015
- Pilon D, Queener M, Lefebvre P, et al. Cost per median overall survival month associated with abiraterone acetate and enzalutamide for treatment of patients with metastatic castration-resistant prostate cancer. J Med Econ 2016; 19:777-84
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988;318:1728-33
- Belozeroff V, Lee A, Tseng S, et al. Cost per responder analysis in patients with secondary hyperparathyroidism on dialysis treated with cinacalcet. J Med Econ 2013;16:1154-62
- Bria E, Cuppone F, Giannarelli D, et al. Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer?: meta-analysis of randomized trials. Cancer 2009;115:3446-56
- Liu Y, Wu EQ, Bensimon AG, et al. Cost per responder associated with biologic therapies for Crohn's disease, psoriasis, and rheumatoid arthritis. Adv Ther 2012;29:620-34
- Martin S, Feldman SR, Augustin M, et al. Cost per responder analysis of ustekinumab and etanercept for moderate to severe plaque psoriasis. J Dermatolog Treat 2011;22:138-43
- Schmitz-Dräger BJ, Weiss C, Ebert T, et al. Skeletal-related events in metastatic prostate cancer and the number needed to treat: a critical consideration. Urol Int 2013;90:329-33
- Wu EQ, Xie J, Signorovitch J, et al. Number needed to treat and treatment cost per fracture avoided with denosumab compared with zoledronic acid in patients with breast cancer with bone metastases. J Clin Oncol 2011;29:abstr 151
- Xie J, Diener M, Wu E, et al. Number needed to treat and risk benefit analysis of denosumab versus zoledronic acid in the treatment of castrate-resistant prostate cancer patients with bone metastasis. J Urol 2012;187:e74
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66:815-25
- National Institute for Health and Care Excellence, London, UK. NICE publishes draft guidance on two prostate cancer treatments. 2015. www.nice.org.uk/news/press-and-media/nice-publishes-draft-guidance-on-two-prostate-cancer-treatments. Accessed January 5, 2016
- Truven Health Analytics, London, UK. Red Book Online. 2016. http://www.redbook.com/redbook/. Accessed March 15, 2016
- Centers for Medicare and Medicaid Services, Maryland, USA. Medicare Physician Fee Schedule. 2015. http://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed January 4, 2016
- Centers for Medicare and Medicaid Services, Maryland, USA. Clinical diagnostic laboratory fee schedule for Medicare. 2015. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/clinlab.html. Accessed January 4, 2016
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152-60
- Adigopula S, Babu V, Parperis KM, et al. Hyperglycemia in heart failure patients is associated with increased length of stay and costs. Circulation 2009;120:S548
- Agency for Healthcare Research and Quality, Maryland, USA. HCUP Nationwide Inpatient Sample. 2013. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed January 4, 2016
- Borker R. Costs associated with adverse events in patients with metastatic renal cell carcinoma. J Med Econ 2014;17:792-97
- Fu az, Zhao Z, Wang S, et al. Hospital costs of adverse events in patients with metastatic colorectal cancer. J Cancer Ther 2013;4:153-8
- Hagiwara M, Borker R, Oster G. Economic burden of adverse events in patients with metastatic renal cell carcinoma. Clin Ther 2013;35:1955-63
- Ivanova JI, Birnbaum HG, Kidolezi Y, et al. Direct and indirect costs associated with epileptic partial onset seizures among the privately insured in the United States. Epilepsia 2010;51:838-44
- Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care 2008;14:317-22
- Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and co-morbidities of atrial fibrillation in a privately insured population. Curr Med Res Opin 2005;21:1693-9
- Food and Drug Administration, Maryland, USA. Provenge product label 2014. 2016. http://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM210031.pdf. Accessed May 14, 2015
- Food and Drug Administration, Maryland, USA. Docetaxel [package insert]. 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022234s03lbl.pdf. Accessed May 28, 2015
- Food and Drug Administration, Maryland, USA. Jevtana [package insert] 2014. 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/201023s011lbl.pdf. Accessed August 24, 2015
- United States Department of Labor, Washington DC, USA. Bureau of Labor Statistics: The Consumer Price Index. 2014. http://www.bls.gov/cpi/. Accessed January 5, 2016
- Muennig P, Bounthavong M. Cost-effectiveness analysis in health: a practical approach, 3rd edn. San Francisco, CA: Jossey-Bass, 2016
- Ellis LA, Lafeuille MH, Gozalo L, et al. Treatment sequences and pharmacy costs of 2 new therapies for metastatic castration-resistant prostate cancer. Am Health Drug Benefits 2015;8:185-95
- Stevens JA, Corso PS, Finkelstein EA, et al. The costs of fatal and non-fatal falls among older adults. Inj Prev 2006;12:290-5
- Schuetz P, Balk R, Briel M, et al. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med 2015;53:583-92
- Verbraecken J, Van de Heyning P, De Backer W, et al. Body surface area in normal-weight, overweight, and obese adults. A comparison study. Metabolism 2006;55:515-24
- Bergman J, Saigal CS, Lorenz KA, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med 2011;171:204-10
- Centers for Medicare and Medicaid Services, Maryland, USA. Health Care Information System (HCIS) Data File. HCIS Data Files for 2011. 2011. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/HealthCareInformationSystem.html. Accessed January 4, 2016