Abstract
Objective: To evaluate the cost-effectiveness of second-line nilotinib vs dasatinib among patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) who are resistant or intolerant to imatinib, from a US third-party perspective.
Methods: A lifetime partitioned survival model was developed to compare the costs and effectiveness of nilotinib vs dasatinib, which included four health states: CP on treatment, CP post-discontinuation, progressive disease (accelerated phase [AP] or blast crisis [BC]), and death. Time on treatment, progression-free survival, and overall survival of nilotinib and dasatinib were estimated using real-world comparative effectiveness data. Parametric survival models were used to extrapolate outcomes beyond the study period. Drug treatment costs, medical costs, and adverse event costs were obtained from the literature and publicly available databases. Utilities of health states were derived from the literature. Incremental cost-effectiveness ratios, including incremental cost per life-year (LY) gained and incremental cost per quality-adjusted life-year (QALY) gained, were estimated comparing nilotinib and dasatinib. Deterministic sensitivity analyses were performed by varying patient characteristics, cost, and utility inputs.
Results: Over a lifetime horizon, nilotinib-treated patients were associated with 11.7 LYs, 9.1 QALYs, and a total cost of $1,409,466, while dasatinib-treated patients were associated with 9.5 LYs, 7.3 QALYs, and a total cost of $1,422,122. In comparison with dasatinib, nilotinib was associated with better health outcomes (by 2.2 LYs and 1.9 QALYs) and lower total costs (by $12,655). Deterministic sensitivity analysis results showed consistent findings in most scenarios.
Limitations: In the absence of long-term real-world data, the lifetime projection could not be validated.
Conclusions: Compared with dasatinib, second-line nilotinib was associated with better life expectancy, better quality-of-life, and lower costs among patients with Ph+ CML-CP who were resistant or intolerant to imatinib.
Introduction
Chronic myeloid leukemia (CML) is a cancer initiating in hematopoietic stem cells and constitutes ∼15% of all adult leukemia cases in the USCitation1. Approximately 95% of patients with CML display a reciprocal translocation between chromosomes 9 and 22, known as the Philadelphia chromosome (Ph)Citation2–4. Currently, inhibition of BCR-ABL kinase activity with tyrosine kinase inhibitors (TKIs) is the mainstay of treatment for Ph positive (Ph+) CMLCitation5. Specifically, the first-generation TKI imatinib has long been a standard-of-care first-line therapy for patients with CML in chronic phase (CP)Citation6–9. When patients develop resistance or intolerance to imatinib, they are then switched to second-generation TKIs such as nilotinib or dasatinibCitation8.
Since their US Food and Drug Administration approvals in 2006, second-line treatments of nilotinib and dasatinib have been compared in several cost-effectiveness analyses (CEAs)Citation10–13. However, these studies included notable data limitations that could be overcome by real-world evidence. First, there was a lack of comparative effectiveness evidence for nilotinib and dasatinib, and these drugs’ respective trials recruited patients based on different criteriaCitation14,Citation15, making indirect comparisons difficult. Second, at the time when these models were constructed, comparative survival data were not yet available. Thus, varying surrogate markers have been used across studies to impute survival endpointsCitation10–13. Because there is no well-defined conversion algorithm mapping these surrogate markets to survival outcomes that can be used across these trials, the imputed survival outcomes may not represent the real survival endpoints.
Newly available real-world evidence on the direct comparative effectiveness between second-line treatment with nilotinib and dasatinibCitation16 can now help overcome the limitations of prior CEAsCitation10–13. Specifically, an online medical chart review by Griffin et al.Citation16 retrospectively collected time on treatment (TOT), progression-free survival (PFS), and overall survival (OS) information on CML-CP patients who switched from imatinib to nilotinib or dasatinib as second-line therapy from 122 US hematologists and oncologists. TKI treatment has established CML as a chronic disease with a high survival rateCitation8. Thus, it is important to re-evaluate the cost-effectiveness of second-line nilotinib vs dasatinib over a lifetime horizon based on this new direct comparative TOT, PFS, and OS dataCitation16. Leveraging the partitioned survival model structure and adopting similar health states as those used in the National Institute for Health and Care Excellence (NICE) health technology assessments published by Rogers et al.Citation10 and Loveman et al.Citation11, this CEA uses direct comparative effectiveness on TOT, PFS, and OS from Griffin et al.Citation16 to assess the economic value of nilotinib vs dasatinib as a second-line therapy over the lifetime of CML-CP patients, and may assist US third-party payers to better evaluate these therapies.
Methods
Model overview
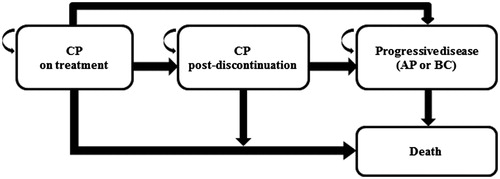
A partitioned survival modelCitation17,Citation18 was developed in Excel (Microsoft, v. 2010) to compare the cost-effectiveness of nilotinib vs dasatinib in Ph+ CML-CP patients who were resistant or intolerant to imatinib, with a starting age of 60 years, and comprised of 35% females. These parameters were selected to be consistent with the effectiveness input sourceCitation16. The model was constructed from a US third-party payer perspective with a lifetime time horizon. Thus, only direct costs, including drug costs, medical costs, and adverse events (AE) costs, were considered. The partitioned survival model consisted of four mutually exclusive heath states (): CP on treatment, CP post-discontinuation, progressive disease (accelerated phase [AP] or blast crisis [BC]), and death. To better estimate the cost and effectiveness of treatments, a half-cycle correction was applied to the modelCitation17,Citation19. Both costs and effectiveness were discounted at 3% annually.
Model assumptions
The model incorporated several key assumptions. First, a hypothetical cohort of Ph+ CML-CP patients who were resistant or intolerant to imatinib (per the drug labels of nilotinib or dasatinib) and received second-line nilotinib or dasatinib started with the CP on treatment state at the time of model entry. Patients with CML who were resistant or intolerant to imatinib were included in order to be consistent with the US FDA-approved indications of nilotinib and dasatinibCitation20,Citation21. Second, all transitions were unidirectional, i.e. patients could only transition into a subsequent health state (e.g. CP on treatment to CP post-discontinuation) but not in the other direction. Patients in CP on treatment, CP post-discontinuation, or progressive disease could all transition directly to death. Third, after the discontinuation of second-line treatment (i.e. CP post-discontinuation), or when entering the progressive disease state, patients were assumed to have received a mixture of commonly-used treatments monthly. Fourth, in addition to monthly treatment costs, the model assumed that, when transitioning to the progressive disease state, a proportion of patients would also incur one-time costs for chemotherapy and bone marrow transplantation. Fifth, medical costs incurred by patients were assumed to be dependent only on health states and independent of treatments, i.e. nilotinib- and dasatinib-treated patients would incur the same medical costs in CP on treatment, CP post-discontinuation, and progressive disease, respectively. Sixth, grade 3/4 AE costs were considered in the model, and costs were added as monthly costs in CP on treatment during the first 24 months, which was consistent with the timeframe of AE inputs from the respective clinical trials (see “Model Inputs” section below). Finally, health utilities were assumed to be dependent only on health states and independent of treatments. No disutilities (decrease in health utility values) due to AEs were considered in the model in order to avoid double-counting in health state utilities (see “Model Inputs” section below).
Model inputs
Effectiveness inputs
Effectiveness inputs, including TOT, PFS, and OS, were derived from the patient-level data of Griffin et al.Citation16, a US chart review study comparing nilotinib (n = 301) and dasatinib (n = 296) as second-line therapies (initiating treatment after October 28, 2007, when both drugs were approved) in Ph+ CML-CP patients who were resistant or intolerant to imatinib. Patients were included in that study if they met the following criteria: a diagnosis of Ph+ CML, at least 18 years of age at CML diagnosis, received imatinib as first-line therapy, switched to either dasatinib or nilotinib therapy after October 28, 2007 and at least 180 days prior to the data collection date, in CML-CP at the time of the switch, not diagnosed with other concurrent malignancies prior to the switch, and followed by the responding physician for at least 30 days after the switchCitation16. Among the Griffin et al.Citation16 patient cohort, the reasons for switching from imatinib to second-line therapy included primary or secondary imatinib resistance, AEs or intolerance of imatinib, and non-adherence to imatinib; more nilotinib patients experienced secondary resistance (p = .05) and more dasatinib patients experienced AEs or intolerance (p = .02)Citation16 (Supplemental Table 1). The median time from CML diagnosis at the index date was 15 months for nilotinib patients and 13 months for dasatinib patients, with a median follow-up time of 11 months and 10 months for nilotinib and dasatinib patients, respectively.
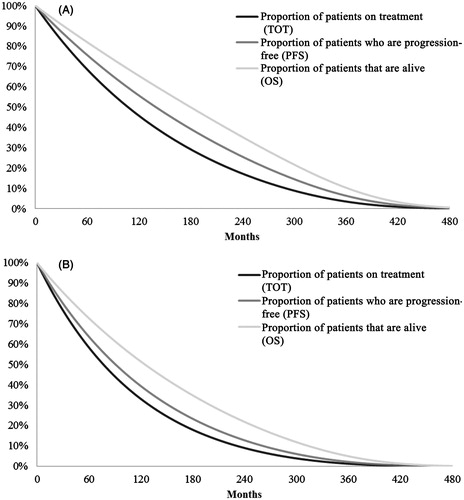
Although Griffin et al.Citation16 reported time to progression (TTP) data, PFS was used instead of TTP because the partitioned survival model intended to estimate the time on progression-free survival and post-progression survival. TTP, not accounting for pre-progression death, was not suitable for this model. The parameter estimates for TOT, PFS, and OS were obtained from the corresponding data using various parametric survival functions, i.e. exponential, log-logistic, log-normal, and Weibull distribution. The goodness-of-fit criteria including the Akaike information criterion (AIC)Citation22–24 and Bayesian information criterion (BIC)Citation23,Citation25,Citation26 were analyzed for each parametric function. The exponential function was selected as the base-case, because it was associated with the best AIC for TOT and OS, and the best BIC for TOT, PFS, and OS. Furthermore, the model incorporated the natural mortality rates from US life tables within the 2010 National Vital Statistics ReportCitation27 into the calculation of OS. The TOT, PFS, and OS projection for both nilotinib and dasatinib, factoring in natural mortality rates, is shown in , where all patients will be deceased at the end of the 480 months following second-line treatment initiation (i.e. when they are 100 years old).
Utility inputs
Utility (a measurement of preferences for specific health-related outcomes) inputs for CP on treatment and progressive disease, were obtained from Szabo et al.Citation28, which was a time trade-off preference survey conducted among patients with CML in the US, UK, Canada, and Australia, and US-specific inputs were adopted in the model. Since Szabo et al.Citation28 incorporated incremental disutilities (decrease in utility values) due to treatment-related toxicities into the estimation of utility, no disutilities due to AEs were further considered in the model. In the absence of information on utility specific to CP post-discontinuation, utility for CP post-discontinuation was assumed to be the same as that in CP on treatment, because patients in CP post-discontinuation were still in CP (i.e. not experiencing disease progression) and had switched to a third-line treatment ().
Table 1. Base-case model inputs.
Cost inputs
The model included the following types of direct healthcare cost inputs: CML-related drug costs, medical costs (including monitoring, resource utilization, and terminal care costs), and AE-related costs. All cost inputs were obtained from the literature or publicly available data (detailed below) and inflation-adjusted to 2015 USD using the medical component of the Consumer Price IndexCitation29 ().
CML-related drug costs
When estimating CML-related drug costs for CP on treatment, the model considered unit drug cost, dosing schedule, average starting dose, and adherence level. The unit cost of nilotinib and dasatinib was obtained from the 2015 Red Book OnlineCitation30 using the wholesale acquisition cost. The dosing schedules for nilotinib (400 mg twice daily) and dasatinib (100 mg/day) for CML-CP were obtained from their corresponding drug labelsCitation20,Citation21. The starting doses were based on Griffin et al.Citation16: of the dasatinib patients, 70.9% were prescribed 100 mg daily, and, of the nilotinib patients, 45.9% received 300 mg twice daily and 44.9% received 400 mg twice daily. Adherence was used as a proxy for relative dose intensity. The adherence levels for both nilotinib and dasatinib were assumed to be 100% in the base-case to be consistent with those in the previous CEAs of second-line nilotinib vs dasatinibCitation10,Citation11. In the absence of strong real-word evidence comparing the adherence to second-line nilotinib vs dasatinib, adherence levels were tested in the sensitivity analysis.
When estimating CML-related drug costs for CP post-discontinuation, the model assumed that patients who initiated nilotinib as second-line treatment could switch to dasatinib in CP post-discontinuation and vice versa, as well as to other therapies including bosutinib, ponatinib, interferon alpha, and omacetaxine, based on the National Comprehensive Cancer Network (NCCN) guidelinesCitation8. Treatment costs were calculated for each monthly cycle (hereinafter referred to as “monthly treatment”). A survey of 100 hematologists and oncologists in the US who had treated Ph+ CML patients with second-line nilotinib or dasatinib after first-line imatinib was conducted to estimate the proportion of patients receiving each treatment for the CP post-discontinuation stateCitation31. For these monthly treatments, the unit cost was obtained from the 2015 Red Book OnlineCitation30 and the dosing schedule was obtained from drug labels and previous CEAsCitation10,Citation11,Citation32–34.
When estimating CML-related drug costs for progressive disease, the model considered both monthly treatment costs and one-time treatment costs, where treatments were selected based on the NCCN guidelinesCitation8. However, the model assumed that patients would not receive nilotinib or dasatinib in the progressive disease state. Monthly treatments included bosutinib, ponatinib, interferon alpha, and omacetaxine. One-time treatments included chemotherapy and bone marrow transplant. The proportion of patients receiving each treatment for progressive disease was also obtained from the aforementioned physician surveyCitation16. For monthly treatments, the unit costs were obtained as described above. For one-time treatments, the unit cost of chemotherapy was obtained from a retrospective claims database analysis using the PharMetrics Plus and MarketScan databasesCitation31; the unit cost of bone marrow transplantation was obtained from Maziarz et al.Citation35,Citation36.
Medical costs
Medical costs consisted of monitoring costs, resource utilization costs, and terminal care costs. For monitoring costs, the types of monitoring tests used were based on the NCCN guidelinesCitation8. The unit costs of monitoring tests were obtained from the Centers for Medicare and Medicaid Services (CMS) Physician Fee Schedule (unit costs: polymerase chain reaction [PCR] test, $223; cytogenetic test, $543)Citation36. The frequencies of monitoring tests for CP on treatment were obtained from Goldberg et al.Citation37. In the absence of information on frequencies of monitoring tests specific to CP post-discontinuation or progressive disease, they were assumed to be the same as those for CP on treatment. For costs related to resource utilization, the types of resources used were based on Reed et al.Citation38. The unit costs of generalist and specialist visits were also obtained from the CMS Physician Fee ScheduleCitation36. Because the unit costs of nurse and inpatient visits were not available from the CMS Physician Fee Schedule, they were obtained from Reed et al.Citation38. The frequencies of resource utilization for CP on treatment and progressive disease were also based on Reed et al.Citation38. In the absence of information on frequencies of resource utilization specific to CP post-discontinuation, they were assumed to be the same as those for CP on treatment. Last, all patients who transitioned to death were assumed to incur terminal care costs in the last cycle before death. Terminal care cost information was obtained from Yabroff et al.Citation39.
AE costs
Grades 3/4 AEs were included in the model if they had a rate of ≥5% over a 24-month timeframe in either of the pivotal trials of second-line nilotinibCitation40 or dasatinibCitation15. The model first converted the 24-month rates to 1-month probabilities, and then applied the monthly AE costs up to 24 months in CP on treatment. Unit costs for neutropenia, thrombocytopenia, and anemia were obtained from Liou et al.Citation41, a systematic review on costs of these hematological AEs in cancer patients, while unit costs for leukopenia, lipase elevation, hyperglycemia, hypophosphatemia, and elevated bilirubin (total) were obtained from the Agency for Healthcare Research and Quality’s HCUPnetCitation42.
Model outputs
Model outputs included total costs and total effectiveness associated with nilotinib and dasatinib, measured by life years (LYs) and quality-adjusted life years (QALYs). LYs were estimated as the time spent in each state, while QALYs were estimated as the time spent in each state weighted by the utility for each state. Incremental cost-effectiveness ratios (ICERs), measured as the incremental costs per LY gained and the incremental costs per QALY gained, were estimated by comparing nilotinib with dasatinib:
Sensitivity analysis
A deterministic sensitivity analysis was conducted to test the model’s robustness by examining the impact of change in one key model input or assumption, while holding others at the base-case value. The model inputs varied were starting age, proportion of females, adherence/dose intensity for both drugs, CP post-discontinuation drug treatment costs, progressive disease drug treatment costs, medical costs, AE costs, and utility for CP post-discontinuation.
Results
Over a lifetime horizon, patients in the nilotinib group were found to have 11.7 LYs and 9.1 QALYs, and to incur a total cost of $1,409,466. Of this total direct healthcare cost, 87.9% was attributed to CML-related drug costs, 11.5% to medical costs, and 0.6% to AE-related costs. Patients in the dasatinib group were found to have 9.5 LYs and 7.3 QALYs, and to incur a total cost of $1,422,122. Of this total direct healthcare cost, 88.5% was attributed to CML-related drug costs, 11.0% to medical costs, and 0.5% to AE costs (). Thus, in comparison with dasatinib, second-line treatment with nilotinib was associated with better health outcomes (with differences of 2.2 LYs and 1.9 QALYs) as well as lower total costs (a difference of $12,655) over a lifetime horizon.
Table 2. Base-case results.
The deterministic sensitivity analysis results showed a consistent finding of better QALY and lower costs for nilotinib vs dasatinib in most of the scenarios (), including variation in sex-ratio, progressive disease treatment costs, medical costs for all health states, AE costs, adherence/dose intensity, and utility for CP post-discontinuation. Out of 18 scenarios tested, the only three scenarios in which the sensitivity analysis resulted in better QALY but higher costs for nilotinib vs dasatinib were after decreasing the starting age of 60 years by 5 years to 55 years, decreasing the adherence of both nilotinib and dasatinib to 80%, and increasing CP post-discontinuation monthly treatment costs by 20%. In these cases, the ICERs were $10,095, $2,055, and $1,492 per QALY, respectively, suggesting that nilotinib was slightly more costly relative to dasatinib per QALY gained.
Table 3. Deterministic sensitivity analysis results.
Discussion
This study re-assessed the cost-effectiveness of second-line nilotinib vs dasatinib among patients with Ph+ CML-CP who were resistant or intolerant to imatinib, based on real-world comparative effectiveness data (TOT, PFS, and OS) from Griffin et al.Citation16. The base-case results showed that, over a lifetime horizon, second-line nilotinib was associated with better life expectancy (LY difference =2.2 years) and better quality-of-life (QALY difference =1.9 years). Because the monthly treatment cost of nilotinib was lower than that of dasatinib, nilotinib was associated with a total lifetime treatment cost savings of $20,691, which exceeded the total medical cost difference ($5,861) and total AE cost difference ($2,174) compared to dasatinib. Therefore, over a lifetime horizon, nilotinib was associated with lower total healthcare cost (by $12,655) relative to dasatinib. In addition, these results remained robust across the various deterministic sensitivity analyses, with the majority of scenarios showing consistent dominance of nilotinib over dasatinib.
The current CEA presents improvements relative to previous CEAs comparing second-line nilotinib vs dasatinibCitation10–13. First, prior CEAs did not use comparative effectiveness evidence between nilotinib and dasatinib due to a lack of data. For example, in the dasatinib manufacturer’s UK NICE submission modelCitation13, effectiveness data for nilotinib and dasatinib were derived based on their respective trials, which recruited patients based on different criteria. Conversely, the current effectiveness data for nilotinib and dasatinib were based on direct comparative evidence from a real-world chart review studyCitation16, which retrospectively collected medical chart information from US hematologists and oncologists on Ph+ CML-CP patients who switched from imatinib to nilotinib (n = 301) or dasatinib (n = 296) as second-line therapy, and compared TOT, PFS, and OS between these two groups of patients. Second, prior CEAs used surrogate markers to impute survival endpoints. Specifically, the dasatinib manufacturer’s model submitted to the UK NICECitation13 used hematologic and cytogenetic response as surrogate markers to impute survival endpoints, Rogers et al.Citation10, Loveman et al.Citation11, and Hoyle et al.Citation43 used major cytogenetic response (MCyR), and Whalen et al.Citation12 used complete cytogenetic response (CCyR) and major molecular response (MMR). In comparison, the current CEA used survival endpoints directly collected from the aforementioned chart-review studyCitation16. A 2014 study by Kulpeng et al.Citation44 found that, among a population of Thai patients with CML-CP resistant or intolerant to imatinib, dasatinib was more cost-effective that nilotinibCitation44. However, treatment with nilotinib resulted in the highest number of QALYs in comparison with dasatinib or high-dose imatinib. While this study came to a different conclusion per comparative cost-effectiveness, drug costs, healthcare access/costs, and patient characteristics vary widely by country and by population, as noted by Kulpeng et al.Citation44. Major differences in healthcare infrastructure and economic parameters exist between Europe/US and Thailand, and the mean age of CML onset in Thailand was 45 years vs ∼69 years in Western populations (the median age in Griffin et al.Citation16 was 60 years). In addition, Kulpeng et al.Citation44 used CCyR as an efficacy indicator, which contrasts with prior studies which used MCyRCitation10,Citation11,Citation43, and came to conclusions that were similar to those of the current study. Finally, Kulpeng et al.’sCitation44 efficacy inputs were derived from three clinical trials, while the current study used real-world data—a major difference which may also account for our differing conclusions.
A 2015 study by Rochau et al.Citation45 assessed the balance of cost-effectiveness and effectiveness of 18 treatment strategies for patients with CML-CP, taking into consideration survival, quality-adjusted survival, and costs. The study assumed patients would transition from first-line TKI (imatinib, bosutinib, nilotinib, and dasatinib) to (1) chemotherapy/SCT, or (2) a second-line TKI followed by chemotherapy/SCT. In this context, the authors found that the most cost-effective treatment strategies involving second-line TKI were imatinib → nilotinib → chemotherapy/SCT ($253,500/QALY), and nilotinib → dasatinib → chemotherapy/SCT ($445,100/QALY). Rochau et al.’sCitation45 study may not be directly comparable to the current analysis, because they considered three lines of therapy while we considered two lines, although they similarly found that second-line nilotinib was the most cost-effective choice in the sequence. The sequencing of therapies is an important topic for healthcare decision-makers, particularly now that imatinib is now generic and can be assumed to be widely used as a cost-effective first-line therapyCitation46.
The results of this study provide economic evidence, in addition to the clinical literature, associated with the second-line treatment of Ph+ CML-CP patients to assist US third-party payers in making reimbursement decisions. US healthcare spending has been rapidly increasing over recent yearsCitation47, and the upward trend in cancer treatment costs is particularly strikingCitation48. Various frameworks, such as the American Society of Clinical Oncology (ASCO) conceptual frameworkCitation49, have been developed to aid healthcare decision-makers such as payers in evaluating the comparative value of cancer treatment options. In the framework, CEA is considered an important method to determine the value of new technologies compared with the current standard of care. Although there is no standard process across third-party US payers to evaluate whether a drug is cost-effective, they have increasingly adopted CEA as an important reference for reimbursement decision-makingCitation50. Therefore, the findings of this real-world data-based CEA, showing that second-line nilotinib was associated with better effectiveness and lower costs relative to dasatinib for Ph+ CML-CP patients, are of particular interest to US payers given their strong need to curb cancer drug spending.
This study is subject to several limitations, some of which are inherent to economic modeling studies. First, the model inputs were obtained from various data sources that were not necessarily based on the study population. However, best efforts were made to select the most fit model inputs based on the existing literature and de novo data collection (i.e. physician survey). Extensive sensitivity analyses were also conducted to test the robustness of the model and showed that, in the majority of scenarios, the results were consistent with the base-case analysis. Second, effectiveness inputs were estimated based on a chart review studyCitation16 with a 24-month follow-up time period, and the estimated effectiveness data fit well against the observed data. However, because long-term real-world data was not available, the lifetime projection could not be validated. Third, the proportion of patients receiving each CP post-discontinuation and progressive disease treatment was estimated based on a physician survey with a relatively small sample size (100 hematologists/oncologists in the US). Thus, the generalizability of the results may be limited. Fourth, due to lack of data, certain assumptions were made in cost and utility inputs, e.g. frequencies of monitoring tests specific to CP post-discontinuation or progressive disease were assumed to be the same as those for CP on treatment. Last, because this analysis used effectiveness inputs from Griffin et al.Citation16, the limitations of that study carry forward to the present study. Namely, these include that no mutation test results were included and that median follow-up was a little less than a year. In addition, a greater proportion of Griffin et al.’sCitation16 patients receiving second-line nilotinib had secondary resistance to imatinib (p = .047), and a greater proportion of those receiving second-line dasatinib had AEs with and intolerance to imatinib (p = .024). Thus, because nilotinib patients were more likely to be resistant to imatinib, our approach is likely biased against nilotinib because these patients are expected to have worse clinical outcomes, and thus is a conservative approach.
Conclusion
The current CEA, based on real-world comparative effectiveness evidence, suggested that, compared with dasatinib, second-line nilotinib was associated with better life expectancy and quality-of-life, and lower total healthcare costs among patients with Ph+ CML-CP who are resistant or intolerant to imatinib. Deterministic sensitivity analysis results were robust, and nilotinib had better outcomes and lower costs relative to dasatinib in all scenarios except when starting age, adherence for both drugs, and CP post-discontinuation costs were changed.
Transparency
Declaration of funding
Funding for this research was provided by Novartis Pharmaceuticals Corporation. The study sponsor was involved in all stages of the study research and manuscript preparation, but all authors participated in the design of the study and contributed to the manuscript development.
Declaration of financial/other relationships
NL, XY, LF, TT, and AG are employees of Analysis Group Inc., which has received consultancy fees from Novartis Pharmaceuticals Corporation. GJ, SB, and LC are employees of Novartis Pharmaceuticals Corporation and own stocks/options. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentation
A synopsis of the current research was presented in poster format at the American Society of Hematology annual meeting, which took place in San Diego, CA during December 3–6, 2015, and at the Academy of Managed Care Pharmacy annual meeting in San Francisco, CA on April 19–22, 2016.
Supplemental Table 1
Download MS Word (14 KB)Acknowledgments
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc., ultimately paid by the sponsor. The authors thank Ankur Khare and Benjamin Cohen for technical review of the CEA model.
References
- NIH National Cancer Institute. Surveillance, epidemiology, and end results program (SEER) stat fact sheets: Chronic Myeloid Leukemia (CML). 2015, http://seer.cancer.gov/statfacts/html/cmyl.html. Accessed October 27, 2015
- Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science 1960;132:1488-501
- Morel F, Ka C, Le Bris MJ, et al. Deletion of the 5′ abl region in Philadelphia chromosome-positive chronic myeloid leukemia. Leukemia 2003;17:473-4
- Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood 1996;88:2375-84
- Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041-51
- Mace ML, Dahl J, Jabbour EJ. Which tyrosine-kinase inhibitor to use first in chronic phase chronic myelogenous leukemia? Expert Opin Pharmacother 2015;16:999-1007
- O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003;348:994-1004
- Radich JP, Deininger M, Abboud CN, et al. NCCN clinical practice guidelines in oncology: Chronic myelogenous leukemia, Version 1; 2016. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf. Accessed March 15, 2016
- Jabbour E, Kantarjian H, Cortes J. Use of second- and third-generation tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia: an evolving treatment paradigm. Clin Lymphoma Myeloma Leuk 2015;15:323-34
- Rogers G, Hoyle M, Thompson Coon J, et al. Dasatinib and nilotinib for imatinib-resistant or -intolerant chronic myeloid leukaemia: a systematic review and economic evaluation. Health Technol Assess 2012;16:1-410
- Loveman E, Cooper K, Bryant J, et al. Dasatinib, high-dose imatinib and nilotinib for the treatment of imatinib-resistant chronic myeloid leukaemia: a systematic review and economic evaluation. Health Technol Assess 2012;16:iii-xiii, 1–137
- Whalen J, Ozer-Stillman I, Ambavane A, et al. Cost-effectiveness analysis (CEA) of sequential treatment with tyrosine kinase inhibitors (TKIs) for chronic myelogenous leukemia (CML) (Poster). 56th ASH Annual Meeting and Exposition; December 6–9, 2014; San Francisco, CA. https://ash.confex.com/ash/2014/webprogram/Paper74905.html. Accessed July 14, 2016
- Bristol-Myers Squibb Pharmaceuticals Ltd. Dasatinib (Sprycel) for the treatment of adults with chronic, accelerated or blast phase CML with resistance or intolerance to prior therapy including imatinib. Multiple Technology Appraisal Submission to the National Institute for Health & Clinical Excellence; 2009
- Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006;354:2542-51
- Shah NP, Guilhot F, Cortes JE, et al. Long-term outcome with dasatinib after imatinib failure in chronic-phase chronic myeloid leukemia: follow-up of a phase 3 study. Blood 2014;123:2317-24
- Griffin JD, Guerin A, Chen L, et al. Comparing nilotinib with dasatinib as second-line therapies in patients with chronic myelogenous leukemia resistant or intolerant to imatinib—a retrospective chart review analysis. Curr Med Res Opin 2013;29:623-31
- Minacori R, Bonastre J, Lueza B, et al. How to model survival in cost-effectiveness analysis? Differences between Markov and partitioned survival analysis models. ISPOR 18th Annual European Congress; November 11, 2015; Milan, Italy. http://www.ispor.org/research_pdfs/51/pdffiles/PRM123.pdf. Accessed March 1, 2016
- Pan F. Evaluation of treatment pathways in oncology: Modeling approaches. ISPOR; May 18–22, 2013; New Orleans, LA. http://www.ispor.org/meetings/neworleans0513/releasedpresentations/W27-Pan.pdf. Accessed March 14, 2014
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409
- Federal Drug Administration (FDA). Highlights of prescribing information. SPRYCEL (dasatinib) tablet for oral use. FDA; August 2015, http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021986s004lbl.pdf
- Federal Drug Administration (FDA). Highlights of prescribing information. TASIGNA (nilotinib) capsules for oral use. FDA; January 2015, http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022068lbl.pdf
- Akaike H, Nakagawa T. Statistical analysis and control of dynamic systems. Boston: Kluwer Academic Publishers; 1988
- Konishi S, Kitagawa G. Information criteria and statistical modeling. New York: Springer; 2008
- Saefken B, Kneib T, van Waveren C-S, et al. A unifying approach to the estimation of the conditional Akaike information in generalized linear mixed models. Electronic Journal of Statistics. 2014;8:201-25
- Schwarz G. Estimating the dimension of a model. Ann Statist 1978;6:461-4
- Fouskakis D, Ntzoufras I, Draper D. Bayesian variable selection using cost-adjusted BIC, with application to cost-effective measurement of quality of health care. Ann App Stat 2009;3:663-90
- Arias E. United States life tables, 2010. Natl Vital Stat Rep 2014;63:1-63
- Szabo SM, Levy AR, Davis C, et al. A multinational study of health state preference values associated with chronic myelogenous leukemia. Value Health 2010;13:103-11
- United States Bureau of Labor Statistics. Consumer Price Index (CPI); United States Bureau of Labor Statistics; 2014, http://www.bls.gov/cpi/
- Truven Health Analytics MicroMedex Solutions. Red Book Online Search. 2015. http://www.redbook.com/redbook. Accessed June 6, 2015
- Li N, Yang C, Fan L, et al., editors. Abstract 2090: Nilotinib vs dasatinib as second-line therapy in patients with Philadelphia-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP) who are resistant or intolerant to imatinib: a cost-effectiveness analysis (CEA) based on real-world data. American Society of Hematology (ASH); December 5–8, 2015; Orlando, FL
- Federal Drug Administration (FDA). Highlights of prescribing information. BOSULIF (bosutinib) tablet for oral use. November 2014, http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203341lbl.pdf
- Federal Drug Administration (FDA). Highlights of prescribing information. ICLUSIG (ponatinib) tablets for oral use. September 2014, http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203469lbl.pdf
- Federal Drug Administration (FDA). Highlights of prescribing information. SYNRIBO (omacetaxine mepesuccinate) for injection, for subcutaneous use. April 2014, http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203585lbl.pdf
- Maziarz RT, Guerin A, Gauthier G, et al., editors. Abstract 872: Five-year direct cost of pediatric patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic stem cell transplantation (HSCT): an analysis from US payers’ perspective. American Society of Hematology (ASH); December 5–8, 2015; Orlando, FL.
- CMS Physician Fee Schedule [Internet]. Centers for Medicare & Medicaid Services (CMS). 2015. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed June 5, 2015
- Goldberg SL, Chen L, Guerin A, et al. Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin 2013;29:1075-82
- Reed SD, Anstrom KJ, Ludmer JA, et al. Cost-effectiveness of imatinib versus interferon-alpha plus low-dose cytarabine for patients with newly diagnosed chronic-phase chronic myeloid leukemia. Cancer 2004;101:2574-83
- Yabroff KR, Lamont EB, Mariotto A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst 2008;100:630-41
- Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011;117:1141-5
- Liou SY, Stephens JM, Carpiuc KT, et al. Economic burden of haematological adverse effects in cancer patients: a systematic review. Clin Drug Investig 2007;27:381-96
- Healthcare Cost and Utilization Project’s (HCUP) HCUPnet [Internet]. Agency for Healthcare Research and Quality, Rockville, MD; 2012. http://hcupnet.ahrq.gov/. Accessed July 10, 2015
- Hoyle M, Rogers G, Moxham T, et al. Cost-effectiveness of dasatinib and nilotinib for imatinib-resistant or -intolerant chronic phase chronic myeloid leukemia. Value Health 2011;14:1057-67
- Kulpeng W, Sompitak S, Jootar S, et al. Cost-utility analysis of dasatinib and nilotinib in patients with chronic myeloid leukemia refractory to first-line treatment with imatinib in Thailand. Clin Ther 2014;36:534-43
- Rochau U, Kluibenschaedl M, Stenehjem D, et al. Effectiveness and cost-effectiveness of sequential treatment of patients with chronic myeloid leukemia in the United States: a decision analysis. Leuk Res Treat 2015;2015:1-12
- Padula WV, Larson RA, Dusetzina SB, et al. Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst 2016;108, https://www.ncbi.nlm.nih.gov/pubmed/26944912
- National Health Expenditures 2014 Highlights. Centers for Medicare & Medicaid Services (CMS); 2015. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/nationalhealthexpenddata/downloads/highlights.pdf. Accessed February 5, 2016
- Tangka FK, Trogdon JG, Richardson LC, et al. Cancer treatment cost in the United States: has the burden shifted over time? Cancer 2010;116:3477-84
- Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 2015;33:2563-77
- Bryan S, Sofaer S, Siegelberg T, et al. Has the time come for cost-effectiveness analysis in US health care? Health Econ Policy Law 2009;4:425-43