Abstract
Objective: Dulaglutide 1.5 mg once weekly is a novel glucagon-like peptide 1 (GLP-1) receptor agonist, for the treatment of type two diabetes mellitus (T2DM). The objective was to estimate the cost-effectiveness of dulaglutide once weekly vs liraglutide 1.8 mg once daily for the treatment of T2DM in Spain in patients with a BMI ≥30 kg/m2.
Methods: The IMS CORE Diabetes Model (CDM) was used to estimate costs and outcomes from the perspective of Spanish National Health System, capturing relevant direct medical costs over a lifetime time horizon. Comparative safety and efficacy data were derived from direct comparison of dulaglutide 1.5 mg vs liraglutide 1.8 mg from the AWARD-6 trial in patients with a body mass index (BMI) ≥30 kg/m2. All patients were assumed to remain on treatment for 2 years before switching treatment to basal insulin at a daily dose of 40 IU. One-way sensitivity analyses (OWSA) and probabilistic sensitivity analyses (PSA) were conducted to explore the sensitivity of the model to plausible variations in key parameters and uncertainty of model inputs.
Results: Under base case assumptions, dulaglutide 1.5 mg was less costly and more effective vs liraglutide 1.8 mg (total lifetime costs €108,489 vs €109,653; total QALYS 10.281 vs 10.259). OWSA demonstrated that dulaglutide 1.5 mg remained dominant given plausible variations in key input parameters. Results of the PSA were consistent with base case results.
Limitations: Primary limitations of the analysis are common to other cost-effectiveness analyses of chronic diseases like T2DM and include the extrapolation of short-term clinical data to the lifetime time horizon and uncertainty around optimum treatment durations.
Conclusion: The model found that dulaglutide 1.5 mg was more effective and less costly than liraglutide 1.8 mg for the treatment of T2DM in Spain. Findings were robust to plausible variations in inputs. Based on these results, dulaglutide may result in cost savings to the Spanish National Health System.
Introduction
Diabetes is a metabolic disorder that is characterized by high blood glucose which can lead to serious health conditions including cardiovascular, renal, and eye diseases. The current prevalence of diabetes in the European region has been estimated at ∼60 million (9.1% of the population aged 20–79), including 23.5 million diagnosed casesCitation1. In 2015, the International Diabetes Federation (IDF Atlas) estimated the prevalence of type 1 and type 2 diabetes in Spain to be 10.4%, one of the highest observed rates in Western EuropeCitation1. Other cross-sectional studies conducted in Spain have reported even higher prevalence rates (up to 13.8%), with almost half (6%) of patients being undiagnosedCitation2. The majority of diabetes patients (∼90%) suffer from type 2 diabetes mellitusCitation1 (T2DM).
The high prevalence and socio-economic consequences of T2DM make this disease an important public health concern worldwide. The life expectancy of patients with diabetes is reduced by up to 8 years compared to the general population, mainly due to the increased risk of cardiovascular death and strokeCitation3,Citation4. In Spain, diabetes-related mortality has been estimated at 22,308 deaths in 2015Citation1. A Spanish study conducted in 2015 estimated the average annual direct healthcare costs for diabetic patients to be €3110.10 vs €1803.60 for non-diabetic patientsCitation5. Costs associated with hospitalizations and medications are a major underlying cost driver, generating ∼70% of the difference between diabetic and non-diabetic patients (cost of hospitalizations are €1306.60 and €801.60, and medication costs are €925.0 and €489.20 in diabetic and non-diabetic patients, respectively, in 2011 values)Citation5.
Dulaglutide is a new GLP-1 receptor agonist administered once weekly via a disposable auto-injection pen with a fixed dosage (1.5 mg). The efficacy and safety of dulaglutide 1.5 mg has been demonstrated in the AWARD clinical trial program. Dulaglutide 1.5 mg exhibits GLP-1-mediated effects, including glucose-dependent potentiation of insulin secretion, inhibition of glucagon secretion, delay of gastric emptying, and weight loss. The combination of these effects results in a decrease in both fasting and postprandial glucose concentrations, thereby leading to improvement in overall glycemic controlCitation6–12.
The objective of this study was to assess the cost-effectiveness of dulaglutide 1.5 mg once weekly when compared to liraglutide 1.8 mg, a once daily GLP-1 receptor agonist, for the treatment of T2DM patients with a body mass index (BMI) ≥ 30 kg/m2 from the perspective of the Spanish National Health System. According to Spanish payers’ restrictions on GLP-1 receptor agonists, prescription is reimbursed only to patients with a BMI ≥30 kg/m2. The analysis was based on direct head-to-head outputs from the AWARD-6 clinical trialCitation8. Liraglutide was chosen as the comparator, since it is the GLP-1 receptor agonist most widely used in SpainCitation13.
Methods
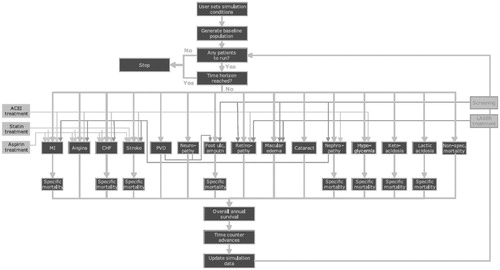
The IMS Core Diabetes Model (CDM), an internet-based computer simulation model, was used to project the long-term health outcomes (life expectancy, quality adjusted life expectancy, cumulative incidence of complications) and economic consequences (annual and cumulative costs per patient, cost of treatments, and complication costs), as well as the incremental cost effectiveness ratio (ICER) of the intervention of interest. The model performs real-time simulations, which take into account the baseline population characteristics, history of complications, and treatment strategies for micro-vascular, end-stage complications, and multi-factorial interventions. The disease progression is based on 17 inter-dependent Markov sub-models that simulate the complications of diabetes (i.e. cardiovascular disease, eye disease, hypoglycemia, nephropathy, neuropathy, foot ulcer, amputation, stroke, ketoacidosis, lactic acidosis, and mortality). Each sub-model incorporates time, state, and diabetes type-dependent probabilities from public sourcesCitation14 (). CDM is a robust and well established simulation model validated against results from both clinical and epidemiological studiesCitation15–17.
Figure 1. Diagrammatic representation of the IMS CORE Diabetes Model of Palmer et al.Citation18
The analyses were conducted from the perspective of the Spanish National Health System capturing all relevant direct medical costs (pharmacy costs plus costs of complications and management). The patient population comprised those with T2DM and reflected the AWARD-6 trial population on which the analyses were based. A targeted literature review was performed in order to identify Spanish-specific unit costs data and inflated to 2015 values, where necessary, using the Consumer Price Index (CPI) published by the Spanish National Statistics InstituteCitation19.
Given the chronic nature of T2DM, a lifetime horizon representing 40 years or death (whichever comes first) was used in the analysis. Treatment effects were based on direct comparative data from the AWARD-6 clinical trial. Costs and benefits were both discounted at 3% annually in accordance with Spanish guidelinesCitation20. A series of outcomes are estimated in the CDM, although the key metric is the incremental cost per quality adjusted life year (QALY) gained.
Baseline characteristics of patients
The patient cohort with BMI ≥30 kg/m2 was defined with baseline demographics, baseline complications, and physiological parameters representative of the patients enrolled in the AWARD-6 head-to-head trial where dulaglutide 1.5 mg was compared against liraglutide 1.8 mg. Other patient characteristics such as the proportion of smokers, cigarettes per day, and alcohol consumption were derived from the World Health OrganizationCitation21,Citation22 ().
Table 1. Baseline cohort characteristics BMI ≥30 kg/m2 (AWARD-6).
reports the modeled rates of baseline diabetes complications. The prevalence of these co-morbidities (renal, retinopathy, ophthalmological, and limb) were estimated based on the T2DM cohort defined in the NICE clinical guideline 28Citation23. Rates were assumed applicable due to similar profiles between cohorts, including baseline HbA1c, age, and duration of diabetes. Rates were also assumed applicable to a Spanish population ().
Table 2. Modeled rates of diabetes complications at baseline.
Treatment effects
Treatment effects associated with both treatments were drawn from the efficacy and safety population of AWARD-6 data (26-week data, background medication metformin). Treatment effects include changes in HbA1c, systolic blood pressure (SBP), total cholesterol (TC), high and low density lipoprotein cholesterol (LHD and HDL), and triglycerides ().
Table 3. Treatment effects for BMI ≥30 kg/m2 (AWARD-6).
In CDM, treatment effects are applied in the first year of treatment. In subsequent years, the UK Prospective Diabetes Study (UKPDS) regression approach is applied to predict the progression of model parameters over the course of the simulation. After 2 years of initial treatment, treatment groups were assumed to switch to basal insulin glargine (at a daily dose of 40 IU as per WHO daily defined dose for basal insulin). This conservative assumption of a 2-year treatment was based upon recent real world evidence from currently marketed GLP-1 receptor analogs, suggesting that most patients switch treatment after a period of ∼2 yearsCitation25,Citation26. Impact of the switch was limited to impact on weight, and no effect was assumed on HbA1c or hypo event rate (hypo event rates were assumed to be “0” on switch, as both treatment arms receive the same therapy). In order to model impact on weight, patients switching to insulin glargine were assumed to rebound to their baseline BMI at the start of year 3 (i.e. the initial BMI effect was reversed, which is a conservative assumption). Scenario analyses explored the impact of eliminating the benefit of dulaglutide on BMI for the duration of the treatment, i.e. increasing the relative treatment benefit for liraglutide vs dulaglutide on BMI. Other parameters were assumed to follow natural progression.
Health state utilities and disutilities
Health state utilities for T2DM and its complications were derived based on a systematic literature review of reported valuesCitation27. Utility values express patient preferences for different health states and typically range from 0 (death) to 1 (full health without complications), although negative values for health states valued worse than death also existCitation28. The disutilities (decrements of utility, range of values from −1 to 0) included in the model were associated with treatment-related events, e.g. nausea, hypoglycemia, and excess BMI were derived based on best available evidence ()Citation29,Citation30. Nausea disutility was applied within the model based on nausea rates observed in the AWARD-6 trial (40% and 33% for patients treated with dulaglutide 1.5 mg and liraglutide 1.8 mg, respectively). The disutility for nausea (−0.04 annually) was taken from Matza et al.Citation30 and was applied only for the first 6 months of the analysis period.
Table 4. Health state and treatment related utilities.
Treatment costs
Treatment costs comprised drug cost, needle cost (where applicable), and the costs associated with self-monitoring of blood glucose (SMBG). Drug costs were estimated based on costs reported in BOTplusCitation31 and calculated on the basis of the pubic price considering 7.5% discount in medications covered by the Spanish Royal Decree Law 8/2010Citation32. Unit costs for drugs are reported in Citation31 and for needle and SMBG costs in Citation33,Citation34.
Table 5. Drug prices (€2014) in Spain.
Table 6. Needle and SMBG prices (€2014) in Spain.
Annual costs were estimated based on expected dose and SMBG requirements. The self-monitoring of blood glucose frequency used in the model followed that recommended by Owens et al.Citation35 for regimens including basal insulin, daily monitoring is recommended. Annual treatment costs are reported in . The cost of background therapies was not included in the analyses, as these therapies were assumed constant across treatment settings.
Table 7. Annual treatment costs (€) in Spain.
Management costs
Costs associated with the management of complications are summarized in . Complications associated with T2DM include CV disease, renal complications, acute events, ophthalmological events, and events related to neuropathy and limb complications. Costs were derived from a combination of literature and published tariffs, using diabetes-specific Spanish costs where availableCitation36–48.
Table 8. Management costs (€annual).
Handling uncertainty
Comprehensive sensitivity analyses were conducted to assess the robustness of model outputs to plausible changes in key model parameters. Treatment effects were varied according to the 95% confidence intervals for HbA1c and BMI for dulaglutide 1.5 mg, while the comparator inputs were kept constant. Alternate treatment durations of 1 and 3 years were assessed. Complication costs were varied by 20% along with alternate discount rates at 0% and 5%. The impact of shorter time horizons was assessed (10 and 20 years). The impact of the removal of disutilities, including BMI, nausea, and hypoglycemia, was also assessed. An alternative approach to treatment switch when HbA1c threshold exceeds 7.5% points was also applied, since exceeding this threshold indicates inadequately controlled diseaseCitation23. In addition, a sensitivity analysis was run to explore the price of liraglutide using costs equal to average daily dose costs for liraglutide 1.5 mg and 1.36 mg, based on published real world evidence of its use in practice (all other inputs including efficacy were kept constant)Citation25,Citation42.
Probabilistic sensitivity analysis (PSA) was conducted to evaluate the uncertainty in model outputs associated with input parameter uncertainty. A second order Monte Carlo simulation was used, during which parameter values were sampled from fixed distributions (see for details).
Table 9. Parameters and distributions used in PSA.
Results
Under base case assumptions dulaglutide 1.5 mg was found to be more effective and less costly, i.e. the dominant treatment. In comparison with liraglutide 1.8 mg, dulaglutide 1.5 mg was projected to increase quality adjusted life expectancy. Quality adjusted life expectancy was 10.281 QALYs for dulaglutide 1.5 mg and 10.259 QALYs for liraglutide 1.8 mg (an increment of 0.021 QALYs). Treatment with dulaglutide 1.5 mg resulted in a total cost reduction of €1,165, of which €993 was attributed to difference in treatment costs between dulaglutide 1.5 mg and liraglutide 1.8 mg. Amongst the diabetes-related complications tracked within the model, dulaglutide was mainly associated with lower cumulative incidence of angina, diabetes-related mortality, foot recurring ulcers, myocardial infarction (MI) events, MI related deaths, and minor hypoglycemic events. Liraglutide was mainly associated with lower cumulative incidence of congestive heart failure events (). In terms of complication incidence, the analysis did not show large differences between treatments. If a revised threshold for differences in cumulative incidence of 0.2 is used, for example, the incidence of myocardial infarction, recurring foot ulcers, angina, and diabetes related mortality favors dulaglutide 1.5 mg and neuropathy favors liraglutide 1.8 mg. Full base case results are reported in .
Table 10. Base case outputs (dulaglutide 1.5 mg – liraglutide 1.8 mg).
Sensitivity analyses
A series of one-way sensitivity analyses indicated that dulaglutide would remain as the dominant treatment, generating both cost savings and improved quality adjusted life years, when treatment effects, economic inputs treatment duration, disutilities, time horizon, discounting, and HbA1c threshold were varied. For all of the scenarios considered, dulaglutide 1.5 mg remained the dominant strategy ().
Table 11. Sensitivity analyses (dulaglutide 1.5 mg – liraglutide 1.8 mg).
Probabilistic sensitivity analyses
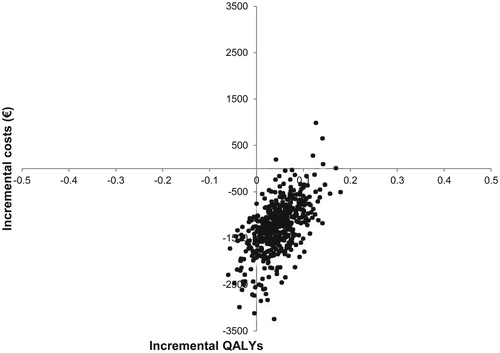
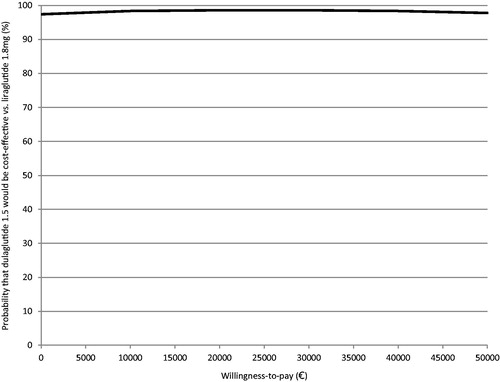
Results of the probabilistic sensitivity analysis were in line with deterministic results, indicating that dulaglutide 1.5 mg was more effective and less costly relative to liraglutide 1.8 mg in the majority of iterations. It was observed that 86.4% of the cost-effectiveness pairs fell in the south-east quadrant of the cost-effectiveness plane. The incremental cost-effectiveness pairs for costs and QALYs gained are plotted in . The cost-effectiveness acceptability curve (CEAC) shows that dulaglutide 1.5 mg is expected to have over 98% probability of being cost-effective at a willingness to pay threshold of €30,000 per QALY gainedCitation50 ().
Discussion
Long-term projections suggested that dulaglutide 1.5 mg once weekly results in improvements in life-expectancy and quality adjusted life years. From a Spanish healthcare payer perspective and under base case assumptions, dulaglutide 1.5 mg was found to be dominant (more effective and less costly) when compared against liraglutide 1.8 mg for patients with BMI ≥30 kg/m2 for whom GLP-1 receptor agonists are prescribed, making it a viable treatment option for patients with type 2 diabetes mellitus.
In the analysis performed against liraglutide 1.8 mg, the model inputs, and efficacy and safety data were based on robust head-to-head data from the AWARD-6 clinical trial programCitation8. The results indicate that dulaglutide 1.5 mg was associated with lower treatment cost and cost of management and complications. Total QALYs generated in the dulaglutide arm were higher than those accumulated in liraglutide 1.8 mg arm (10.281 vs 10.259, respectively). These findings were robust to plausible changes in initial treatment effect (treatment effect was varied between the trial generated 95% confidence intervals in sensitivity analysis).
Exploration of uncertainty through one-way sensitivity analyses and probabilistic sensitivity analysis suggested that results were robust to plausible variations in the input parameters, with dulaglutide remaining less costly and more effective (dominant) in all cases.
There is limited evidence on studies conducted in the Spanish setting with which to compare the outcome of these analyses. Studies by Raya et al.Citation51 and Perez et al.Citation52 assessed the cost-effectiveness of liraglutide 1.2 mg and liraglutide 1.8 mg vs sitagliptin in Spain, revealing the increasing trend to assess the cost-effectiveness of various GLP-1 receptor agonists for the treatment of T2DM. While not directly comparable in terms of country setting, a previous analysis conducted by Raibouaa et al.Citation53 to estimate the cost-effectiveness of dulaglutide 1.5 mg vs liraglutide 1.8 mg in a Swedish setting resulted in the same conclusions. In this analysis, dulaglutide 1.5 mg was found to be dominant against liraglutide 1.8 mg, resulting in societal cost savings in Sweden.
This analysis was subject to limitations common to most diabetes modeling analyses. First, as with any cost-effectiveness model, in the absence of lifetime data, a number of core assumptions were applied within the CDM in order to extrapolate these short-term clinical trial data; however, these assumptions were consistent with disease progression and based on established risk equations. Emerging real world evidence on the duration of treatment and long-term treatment effects will allow for better future estimates of core model inputsCitation42. However, the current analysis suggests that the dominance of dulaglutide 1.5 mg is a robust finding.
The analysis specifically focuses on patients with BMI ≥30 kg/m2, which we consider to be a strength of the analysis, as physicians can only prescribe GLP-1 analogs in this specific population. Therefore, our analysis is informative to the Spanish decision-makers since the relevant patient sub-group was used to explore the cost-effectiveness.
In this analysis, patients switching to insulin glargine were assumed to rebound to their baseline BMI at the start of year 3, reversing the initial BMI effect. Scenario analyses explored the impact of eliminating dulaglutide’s treatment effect on BMI, i.e. increasing the relative treatment benefit for liraglutide vs dulaglutide on BMI. Scenario analysis using the upper and lower bounds of the BMI treatment effect were applied to the dulaglutide arm. In both scenarios, dulaglutide 1.5 mg remained the dominant treatment, in line with base case results. This suggests results are not sensitive to plausible changes in BMI treatment effect, even where the relative effect of liraglutide is increased vs dulaglutide.
Identification of precise, coherent, up-to-date, and relevant data on the many inputs required to model T2DM represents a challenge. Nevertheless, the best available data were used and tested thoroughly by means of sensitivity analyses, with a clear focus on use of local data where possible. The primary limitations of the analysis identified are common to other T2DM analyses, and computer simulation modeling remains the best option currently available to estimate the clinical and economic consequences of therapeutic interventions in the medium-to-long-term. A limitation of the analysis was that data on non-cardiovascular (CV) co-morbidities were not collected in the AWARD studies; however, non-cardiovascular events were modeled using published literature which informed the risk of these events occurring in the modelCitation54. Associated life time cost and health benefits of dulaglutide 1.5 mg once weekly and liraglutide 1.8 mg once daily for T2D are likely to vary across regions due to variations in the prevalence between localities and population heterogeneity.
Conclusion
This analysis indicated that, from the Spanish healthcare perspective, dulaglutide 1.5 mg would be considered a dominant treatment alternative to liraglutide 1.8 mg in type 2 diabetes mellitus patients with a BMI ≥30 kg/m2. The introduction of dulaglutide 1.5 mg provides additional health benefit for T2DM patients and may result in cost-savings to the Spanish payers. Although modest, the per patient cost savings and QALY gains may translate into wider cost and clinical outcome benefits for the overall Spanish healthcare system, if dulaglutide 1.5 mg is administered in clinical practice.
Transparency
Declaration of funding
The project was conducted by IMS Health and was funded by Eli Lilly and Company.
Declaration of financial/other competing interests
TD and KN are employees of Eli Lilly and Company. JL was an employee of IMS Health, who served as a paid consultant to Eli Lilly and Company during the development of this study and manuscript. DA, IC, and RA are employees of IMS Health, who served as paid consultants to Eli Lilly during the development of this study and manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors wish to acknowledge the reviewers and the editor for their detailed and helpful comments, which have contributed to the strength of this research. The authors would also like to thank Eli Lilly and Co. for supporting and funding this study.
References
- International Diabetes Federation. IDF Diabetes Atlas, 7th Edition. 2015. Belgium http://www.idf.org/diabetesatlas. Accessed April 4, 2016
- Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Diabetes Study. Diabetologia 2012;55:88-93
- Almdal T, Scharling H, Jensen JS, et al. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med 2004;164:1422-6
- Roper NA, Bilous RW, Kelly WF, et al. Excess mortality in a population with diabetes and the impact of material deprivation: longitudinal, population based study. BMJ 2001;322:1389-93
- Mata-Cases M, Casajuana M, Franch-Nadal J, et al. Direct medical costs attributable to type 2 diabetes mellitus: a population-based study in Catalonia, Spain. Eur J Health Econ 2015;15:1-10
- Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet 2015;385:2057-66
- Dungan KM, Povedano ST, Forst T, et al. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 2014;384:1349-57
- Eli Lilly and Company. A study comparing the effect of dulaglutide with liraglutide in type 2 diabetes (AWARD-6). Eli Lilly and Company; 2014. London, United Kingdom, https://clinicaltrials.gov/ct2/show/NCT01624259 Accessed Jan 15th 2016
- Giorgino F, Benroubi M, Sun JH, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2). Diabetes Care 2015;38:2241-9
- Nauck M, Weinstock RS, Umpierrez GE, et al. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5). Diabetes Care 2014;37:2149-58
- Umpierrez G, Povedano ST, Manghi FPr, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37:2168-76
- Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014;37:2895
- IMS. IMS MAT 2016 for Spain. IMS; United Kingdom, 2016. http://www.imspadds.com/midas/ IMS Health Data Accessed July 1, 2016
- Grant D, Foos V, Palmer J, et al. Long-term validation of the IMS CORE Diabetes Model in Type 1 and Type 2 diabetes. New Jersey, United States of America: Value in Health Journal, 1203-p
- Hornberger J. Computer modeling of diabetes and its complications: a report on the fifth mount hood challenge meeting. Value Health 2013;16:453-4
- Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(Suppl 1):S27-S40
- Palmer AJ, Mount H. Computer modeling of diabetes and its complications: a report on the Fifth Mount Hood challenge meeting. Value in Health 2013;16:670-85
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(Suppl 1):S5-S26
- Instituto Nacional de Estadistica. Consumer Prices: All Items. 2014. Madrid, Spain http://www.ine.es/en/welcome_en html Accessed Nov 2014
- Lopez-Bastida J, Oliva J, Antoñanzas F, et al. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 2010;11:513-20
- World Health Organisation. WHO Report on the Global Tobacco Epidemic, 2013; Country profile Spain Geneva Switzerland: WHO Press. 2013
- World Health Organisation. World Health Organisation Country Statistics: Spain. Alcohol Consumption: Levels and Patterns. 2014 Geneva Switzerland: WHO Press
- NICE. Type 2 diabetes in adults: management. 2015. United Kingdom https://www.nice.org.uk/guidance/ng28 Accessed Jan 2016
- Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006;69:2057-63
- Divino V, DeKoven M, Hallinan S, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther 2014;5:499-520
- Levin PA, Wei W, Zhou S, et al. Outcomes and treatment patterns of adding a third agent to 2 OADs in patients with type 2 diabetes. J Managed Care Pharm 2014;20:501-12
- Beaudet A, Clegg J, Thuresson PO, et al. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462-70
- Brazier J. Measuring and valuing health benefits for economic evaluation. Toronto, Canada: Oxford University Press; 2007
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ 2011;12:219-30
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- Consejo General de Colegios Oficiales de Farmaceuticos. BOTPlus. 2015. Madrid, Spain https://botplusweb portalfarma com/. Accessed November 27, 2015
- Spanish Government. Real Decreto-ley 8/2010. Madrid, Spain 2010. http://www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf Accessed November 2014
- Guisasola A. Análisis de costes del tratamiento de la diabetes mellitus tipo 2 con insulina glargina o detemir. Av Diabetol 2010;26:430-5
- Farmacia Encasa. Farmacia Encasa. 2014. Madrid, Spain https://wwwfarmaciaencasaonline.es Accessed November 2014
- Owens D, Barnett A, Pickup J. Blood glucose self-monitoring in type 1 and type 2 diabetes: reaching a multidisciplinary consensus. Diabetes Primary Care 2004;6:8-19
- Consellería de Sanidade. Diario Oficial de Galicia n° 222, 21 noviembre 2012. Galicia, Spain 2012
- Departament de Salut. Diari Oficial de la Generalitat de Catalunya n° 6387. Catalonia, Spain 2013
- Departamento de Sanidad. Boletín Oficial del País Vasco 30 julio 2007 n° 145. Pais Vasco, Spain 2007
- Diario Oficial de Castilla- La Mancha n° 267. Servicio de Salud de Castilla-La Mancha (2008). Castilla- La Mancha, Spain 2008
- Goodall G, Costi M, Timlin L, et al. Cost-effectiveness of exenatide versus insulin glargine in Spanish patients with obesity and type 2 diabetes mellitus. Endocrinol Nutr (English edn) 2011;58:331-40
- Hernández-Pastor L. Cost-effectiveness of ranibizumab compared with photodynamic treatment of neovascular age-related macular degeneration. Clin Ther 2008;30:2436-51
- Mezquita-Raya P, Reyes-Garcia R, Moreno-Perez O, et al. Clinical effects of liraglutide in a real-world setting in Spain: eDiabetes-Monitor SEEN Diabetes Mellitus Working Group Study. Diabetes Ther 2015;6:173-85
- Ministerio de Sanidad PSeI. Registro de altas. CIE9 MC – CMBD 2011. 2013. Madrid, Spain http://pestadistico.msc.es Accessed November 2014
- Osakidetza. Servicio Vasco de Salud. 2013. Pais Vasco, Spain http://www osakidetza euskadi.net/r85 Accessed November 2014
- Parra-Moncansi E, Arenas Jimenez MD, Alonso M, et al. Multicentre study of haemodialysis costs. Nefrologia 2011;31:299-307
- Piñol C, Roze S, Valentine W, et al. [Cost-effectiveness of the addition of acarbose to the treatment of patients with type-2 diabetes in Spain]. Gac Sanit 2007;21:97-104
- Ray JA, Valentine WJ, Secnik K, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr Med Res Opin 2005;21:1617-29
- Schwander B, Gradl B, Zollner Y, et al. Cost-utility analysis of eprosartan compared to enalapril in primary prevention and nitrendipine in secondary prevention in Europe—the HEALTH model. Value Health 2009;12:857-71
- World Health Organisation. WHO ATC/DDD Index 2015. Oslo, Norway: WHO; 2015. http://www.whocc.no/atc_ddd_index/. Accessed November 27, 2015
- Sacristan JA, Oliva J, del Llano J, et al. What is an efficient health technology in Spain? Gaceta Sanitaria 2002;16:334-43
- Mezquita-Raya P, Perez A, Ramirez de Arellano A, et al. Incretin therapy for type 2 diabetes in Spain: a cost-effectiveness analysis of liraglutide versus sitagliptin. Diabetes Ther 2013;4:417-30
- Perez A, Mezquita-Raya P, Ramirez de Arellano A, et al. Cost-effectiveness analysis of incretin therapy for type 2 diabetes in Spain: 1.8 mg liraglutide versus sitagliptin. Diabetes Ther 2015;6:61-74
- Raibouaa A, Borgeke H, Alexiou D, et al. Cost-effectiveness of dulaglutide 1.5 mg once weekly for the treatment of patients with type two diabetes mellitus in Sweden. Value Health J Int Soc Pharmacoecon Outcomes Res 2015;18:A607
- Foos V, Palmer JL, Grant D, et al. The IMS CORE diabetes model: model user guide. IMS Health. August 27. Basel, Switzerland http://www.core-diabetes.com/cdm.asp. Accessed April 1 2015