Abstract
Aim: To estimate real-world cardiovascular disease (CVD) burden and value-based price range of evolocumab for a US-context, high-risk, secondary-prevention population.
Materials and methods: Burden of CVD was assessed using the UK-based Clinical Practice Research Datalink (CPRD) in order to capture complete CV burden including CV mortality. Patients on standard of care (SOC; high-intensity statins) in CPRD were selected based on eligibility criteria of FOURIER, a phase 3 CV outcomes trial of evolocumab, and categorized into four cohorts: high-risk prevalent atherosclerotic CVD (ASCVD) cohort (n = 1448), acute coronary syndrome (ACS) (n = 602), ischemic stroke (IS) (n = 151), and heart failure (HF) (n = 291) incident cohorts. The value-based price range for evolocumab was assessed using a previously published economic model. The model incorporated CPRD CV event rates and considered CV event reduction rate ratios per 1 mmol/L reduction in low-density lipoprotein-cholesterol (LDL-C) from a meta-analysis of statin trials by the Cholesterol Treatment Trialists Collaboration (CTTC), i.e. CTTC relationship.
Results: Multiple-event rates of composite CV events (ACS, IS, or coronary revascularization) per 100 patient-years were 12.3 for the high-risk prevalent ASCVD cohort, and 25.7, 13.3, and 23.3, respectively, for incident ACS, IS, and HF cohorts. Approximately one-half (42%) of the high-risk ASCVD patients with a new CV event during follow-up had a subsequent CV event. Combining these real-world event rates and the CTTC relationship in the economic model, the value-based price range (credible interval) under a willingness-to-pay threshold of $150,000/quality-adjusted life-year gained for evolocumab was $11,990 ($9,341–$14,833) to $16,856 ($12,903–$20,678) in ASCVD patients with baseline LDL-C levels ≥70 mg/dL and ≥100 mg/dL, respectively.
Conclusion: Real-world CVD burden is substantial. Using the observed CVD burden in CPRD and the CTTC relationship, the cost-effectiveness analysis showed that, accounting for uncertainties, the expected value-based price for evolocumab is higher than its current annual cost, as long as the payer discount off list price is greater than 20%.
Introduction
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality, despite efforts to promote preventative measures that minimize the risk and impact of CVDCitation1. Worldwide, CVD causes an estimated 17.3 million deaths annually; by 2030, this number is projected to increase to >23.6 million. In the US, CVD is the underlying cause of approximately one-third of deathsCitation2. Atherosclerosis is the underlying cause of most clinical CV eventsCitation3, including coronary heart disease (CHD), acute coronary syndromes (ACS) such as myocardial infarction (MI) or unstable angina (UA), peripheral artery disease (PAD), heart failure (HF), transient ischemic attack, ischemic stroke (IS), and CV-related deathCitation1,Citation4. Recent studies have shown that 15%–43% (over 2–3 years) of patients with a primary CV event will experience a second CV eventCitation5,Citation6. In addition to the clinical burden of CVD, these diseases and their treatments are associated with high costs. A recent analysis reported mean costs associated with a second CV event (ACS, IS, or HF) at ∼$45,000, and with revascularization procedures at ∼$55,000Citation5.
Elevated low-density lipoprotein-cholesterol (LDL-C) is a key risk factor for CVD, and reduction of LDL-C is recommended in clinical practice guidelines to reduce morbidity and mortalityCitation1,Citation4. The primary treatment used to reduce LDL-C is statin therapy, which has been shown to reduce the rate of CV events in both primary- and secondary-prevention patientsCitation7. Recent meta-analyses of statin trials by the Cholesterol Treatment Trialists Collaboration (CTTC) have shown that statins reduce vascular mortality by 12% and major CV events by 21% per 1 mmol/L (38.67 mg/dL) of LDL-C reductionCitation8,Citation9. Statin therapy is effective in reducing LDL-C in most patients. However, most high-risk patients are unable to achieve optimal LDL-C reduction. A recent US database analysis suggested that 70% of patients with atherosclerotic CVD (ASCVD) on high/moderate-intensity statins do not achieve LDL-C level <70 mg/dLCitation10.
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have been shown to substantially reduce levels of LDL-C in patients with hypercholesterolemiaCitation11. Evolocumab is a monoclonal antibody that inhibits PCSK9 and reduces LDL-C by ∼60% across populations and background therapiesCitation12–17. Evolocumab is currently indicated in the US as an adjunct to diet and maximally tolerated statin therapy for use in patients with heterozygous or homozygous familial hypercholesterolemia or clinical ASCVD who require additional lowering of LDL-C levelsCitation18. The Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial is assessing the effect of evolocumab on the incidence of major CV events in patients with elevated LDL-C, clinically-event CVD, and additional CV risk factorsCitation19.
Formulary decisions are based on both clinical and economic considerationsCitation20. Given the robust clinical evidence on evolocumab, discussion on its access often centers on product economic value. Whereas several economic analyses have been conducted on the PCSK9 inhibitor class, they gave different conclusions, mainly due to differences in target population and the corresponding CV risk. Thus, in order to accurately assess the economic value of evolocumab, it is important to identify the appropriate patients and their disease burden. Patients enrolled in FOURIER represent high-risk patients who would be expected to benefit from additional LDL-C reduction with evolocumab. Therefore, FOURIER eligibility criteria were applied to identify patients in a real-world database, and their CV burden was evaluated. Economic modeling was then performed to explore the value-based price range, an increasingly relevant metric in economic evaluation, for evolocumab, given the real-world CV burden of appropriate patients and the CVD reduction per 1 mmol/L of LDL-C reduction rate ratios from the CTTC meta-analyses of statin trials (hereinafter, the CTTC relationship).
Methods
Estimation of CVD burden in high-risk patients
Data sources
The integrated linked primary and secondary care data from the UK were used to estimate CV event rates in a real-world population identified by applying inclusion criteria similar to those used in the FOURIER trialCitation19. Primary care data were obtained from the Clinical Practice Research Datalink (CPRD), an ongoing database of anonymized UK medical records. Linked secondary care and cause of death data were obtained from the Hospital Episode Statistics (HES) and the Office for National Statistics (ONS), respectively. Linkage between these datasets covers 70% of the contributing practices in the UK and accounts for ∼55% of the total CPRD patient population. All conditions in the CPRD data were identified using READ codes. Diagnosis codes for CV events are listed in Supplemental Table 1.
The CPRD is one of the most widely used research databases globally, where patients are representative of the UK general population and are retained in the databases for many years. In addition, given the broad linkage among CPRD and secondary care and cause of death data from HES and ONS, there is a comprehensive capture of patients' CV burden including CV mortality. In the present analysis, we used CPRD to estimate CVD burden in the US context, because there are great similarities in demography between the UK and US, whereas US databases often lack continuity and CV mortality data. The limitations of such an approach are discussed in the Discussion section.
Study design
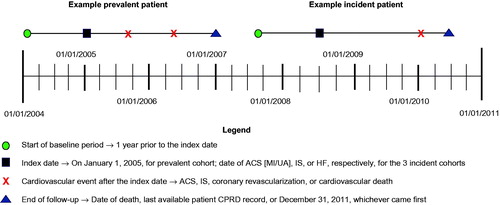
CPRD data from January 1, 2004, through January 1, 2011, were evaluated (). All patients were followed until the last available patient CPRD record, death, or December 31, 2011, whichever came first. Data for at least 2 years before the index date were required for study inclusion. Laboratory values, medication use, and vital signs were identified during the 1-year pre-index period. The mean of lipid and blood pressure levels in the 1-year pre-index period was used as the index date value. Medical history was based on all READ codes prior to the index date.
Patient populations
Patients who had already experienced a CV event as of January 1, 2005, were identified in the CPRD (high-risk prevalent ASCVD). Additionally, three incident cohorts defined by CVD diagnosis (ACS, IS, and HF) were identified.1 For the high-risk ASCVD cohort, index date was January 1, 2005. For incident cohorts, the index date (date of qualifying CV hospitalization) was between January 1, 2005, and December 31, 2010. Patients in incident cohorts were required to survive 30 days after the index hospitalization. Patients in the HF cohort were required to have a history of ACS, IS, or PAD before their first HF hospitalization.
To apply the FOURIER eligibility criteria, patients identified in CPRD were ≥40 years old at index date; had received ≥1 high-intensity statin prescription within 1 year before the index date; had a diagnosis of MI, IS, or PAD; had ≥1 major or >/+2 minor risk factors (); had an LDL-C level ≥70 mg/dL or non-high-density lipoprotein-cholesterol level ≥100 mg/dL; and had a triglyceride level ≤400 mg/dL during the 1-year pre-index period. Statin intensity was based on American College of Cardiology/American Heart Association (ACC/AHA) 2013 blood cholesterol guidelines definitionCitation4.
Table 1. FOURIER eligibility criteria applied to study cohorts.
Study outcomes
The primary outcome, which mirrored the primary end-point in FOURIER, was a composite of CV events defined as ACS (MI or UA), IS, coronary revascularization (coronary artery bypass graft [CABG] or percutaneous coronary intervention [PCI]), or CV-related death. A sensitivity analysis composite outcome excluded coronary revascularization.
Statistical considerations
Incidence rates were calculated for the outcomes of interest as the number of first events divided by the total follow-up person-time at risk until the event or the end of follow-up, and were expressed per 100 patient-years. Multiple-event rates were calculated as the total number of events divided by the total follow-up time at risk until the end of follow-up, and were expressed per 100 patient-years. All analyses were conducted in R version 3.2.4 softwareCitation22.
Model to assess the value-based price range of evolocumab
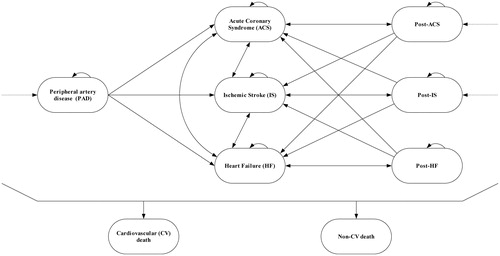
The cost-effectiveness model used to generate the value-based price range estimates for evolocumab has been previously describedCitation23. This model has also been submitted to health-technology assessment agencies around the world. Briefly, a Markov state-transition cohort model () was used to compare evolocumab + SOC vs SOC alone. SOC was defined as the use of high-intensity statins. Patients were evaluated in the context of first-year (ACS, IS, and HF) and subsequent-years (post-ACS, post-IS, and post-HF) health states, as well as combined health states that captured recurrent CV events. Patients entered into the model at the post-ACS, post-IS, or PAD health states, matching FOURIER inclusion criteria.
Model input data included patient baseline characteristics (age, LDL-C, and history of CV events) (Supplemental Table 2), the assumed 60% LDL-C reduction with evolocumab, and an annual non-compliance rate of 10% from the FOURIER design paperCitation19; rates and distribution of CV events as observed in the PAD prevalent cohort and the three (ACS, IS, and HF) incident cohorts in the CPRD; the CTTC relationshipCitation8,Citation9; and health state utility values, direct costs, and non-CV mortality from a prior cost-effectiveness analysis (, Supplemental Table 3).
Table 2. Cost-effectiveness model inputs: rate ratio of CV events per 1 mmol/L of LDL-C reduction, utility values, and direct costs.
Model outcomes included CV event rates, costs, quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratio (ICER) per QALY gained with the addition of evolocumab to SOC relative to SOC alone as a measure of cost-effectiveness at the current US annual cost of evolocumab ($14,100 at list price before any discount or rebate) and SOC ($68). Costs, LYs, and QALYs were each discounted at 3%, as recommended by the Second US Panel on Cost-Effectiveness in Health and MedicineCitation27.
Cost-effectiveness analysis is an available method to assess value, but making statements on value requires the use of willingness-to-pay threshold (WTPT). In the US, there is no established WTPT. Per the recommendation from the Second Panel on Cost-Effectiveness in Health and MedicineCitation27, a range of WTPTs were considered ($100,000–$200,000 per QALY gained), where a WTPT of $150,000 per QALY gained was used as the base case according to the ACC/AHA guideline on cost and value methodology and consistent with widespread practiceCitation28,Citation29. We then estimated the value-based price range of evolocumab in populations with LDL-C ≥70 mg/dL and ≥100 mg/dL. Of note, though clinically LDL-C ≥70 mg/dL is often an accepted threshold for requiring additional LDL-C lowering in patients with ASCVD, many US payer prior-authorization criteria require LDL-C ≥100 mg/dLCitation30–32. The value-based price is defined as the maximum price at which evolocumab + SOC is cost-effective when compared to SOC alone, given a WTPT.
All the analyses above considered the uncertainty in model input parameters through probabilistic analyses. Values for the input parameters were randomly sampled 1000 times from distributions reflecting their uncertainty—rates of events (normal), LDL-C relative reductions (beta), the CTTC relationship (lognormal), and health state costs (gamma) and utility values (beta)Citation33. Model outcomes were summarized with the mean and 95% credible intervals (CrI), corresponding to the 2.5th and 97.5th percentile of the outcomes probability distributions. Deterministic univariate sensitivity analyses were also performed in order to identify the main drivers of evolocumab value-based price.
Results
CVD burden among high-risk patients in CPRD
Based on the analysis from the CPRD, 1,448 patients in the high-risk ASCVD cohort, 602 in the ACS incident cohort, 151 in the IS incident cohort, and 291 in the HF incident cohort were included (). The high-risk ASCVD cohort had a mean (standard deviation [SD]) follow-up of 5.4 (2.2) years, and the incident cohorts had 2.7–2.8 years of follow-up. In the high-risk ASCVD cohort, the mean (SD) age was 67.0 (9.7) years; 60% of patients were male; 93% had hypertension; and 29% had diabetes mellitus. Their baseline mean (SD) LDL-C level was 103.2 (32.1) mg/dL. All cohorts had similar demographic and clinical characteristics except that the three incident cohorts had a higher proportion of patients with diabetes mellitus than the high-risk ASCVD cohort; the IS and HF cohorts had a higher proportion of patients with atrial fibrillation ().
Table 3. Demographic and clinical characteristics of CPRD cohorts at baseline.
Approximately 33% of patients in the high-risk ASCVD cohort experienced a new CV event during follow-up () (i.e. ≥ 2 total CV events after the index event). Among patients with ≥1 new event during follow-up, 42% (203/482) had a subsequent event. Similarly, among patients with ≥2 new events during follow-up, 44% (89/203) had a subsequent event; among patients with ≥3 new events during follow-up, 47% (42/89) had a subsequent event; and among patients with ≥4 events, 48% (20/42) had a new event during follow-up. The incidence rate was 7.5 per 100 patient-years, and the multiple-event rate was 12.3 per 100 patient-years (). The sensitivity analysis regarding the definition of the composite outcome (excluding revascularization) provided similar results.
Table 4. Cumulative incidence of CV events among the different cohorts in the observational study.
Table 5. CV event incidence rates per 100 patient-years.
The proportion of patients with ≥1 CV event during follow-up was 40% in the ACS incident cohort, 27% in the IS incident cohort, and 33% in the HF incident cohort (). The incidence rate per 100 patient-years was 21.1 in the ACS cohort, 11.9 in the IS cohort, and 16.3 in the HF cohort (). The multiple-event rates per 100 patient-years were 25.7, 13.3, and 23.3 in the ACS, IS, and HF incident cohorts, respectively. Results from the sensitivity analysis were slightly lower.
Value-based price range of evolocumab
In patients who received SOC alone, estimated overall rates for any lifetime major CV event (ACS, IS, or CV-related death) and CV-related death per patient were, respectively, 1.92 and 0.53 for patients with LDL-C ≥70 mg/dL, and 2.17 and 0.59 for patients with LDL-C ≥100 mg/dL (Supplemental Table 4). The rate of CV events in the first 5 years was ∼6.7/100 patient-years, which is consistent with the burden in the registry data as estimated in the retrospective analysis. Supplemental Table 4 displays CV event rates with evolocumab + SOC vs evolocumab SOC alone. Using the current annual cost of evolocumab, the incremental lifetime costs (CrI) of adding evolocumab to SOC were $127,088 ($112,827–$141,830) and $110,916 ($91,632–$129,449) among patients with baseline LDL-C ≥70 mg/dL and ≥100 mg/dL, respectively; lifetime QALYs gained (CrI) by adding evolocumab to SOC were 0.68 (0.51–0.87) and 0.95 (0.73–1.19) (). The ICER (CrI) of evolocumab + SOC vs SOC alone was $190,440 ($138,943–$262,158) in patients with baseline LDL-C ≥70 mg/dL, and $118,905 ($83,491–$167,391) in patients with baseline LDL-C ≥100 mg/dL.
Table 6. Cost-effectiveness results for EvoMab added to SOC vs SOC alone.
The value-based price range (CrI) under the WTPT of $150,000/QALY gained was from $11,990 ($9,341–$14,833) to $16,856 ($12,903–$20,678) in patients with LDL-C ≥70 mg/dL and ≥100 mg/dL, respectively. As scenario analyses, alternative WTPTs of $100,000/QALY gained and $200,000/QALY gained were considered. The value-based price (CrI) ranged from $9,064 ($6,869–$11,254) for patients with LDL-C ≥70 mg/dL, to $12,775 ($10,029–$15,497) for patients with LDL-C ≥100 mg/dL under a WTPT of $100,000/QALY, and from $14,850 ($11,371–$18,395) to $21,029 ($16,366–$25,936) under a WTPT of $200,000/QALY.
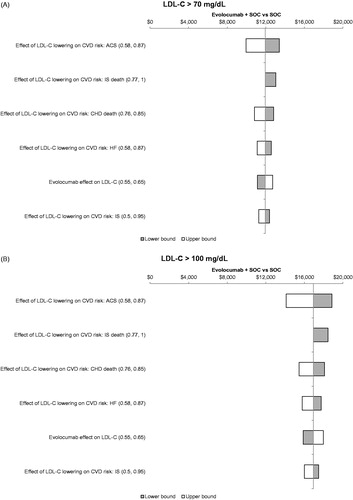
In addition, deterministic sensitivity analysis results showed that the value-based price range of evolocumab was generally consistent when univariate changes in model parameters were considered. Parameters affecting the value-based price more than 5% included the CV event-specific rate ratios capturing the CTTC relationship and evolocumab LDL-C relative reduction ().
Figure 3. Tornado diagram for estimated value-based price range at a WTPT of $150,000 per QALY gained by baseline LDL-C (a) ≥ 70 mg/dL and (b) ≥100 mg/dL. Only factors that affected the ICER by ≥5% are displayed. ACS: acute coronary syndrome; CHD: coronary heart disease; CVD: cardiovascular disease; HF: heart failure; ICER: incremental cost-effectiveness ratio; IS: ischemic stroke; LDL-C: low-density lipoprotein-cholesterol; QALY: quality-adjusted life-year; SOC: standard of care; WTPT: willingness-to-pay threshold.
Discussion
In a real-world, high-risk population identified in the CPRD, the CVD burden on patients was substantial. In the high-risk ASCVD cohort, over 30% of patients had a new CV event, including ACS, IS, revascularization, or CV-related death during a mean follow-up of 5.4 years. Among those who had a new CV event, 42% had at least one subsequent event. The rate of CV events was 12.3 per 100 patient-years for the high-risk ASCVD cohort. CV burden was higher in the incident cohorts: ∼ 40% of the ACS, 27% of the IS, and 33% of the HF incident cohorts experienced a new CV event over a mean follow-up of almost 3 years (adjusted rates: 25.7 per 100 patient-years in the ACS incident cohort, 13.3 in the IS incident cohort, and 23.3 in the HF incident cohort).
A previously published cost-effectiveness model was used to estimate value-based price range for evolocumab based on this real-world CVD burdenCitation23. Of note, the National Institute for Health and Care Excellence issued a positive recommendation on the use of evolocumab in England and Wales informed by the cost-effectiveness results from this modelCitation34, the inputs for which we adapted for the present analysis. Accounting for input parameter uncertainties, the expected value-based price (CrI) identified for evolocumab in this study varied between $11,990 for patients with LDL-C ≥70 mg/dL and $16,856 for patients with LDL-C ≥100 mg/dL, which is higher than the current annual cost ($14,100) as long as the payer discount off list price is greater than 20%. Although the discount rates with individual payers are often confidential, a recent report from Credit Suisse indicated an average discount off list price of 29% in branded pharmaceuticalsCitation35. Of note, the present study assessed the cost-effectiveness of evolocumab in the US payer context. Therefore, these results cannot be directly translated to different settings where model inputs, such as the healthcare costs, LDL-C threshold, and cost-effectiveness thresholds could be different. The cost-effectiveness of evolocumab should be assessed according to the appropriate context.
In addition to the published evolocumab-specific cost-effectiveness model, there are two other recent publications that estimate the value of PCSK9 inhibitors in the US contextCitation36,Citation37. The analysis by Jena et al.Citation36 found that the social value of PCSK9 inhibitors would range from $3.4 trillion to $5.1 trillion (1.9–2.8 million deaths averted) or $12,000 to $17,000 per patient-year of treatment, which is largely aligned with the present value-based price range analysis. In contrast, Kazi et al.Citation37 concluded that the current annual cost of PCSK9 inhibitors did not provide net monetary benefit in the US context and was not at value-based price ($6,904) assuming a WTPT of $150,000 per QALY gained. One notable difference among the three analyses is the baseline CV event rates. Kazi et al.Citation37 used rates that are comparable with rates observed in statin trials. The present analysis and that of Jena et al.Citation36 used CV event rates derived from large real-world databases. For example, the rates of MI- and CV-related death per 100 patient-years in an ASCVD population were, respectively, 0.99 and 0.88 over 5 years in the report by Kazi et al.Citation37, and 2.8 and 2.4 over 7 years in the report by Jena et al.Citation36. The use of data from routine clinical practice (as done in this analysis) could help capture the real-world CV burden, which is an important input to the cost-effectiveness analysis. However, it is important to note that this analysis used LDL-C reduction achieved in the randomized studies of evolocumab, which may differ from what occurs in the real-world setting.
US databases have important limitations, including a lack of continuity between payers’ data, including a lack of continuity with limited follow-up for individuals. This limitation makes most US databases unsuitable for capturing overall CV burden over time. US databases also lack information on CV mortality—a critical component of CV disease burden. The UK CPRD was, therefore, used. CV event risk in the CPRD patient population was generally similar to that reported in other real-world studies. The proportion of patients with ≥1 subsequent CV event (33% in the high-risk ASCVD cohort) was similar to other studies. Two US claims database analysesCitation6,Citation38 reported a 43% subsequent CV event risk over 2–3 years of follow-up for patients with a history of MI, stroke, UA, or revascularization. CV event risk was 49% over 2 years among a Swedish cohort of patients with a history of CVDCitation39. The proportion of patients with ≥1 subsequent CV event in the ACS and IS incident cohorts (40% and 27% over 2.8 years) is slightly higher than other studies. Subsequent CV event risks were 21% for recent ACS patients and 15% for recent stroke patients in 36 months in a US claims database studyCitation5. Subsequent CV event risks among recent ACS and stroke patients were 33% in 2 yearsCitation39 and 20.5% in 1 yearCitation40 in two studies of Swedish patients. A recent study using the Japan Medical Data Center database found a CV event risk of 20.4% (35.2 per 100 patient-years) among recent ACS and stroke patientsCitation41. However, each of the studies differed from the current analysis in length of follow-up, inclusion/exclusion criteria for the study population, and definition of CV event outcomes, which may affect the comparison of CV event risks. In addition, in the current cost-effectiveness analysis, to help further mitigate the differences in the UK patient population compared to US patients, patients’ age, LDL-C level, and history of CV events were based on the FOURIER population, and the baseline CV event rates were adjusted accordingly.
CV event rates in the present study, along with rates reported in other real-world studies, are higher than those reported for randomized clinical trials of secondary prevention evaluating high-intensity statin therapy, indicating that the CV burden is higher in the real-world setting than the burden among selected trial participants in the well-controlled, high-adherence clinical trial setting. The Treating to New Targets (TNT) clinical trial showed that 8.7% of patients with clinically evident CHD and LDL-C ≤ 130 mg/dL treated with 80 mg of atorvastatin over a median follow-up of 4.9 years had a subsequent CV eventCitation42. The CTTC meta-analyses reported CV event per year estimates for patients with CHD on statin/more statin of 4.5% per annumCitation8, which is about one-half to one-third of CV event rates from the high-risk ASCVD cohort in the present study.
Limitations to the current analysis include that the study population was restricted to patients with high-intensity statin use, and rates of CV events may not be generalizable to patients on non-statin lipid-lowering therapies or on low- or moderate-intensity statins. Furthermore, an optimal use of the SOC could help identify appropriate patients for evolocumab. However, many patients have to discontinue or modify highly effective treatments (e.g. high-intensity statin + ezetimibe), due to a variety of circumstances, including statin-related muscle symptoms. Additionally a broader use of ezetimibe is limited in clinical practice in part by its moderate LDL-C reduction and its modest impact on acute CV events in the IMPROVE-IT trialCitation43. We did not analyze information on prescription fills, so the present analysis did not take compliance into account. Differences in US and UK treatment guidelines may influence the rates of CABG and PCI between the two countriesCitation44. Notably, the HES data that we analyzed covered inpatient stays only, and many PCI procedures are performed in the outpatient setting in the US, so actual US primary composite outcome rates may be higherCitation44. Also, revascularization rates are substantially higher in the US compared with the UKCitation45,Citation46. As a result, the value-based price for evolocumab could have been under-estimated. Furthermore, in the model, we adjusted the observed rates by key patient characteristics (e.g. age, LDL-C, and history of CV events). However, other differences between UK and US patients may affect the underlying CV event rates used in the model. Finally, because of the current absence of outcomes data with PCSK9 inhibitors, CV event rate ratios per 1 mmol/L reduction in LDL-C were based on the CTTC meta-analyses of statin trialsCitation8,Citation9. Alternative evidence sources could be explored, such as the analysis reported by Law et al.Citation47, which found an increasing temporal pattern of CV event reduction per 1 mmol/L of LDL-C lowering. The CTTC relationship incorporates many studies of different durations, therefore capturing in a single estimate such temporal patterns. The 36% relative risk reduction that Law et al. observed after year 5 is actually beyond our more conservative assumptions and would improve the cost-effectiveness results. The CV outcome data from the FOURIER trial is expected to be released in early 2017 and will further inform the treatment benefit and CV risk reduction and, subsequently, the cost-effectiveness of evolocumab.
Conclusions
In this real-world analysis of high-risk patients with clinically evident CVD and elevated LDL-C despite statin therapy, a substantial CV event burden was observed over time. Using the observed CVD burden in CPRD and the CTTC relationship, the cost-effectiveness analysis showed that, accounting for input parameter uncertainties, the expected value-based price for evolocumab is higher than the current annual cost ($14,100), as long as the payer discount off list price is greater than 20%.
Transparency
Declaration of funding
This study was sponsored by Amgen Inc.
Declaration of financial/other interests
PPT is a member of the speakers’ bureau, is a consultant for, and has received speaking fees from Amarin, Amgen Inc., Kowa, Merck, and Regeneron-Sanofi; has received consulting fees from AstraZeneca and Gemphire Therapeutics Inc. MD analyzed data for this analysis under contract with Amgen Inc. GV, YQ, and AL are employees and stockholders in Amgen Inc. AB is now a former employee of Amgen. JPJ is an employee of Precision Health Economics, which received funding associated with the involvement in this study. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental material
Download MS Word (45.1 KB)Acknowledgments
Tim Peoples, MA, ELS, CMPP, of Amgen Inc. provided medical writing support. We thank Peter Neumann, MD, for an early review of the analysis.
Note
Notes
1 The sub-group of patients in the high-risk ASCVD cohort with PAD was used as an entry health state in the economic model, reflecting the FOURIER eligibility criteria.
References
- Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2999-3058. DOI:10.1093/eurheartj/ehw272
- Smith SC Jr, Collins A, Ferrari R, et al. Our time: a call to save preventable death from cardiovascular disease (heart disease and stroke). Eur Heart J 2012;33:2910-16
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation 2015;131:e29-e322
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1-S45
- Bonafede MM, Johnson BH, Richhariya A, et al. Medical costs associated with cardiovascular events among high-risk patients with hyperlipidemia. Clinicoecon Outcomes Res 2015;7:337-45
- Punekar RS, Fox KM, Richhariya A, et al. Burden of first and recurrent cardiovascular events among patients with hyperlipidemia. Clin Cardiol 2015;38:483-91
- Wadhera RK, Steen DL, Khan I, et al. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J Clin Lipidol 2016;10:472-89
- Cholesterol Treatment Trialists (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81
- Cholesterol Treatment Trialists (CTT) Collaboration, Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015;385:1397-405
- Aggarwal J, Patel J, Yu J, et al. LDL-C goal achievement after adding or switching to ezetimibe in patients with clinical atherosclerotic cardiovascular disease or probable HeFH [abstract]. J Manag Care Spec Pharm 2016;22:S86
- Gouni-Berthold I, Descamps OS, Fraass U, et al. Systematic review of published phase 3 data on anti-PCSK9 monoclonal antibodies in patients with hypercholesterolaemia. Br J Clin Pharmacol 2016;82:1412-43
- Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA 2012;308:2497-506
- Koren MJ, Lundqvist P, Bolognese M, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2531-40
- Robinson JG, Nedergaard BS, Rogers WJ, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014;311:1870-83
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331-40
- Blom DJ, Djedjos CS, Monsalvo ML, et al. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52-week, phase 3, double-blind, randomized, placebo-controlled DESCARTES study. Circ Res 2015;117:731-41
- Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580-90
- Hirayama A, Honarpour N, Yoshida M, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J 2014;78:1073-82
- Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J 2016;173:94-101
- Tyler LS, Cole SW, May JR, et al. ASHP guidelines on the pharmacy and therapeutics committee and the formulary system. Am J Health Syst Pharm 2008;65:1272-83
- Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention. National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640-5
- Mearns BM. Dyslipidaemia: 1-year results from OSLER trial of anti-PCSK9 monoclonal antibody evolocumab. Nat Rev Cardiol 2014;11:63
- Gandra SR, Villa G, Fonarow GC, et al. Cost-effectiveness of LDL-C lowering with evolocumab in patients with high cardiovascular risk in the United States. Clin Cardiol 2016;39:313-20
- Cholesterol Treatment Trialists (CTT) Collaborators, Mihaylova B, Emberson J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581-90
- Fox KM, Wang L, Gandra SR, et al. Long-term economic burden associated with cardiovascular events among high-risk patients with hyperlipidemia. Value Health 2015;18:A139-40
- Lipinski MJ, Cauthen CA, Biondi-Zoccai GG, et al. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am J Cardiol 2009;104:1708-16
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093-103
- Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 2014;129:2329-45
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- CVS/caremark. Repatha (evolocumab) FEP Clinical Criteria [Internet]. Lee's Summit, MO: CVS/caremark. http://www.caremark.com/portal/asset/FEP_Criteria_Repatha.pdf. Accessed November 14, 2016
- Prime Therapeutics LLC. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors prior authorization with quantity limit criteria program. Summary [Internet]. St. Paul, MN: Blue Cross and Blue Shield of Minnesota; updated May 2015. https://www.bluecrossmn.com/healthy/public/portalcomponents/PublicContentServlet?contentId=P11GA_13569744. Accessed November 14, 2016
- United HealthCare Services Inc. Prior authorization/medical necessity, program 2016 P 2063-5: RepathaTM (evolocumab) [Internet]. Minnetonka, MN: UnitedHealth Group, Incorporated; updated Aug 2016. https://www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Assets/ProviderStaticFiles/ProviderStaticFilesPdf/Tools%20and%20Resources/Pharmacy%20Resources/Clinical%20Programs/PA_Med_Nec_Repatha.pdf. Accessed November 14, 2016
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006
- National Institute for Health and Care Excellence (NICE). Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia. Technology appraisal guidance [TA394] [Internet]. London, UK: NICE; updated June 2016. https://www.nice.org.uk/guidance/TA394/. Accessed October 17, 2016
- Credit Suisse. Equity research, major pharmaceuticals: Global pharma [Internet]. Zürich, Switzerland: Credit Suisse Group AG; updated May 2015. https://doc.research-and-analytics.csfb.com/docView?language=ENG&format=PDF&source_id=csplusresearchcp&document_id=1047911451&serialid=hfXhzuK6sK6xRrBsMZzLqZ96QTl7v2YM0kkwWJRGRVU%3D. Accessed October 17, 2016
- Jena AB, Blumenthal DM, Stevens W, et al. Value of improved lipid control in patients at high risk for adverse cardiac events. Am J Manag Care 2016;22:e199-e207
- Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743-53
- Henk HJ, Paoli CJ, Gandra SR. A retrospective study to examine healthcare costs related to cardiovascular events in individuals with hyperlipidemia. Adv Ther 2015;32:1104-16
- Hallberg S, Gandra SR, Fox KM, et al. Healthcare costs associated with cardiovascular events in patients with hyperlipidemia or prior cardiovascular events: estimates from Swedish population-based register data. Eur J Health Econ 2016;17:591-601
- Jernberg T, Hasvold P, Henriksson M, et al. Cardiovascular risk in post-myocardial infarction patients: nationwide real world data demonstrate the importance of a long-term perspective. Eur Heart J 2015;36:1163-70
- Davis KL, Meyers J, Zhao Z, et al. High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population: Prevalence, cardiovascular event rates, and costs. J Atheroscler Thromb 2015;22:1287-304
- LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425-35
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387-97
- Kolh P, Kurlansky P, Cremer J, et al. Transatlantic Editorial: a comparison between European and North American guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2016;49:1307-17
- Kaul S, Chang WC, Lincoff AM, et al. Optimizing use of revascularization and clinical outcomes in ST-elevation myocardial infarction: insights from the GUSTO-V trial. Eur Heart J 2006;27:1198-206
- Gusmano M, Allin S. Health care for older persons in England and the United States: a contrast of systems and values. J Health Polit Policy Law 2011;36:89-118
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423