Abstract
Background: Fidaxomicin is a macrocyclic antibiotic with proven efficacy against Clostridium difficile infection (CDI) in adults. It was licensed in France in 2012, but, due to higher acquisition costs compared with existing treatments, healthcare providers require information on its cost/benefit profile.
Objective: To compare healthcare costs and health outcomes of fidaxomicin and vancomycin, as reference treatment for CDI.
Methods: A Markov model was used to simulate the treatment pathway, over 1 year, of adult patients with CDI receiving fidaxomicin or vancomycin. Several patient sub-groups (severe CDI; recurrent CDI; concomitant antibiotics; cancer; renal failure; elderly) were evaluated. Cost-effectiveness was analyzed based on cure and recurrence rates derived from published randomized clinical trials comparing fidaxomicin and vancomycin, and costs calculated from the payer perspective using French hospitalization data and drug cost databases. Model outputs included costs in euros (reference year 2014) and health outcomes (recurrence; sustained cure rates). Alternative scenario and sensitivity analyses were performed using data from other clinical trials in CDI, including one conducted in real-life clinical practice in France.
Results: Drug acquisition costs were €1,692 higher in fidaxomicin-treated patients, but this was offset by the lower hospitalization costs with fidaxomicin, which were reduced by €1,722. The reduction in the cost of hospitalization was driven by the significantly lower number of recurrences in fidaxomicin-treated patients, offsetting the acquisition cost of fidaxomicin in all sub-groups except recurrent CDI and concomitant antibiotics.
Conclusion: This study demonstrated that, despite higher acquisition costs, the lower recurrence rate with fidaxomicin resulted in cost savings or low incremental costs compared with vancomycin.
Introduction
Clostridium difficile infection (CDI) is the main cause of antimicrobial- and healthcare-associated diarrheaCitation1, and represents a substantial burden to both patients and healthcare systems. In the US, it was estimated that C. difficile was responsible for almost half a million infections in 2011Citation2. In addition to the significant morbidity associated with infectionCitation3, it also carries a substantial risk of mortality. In the US in 2011, CDI was associated with an estimated 29,000 deaths, corresponding to a mortality rate of ∼6%Citation2. In Europe, the CDI-associated mortality rate varies between countries, ranging from 2–40%Citation4.
Until recently, treatment options for CDI were limited mainly to metronidazole and vancomycin. Oral metronidazole is recommended in non-severe CDI, while oral vancomycin is recommended for patients with severe diseaseCitation5,Citation6. Although the treatments are efficacious, both are associated with high recurrence rates of 20–25%Citation7–9. The risk of recurrence is particularly high in certain sub-groups of patients, including those with cancerCitation10, renal impairmentCitation11,Citation12, previous recurrenceCitation11,Citation13, or severe CDICitation14, patients aged over 65 yearsCitation11, and those taking concomitant antibioticsCitation11,Citation15. Recurrent CDI is associated with a longer hospital stay, greater healthcare utilization, and increased mortality compared with non-recurrent diseaseCitation16–18.
Fidaxomicin is a first-in-class macrocyclic antibiotic with targeted bactericidal activity against C. difficileCitation19. Compared with metronidazole and vancomycin, it has minimal effects on the normal intestinal microbiome, thereby reducing the risk of C. difficile colonization, re-infection, and recurrenceCitation19–21. Indeed, while two randomized controlled trials in patients with CDI demonstrated that fidaxomicin was associated with similar clinical cure rates to vancomycin, recurrence rates were significantly lower and, hence, sustained cure rates were significantly higherCitation9,Citation22. There are no head-to-head comparative trials with fidaxomicin and metronidazole, but an indirect treatment comparison demonstrated that fidaxomicin also provides improved sustained cure rates compared with metronidazoleCitation23.
According to recent guidelines from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), there is strong support for first-line use of metronidazole in non-severe CDI and vancomycin in severe CDICitation6. There is moderate support for the first-line use of fidaxomicin in all patient groups, and the use of either fidaxomicin or vancomycin in those with first recurrence or at risk of recurrent CDI and in those with multiple recurrencesCitation6. The guidelines also highlight the reduced recurrence rate for fidaxomicin vs vancomycin observed in the clinical studies described aboveCitation6.
Fidaxomicin is associated with higher acquisition costs than vancomycin. In France, the total cost for a 10-day course of fidaxomicin is €1,387, compared with €92 for vancomycinCitation24. In 2014, fidaxomicin was added to the Tarification à l’Activité (T2A) tariff list in France. This list includes expensive hospital-only drugs that are charged to health insurersCitation25.
With increasing pressure to control healthcare costs, economic evaluation—and, more specifically, cost-effectiveness analysis—has been recognized as a useful approach to inform decisions related to the provision of new treatments in France. The Haute Autorité de Santé (HAS) introduced guidelines for economic evaluation in 2011Citation26, and the Inspection Générale des Affaire Sanitaires (IGAS) recently recommended wider use of economic evaluation of healthcare interventionsCitation27. Although four fidaxomicin cost-effectiveness studies have been undertaken elsewhereCitation28–31, there are currently no data available on the cost-effectiveness of fidaxomicin in France.
This study was undertaken to compare the healthcare costs and health outcomes attributable to fidaxomicin and vancomycin in treating adult patients with CDI in French clinical practice.
Methods
Model structure
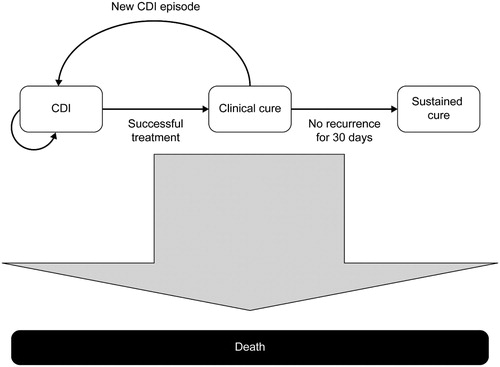
A Markov model, programmed in Excel (Microsoft, Redmond, WA), simulated the treatment pathway for patients with CDI receiving first-line, orally administered treatment with either fidaxomicin (200 mg twice daily) or vancomycin (125 mg four times daily) for 10 days (). All patients entered the model in the CDI state and received first-line treatment. If “clinical cure” was not achieved, vancomycin (125 mg four times daily for 10 days) was given second-line, irrespective of the first-line treatment; patients not cured after second-line treatment were assumed to remain in hospital until cured.
The definition of “clinical cure” was based on that used in clinical trials: resolution of diarrhea (≤3 unformed bowel movements/day for 2 consecutive days), maintained for the duration of therapy and with no further requirement for treatment up to 2 days after the end of therapyCitation9,Citation22. One of the modeling assumptions was that a patient with no recurrence within 30 days of clinical cure moved to the “sustained cure” state, whereupon they were assumed to be at no further risk of infection. Patients with recurrent CDI moved back into the CDI state, where they were reassigned to their first-line treatment and were assumed to follow the same treatment pathway used to treat the original infection. At the third recurrence, which would occur in only a small proportion of patients, patients received rescue treatment, which was assumed to have a 100% cure rate; this was to prevent very small proportions of the population (<0.0001%) continuing in the model ad finitum. No specific treatment costs for rescue medication were incurred, but patients were assumed to have a prolongation of hospital stay or be re-hospitalized (in the same way as for first and second recurrences) until cured.
Transition from the CDI state to the clinical cure state was possible after 10 days, in line with the duration of treatment. However, a 1-day cycle length was used to assess recurrence. Based on previous modelsCitation30,Citation32, a 1-year time horizon was adopted to capture all clinical costs and benefits associated with treatment.
Patient groups
The population considered in the base-case analysis was adults with CDI, of whom 83.9% had an initial infection and 16.1% a recurrent infection (based on data from the clinical trials used to inform efficacy estimatesCitation9,Citation22). Additional analyses were performed for six patient sub-groups at high risk of recurrenceCitation10–15 and for which clinical trial data are available for fidaxomicin vs vancomycin: severe CDI (≥10 unformed stools/day or white blood cell count ≥15,001/mm3); first CDI recurrence (second CDI episode); cancer; age ≥65 years; renal failure; and concomitant antibioticsCitation9,Citation10,Citation12,Citation22,Citation33.
Model inputs
Clinical
Clinical cure and recurrence rates for all patients and sub-groups were derived from the two published fidaxomicin trialsCitation9,Citation22, as provided in . All rates were converted into probabilities using standard formulaeCitation34.
Table 1. Efficacy inputs as 10-day probabilities used in the base-case model and sub-group analyses derived using data from published studiesCitation9,Citation10,Citation12,Citation22,Citation33.
The annual all-cause mortality rate (1.26%), derived from French Life TablesCitation35, was applied to all patients who achieved sustained cure in the model. The 30-day CDI-attributable mortality rate was 3.0% for the base-case analysisCitation36, and 8.0% in the sensitivity analysisCitation37. For the sub-group analyses, CDI-attributable mortality rates were 8.6% for elderly (based on the weighted average of data from patients aged ≥61 yearsCitation37) and 19.7% for patients with cancerCitation38. In the absence of specific evidence of increased mortality attributable to CDI in the other sub-groups, the rate was assumed equal to the base-case.
Economic
Costs in Euros (2014 figures) were calculated from the French collective payer perspective (i.e. all direct healthcare costs, whether paid by patients, national or private insurance). The excess costs of hospitalization due to CDI (€9,024) or CDI recurrence (€10,093) were derived from a large retrospective study of French hospitalization dataCitation39. For each stay, additional costs attributable to CDI were estimated by comparing with age- and sex-matched controls without CDI extracted from the national Diagnostic Related Groups (DRG) database. When CDI was the primary diagnosis, full costs of stay were used. Drug acquisition costs for a 10-day course (fidaxomicin, €1,387; vancomycin, €91.8) and the cost of a general practitioner visit (€23) were derived from a French insurance databaseCitation24,Citation40. The acquisition cost of vancomycin was based on the i.v. formulation (administered via the oral route). No specific treatment costs for rescue medication were incurred, given the very small proportion of patients requiring this treatment. Nevertheless, it was considered to be a conservative assumption, because more patients in the vancomycin arm of the model would be expected to require rescue treatment in view of the higher recurrence rate associated with vancomycin.
Model outputs
The model outputs included cost and health outcomes (recurrences/re-infections within 30 days; sustained cure rates) in all patients and in the pre-determined sub-groups. The breakdown of costs between treatment and hospitalization associated with the initial infection and recurrences, as well as total costs over 12 months, were calculated for both treatment pathways. As the acquisition cost of fidaxomicin is not included in the T2A tariff, treatment costs were reported separately from hospitalization costs; for vancomycin, the cost of treatment was included within hospitalization costs. Incremental cost-effectiveness ratios (ICERs) were expressed as the cost per CDI episode avoided for fidaxomicin vs vancomycin.
Sensitivity analyses
Scenario analyses were performed using recurrence data from two alternative data sourcesCitation13,Citation39, which report lower recurrence rates than those provided in the two clinical studies used in the base-case analysisCitation9,Citation22. In ECODIFCitation39, a retrospective real-world study that was conducted in French acute care hospitals before fidaxomicin was available, the estimated rate of recurrence was 12%. However, as long-term care hospitals were excluded from this study, it is likely that elderly people are under-represented in the sample. For this reason, the rate of 12% is likely to under-estimate the true recurrence rate in France, and, as such, was considered to represent a lower bound. Fekety et al.Citation13 reported a recurrence rate of 20%, based on data from nine clinical studies of metronidazole or vancomycin. Hence, for the sensitivity analyses, recurrence rates for vancomycin were set at 12% (lower bound, based on real-world ECODIF studyCitation39) and 20% (upper bound, based on Fekety et al.Citation13 data). For fidaxomicin recurrence rates, the relative difference between fidaxomicin and vancomycin, as observed in clinical trials, was assumed to be maintained. These sensitivity analyses were conducted for the “all CDI patients” group only, as data for patient sub-groups were not available.
Deterministic one-way sensitivity analyses were performed to assess the sensitivity of the model to uncertainty in individual parameters and assumptions, and to identify those with the greatest impact. All model inputs were varied within 95% confidence intervals (CIs), obtained from assigned statistical distributions (beta-distribution for probabilities, and gamma-distribution for costs).
A probabilistic sensitivity analysis (PSA) was undertaken, in which all model parameters were varied simultaneously, in a Monte Carlo simulation, based on random draws from the assigned statistical distributions (shown in ). Using this method, 95% CIs around incremental costs and outcomes were estimated.
Table 2. Parameter inputs and distributions used in deterministic and probabilistic sensitivity analysis.
Results
Base-case scenario
The base-case analysis reported the cost-effectiveness of fidaxomcin vs vancomycin for the group “All CDI patients”. For all CDI patients, the costs per patient, from the French collective payer perspective, for the management of the initial infection and recurrences are summarized in . The reported total costs for the “all CDI group” for the initial infection were greater for fidaxomicin than vancomycin (+€1,128), but lower for recurrent infections for fidaxomicin (–€1,158), despite the higher drug acquisition costs. This reflects the reduced costs for hospitalization associated with fidaxomicin use, due to the avoidance of recurrences. Overall (taking into account the cost of managing both the initial episode and recurrences), the fidaxomicin acquisition costs (€1,692) were fully offset by the reduced cost of hospitalization (–€1,722).
Table 3. Cost breakdown per patient by treatment strategy for the base-case analysis (all patients).
For the “all CDI group”, fidaxomicin reduced the predicted number of recurrences/re-infections compared with vancomycin () based on the model results over a 12-month time horizon. In this group, fidaxomicin was dominant compared with vancomycin for the incremental cost per CDI episode avoided.
Table 4. Total costs and health outcomes at 12 months by treatment strategy, base case and patient sub-groups, reported as deterministic value (probabilistic mean [95% CI]).
The incremental costs per CDI episode avoided with fidaxomicin vs vancomycin were €2,107 for patients with first recurrence and €6,040 for patients taking concomitant antibiotics. Incremental costs are not reported for the other sub-groups (severe CDI, cancer, renal failure, elderly), as fidaxomicin was dominant in these groups (less expensive and more effective). As a result of the probabilistic modeling, the proportion of patients who experienced a recurrence () is higher than the recurrence reported in the fidaxomicin clinical trials. The results () include any second or third recurrences that the model estimates will occur over a 12-month time horizon, based on the rates of recurrence seen in the 30-day follow-up of the trials.
Sensitivity analysis
In the sensitivity analysis using the baseline recurrence rates from the ECODIF studyCitation39, predicted total costs were €11,784 for fidaxomicin and €11,160 for vancomycin. Over 12 months, fidaxomicin reduced the predicted rate of recurrences compared with vancomycin (11% vs 20%); the incremental cost per CDI episode avoided was €6,933 for all patients. When applying the upper bound estimate of recurrence rate of 20%Citation13, the incremental cost per CDI episode avoided was €2,333.
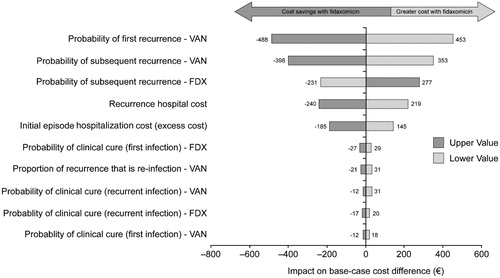
Results of deterministic sensitivity analyses, presented as a tornado chart (), show that the key drivers underlying total cost variations were the probabilities of recurrence in the two treatment arms and the unit cost of recurrence.
Figure 2. Impact of parameters and assumptions on cost difference between fidaxomicin (FDX) and vancomycin (VAN).
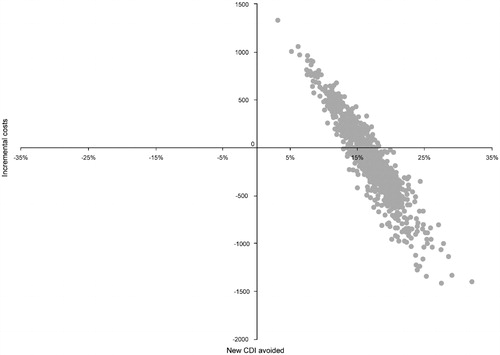
The scatter plot of PSA results () shows that there is a 58% chance of the fidaxomicin treatment strategy being cost-saving compared with vancomycin. The shape of the scatter plot indicates a linear relationship between efficacy and cost; that is, the greater the number of CDI cases avoided, the lower the incremental cost.
Discussion and conclusions
This study was undertaken to provide healthcare professionals in France with a cost-effectiveness analysis of fidaxomicin vs vancomycin in the treatment of CDI. The study showed that the higher acquisition costs for fidaxomicin (+€1,692) were completely offset by the lower hospitalization costs (–€1,722) due to avoided recurrences. Similar results were observed in most of the sub-groups at high risk of recurrence.
Our findings are consistent with those of a recently published cost-effectiveness analysis of fidaxomicin vs vancomycin in the treatment of CDI in ScotlandCitation30. Cost-effectiveness was evaluated in two sub-groups: patients with severe CDI and patients with a first recurrence. Total costs in patients with a first recurrence were similar for fidaxomicin and vancomycin (£16,535 and £16,926, respectively, in the Scottish model vs €14,084 and €13,810 in our study), and in patients with severe CDI (£14,515 and £14,344, respectively, in the Scottish model vs €12,348 and €12,982 in our study). However, unit costs are not directly comparable between the two models, as the Scottish model incorporated costs for managing severe complications of CDI, whereas the French model did not. This may explain why the hospitalization costs were higher in Scotland than in France.
In another cost-effectiveness analysis conducted from a US payer perspective, the total cost per patient was $1,117 higher in patients treated with fidaxomicin compared with vancomycin ($12,306 vs $13,422), resulting in an ICER of $67,576 per quality-adjusted life-year (QALY) gainedCitation29. There are several differences between our model and the US model; for example, the US study used a 23-year time-horizon (compared with 1 year in our study). However, the key reason for the differing results lies in the unit costs: the cost of a 10-day course of fidaxomicin treatment was higher in the US compared with France ($2,800, or €2,200 in 2014 euros, vs €1,387 in France), and the hospitalization cost was lower in the US ($10,793, or €8,462, vs €10,093 for recurrence in France). Therefore, the proportion of drug acquisition costs offset by hospitalization costs was lower in the US. Nevertheless, fidaxomicin was shown to be cost-effective in the US, as the ICER ($67,576 per QALY) was lower than the willingness-to-pay threshold of $100,000Citation29.
Most recently, a cost-effectiveness analysis of fidaxomicin for the treatment of CDI was conducted in GermanyCitation31. Fidaxomicin was compared with vancomycin for treatment of patient sub-groups with CDI at increased risk of recurrence (patients with ≥1 recurrence; with severe CDI; with cancer; with renal failure; those receiving concomitant antibiotics; those aged ≥65 years). Despite higher drug acquisition costs, fidaxomicin was dominant in the cancer sub-group (less costly and more effective) and cost-effective in the other sub-groups, with incremental cost-effectiveness ratios vs vancomycin ranging from €26,900 to €44,500Citation31.
The results of the deterministic sensitivity analysis demonstrated that one of the key drivers in the current model was the recurrence rate, confirming that the clinical efficacy of fidaxomicin (in terms of reduced recurrence rates vs vancomycin) has a direct impact on its cost-effectiveness via the reduction in hospitalization rates. However, it is important to note that the benefits of avoiding recurrences are not limited to reduced hospitalization costs; they also include avoidance of the detrimental effects of CDI on quality-of-lifeCitation41 and CDI-associated mortality, which is greater in recurrent vs non-recurrent diseaseCitation17,Citation37. Furthermore, CDI in general, and CDI recurrences in particular, can disrupt the treatment strategy for a patient admitted to hospital for another reason, leading to, for example, postponement of an intervention; this adds to the impact of disease on quality-of-life and creates inefficiencies in healthcare servicesCitation42. All of these aspects should be considered when assessing the value of a recurrence avoided. Furthermore, loss of productivity, which was not accounted for in the current model, can also have an impact on the overall cost of managing CDI. However, most patients with CDI are over 60 years of ageCitation43, so this is likely to be less of an issue than a condition affecting younger subjects. Nevertheless, its inclusion would likely favor fidaxomicin, given the lower rate of recurrence vs vancomycin; indeed, it has been shown, using a questionnaire developed specifically to evaluate quality-of-life in patients with CDI, that recurrent infection has a significantly greater impact on both physical and mental functioning than initial infectionCitation44. Consequently, the current model provides a conservative estimate of the cost-effectiveness of fidaxomicin.
Given the importance of recurrence in driving overall costs, the results are sensitive to any variations in the recurrence rates used. In the current model, the rates were derived from two large phase III studies comparing fidaxomicin and vancomycinCitation9,Citation22. Sensitivity analyses were, therefore, conducted using data from ECODIF, a real-life study of CDI conducted in FranceCitation39. Based on these data (recurrence rate of 12%), total costs associated with CDI were higher with the fidaxomicin strategy, with an incremental cost of €6,933 per CDI episode avoided. However, the recurrence rate from ECODIF is likely to be an under-estimate, as it represents patients treated in an acute setting only; those in rehabilitation and long-term care facilities were not included. Based on an intermediate estimate of recurrence rates (20%Citation13), which is likely to be more realistic than the rate from ECODIF, the estimated ICER per episode avoided was €2,333.
Metronidazole is frequently used as first-line treatment of CDI in France. However, vancomycin was used in the current model because direct comparative data are available vs fidaxomicin. Vancomycin is associated with improved clinical outcomes compared with metronidazole, particularly in those with severe diseaseCitation5,Citation45–47, but there are no head-to-head studies comparing fidaxomicin vs metronidazole. Nevertheless, an indirect treatment comparison demonstrated that fidaxomicin improved clinical outcomes vs metronidazole, including a significant 58% reduction in recurrenceCitation23. It is also notable that the recent ESCMID guidelines only recommend metronidazole for first-line use in patients with non-severe CDICitation6. This is echoed by recommendations from Public Health England, who note the relatively high failure rates of metronidazole in severe CDICitation48.
In addition to the rate of recurrence, the cost of recurrence was one of the main sources of variability in the model results. This cost was based on payments received by hospitals for patients with recurrent CDI, which reflect the cost of a hospital stay attributable to the CDI infection. However, these payments may be lower than the true costs of recurrence associated with CDI, as costs accrued by patients upon discharge are not accounted for; for example, subsequent recurrence requiring treatment in long-term care facilities would not be included in the cost of recurrence. This may, therefore, have led to an under-estimation of savings in hospitalization costs associated with fidaxomicin.
In conclusion, this study demonstrated that, despite higher acquisition costs, fidaxomicin is associated with cost savings or low incremental costs compared with vancomycin, both in the overall population and in patient sub-groups at high risk of recurrence. This is driven by a lower recurrence rate and, therefore, reduced hospitalization.
Transparency
Declaration of funding
This study was initiated and funded by Astellas Pharma, Inc.
Declaration of financial/other relationships
MW is a full employee of Astellas EMEA. AD, ALM, and PT have received lecture or advisory board honoraria or research grants from Astellas. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgments
We thank Julia Donnelly (on behalf of Creativ-Ceutical) and Nicky French (on behalf of Bioscript Medical) for medical writing support, which was funded by Astellas Pharma, Inc.
References
- Knight DR, Elliott B, Chang BJ, et al. Diversity and evolution in the genome of Clostridium difficile. Clin Microbiol Rev 2015;28:721-41
- Lessa FC, Winston LG, McDonald LC; Emerging Infections Program C. difficile Surveillance Team. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015;372:2369-70
- Cohen SH, Gerding DN, Johnson S, et al.; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431-55
- Wiegand PN, Nathwani D, Wilcox MH, et al. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect 2012;81:1-14
- Zar FA, Bakkanagari SR, Moorthi KM, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302-7
- Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014;20(Suppl 2):1-26
- Bouza E, Dryden M, Mohammed R, et al.; for the Polymer Alternative for CDAD Treatment (PACT) Investigators. Results of a phase III trial comparing tolevamer, vancomycin and metronidazole in patients with Clostridium difficile-associated diarrhoea. Clin Microbiol Infect 2008;14(Suppl 7):S103-S4
- Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197-205
- Louie TJ, Miller MA, Mullane KM, et al.; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364:422-31
- Cornely OA, Miller MA, Fantin B, et al. Resolution of Clostridium difficile-associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol 2013;31:2493-9
- Surawicz CM, Alexander J. Treatment of refractory and recurrent Clostridium difficile infection. Nat Rev Gastroenterol Hepatol 2011;8:330-9
- Mullane KM, Cornely OA, Crook DW, et al. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. Am J Nephrol 2013;38:1-11
- Fekety R, McFarland LV, Surawicz CM, et al. Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 1997;24:324-33
- Eyre DW, Walker AS, Wyllie D, et al.; Infections in Oxfordshire Research Database. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Clin Infect Dis 2012;55(Suppl 2):S77-87
- Mullane KM, Miller MA, Weiss K, et al. Efficacy of fidaxomicin versus vancomycin as therapy for Clostridium difficile infection in individuals taking concomitant antibiotics for other concurrent infections. Clin Infect Dis 2011;53:440-7
- Bouza E. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 2012;18(Suppl 6):5-12
- Escobar GJ, Baker M, Li SX, et al. Clinical and economic burden of recurrent Clostridium difficile infections - a 10-year retrospective large database analysis. ICAAC, Washington DC, September 5–9, 2014 [Abstract K-362]
- Heimann SM, Vehreschild JJ, Cornely OA, et al. Economic burden of Clostridium difficile associated diarrhoea: a cost-of-illness study from a German tertiary care hospital. Infection 2015;43:707-14
- Chaparro-Rojas F, Mullane KM. Emerging therapies for Clostridium difficile infection – focus on fidaxomicin. Infect Drug Resist 2013;6:41-53
- Tannock GW, Munro K, Taylor C, et al. A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology 2010;156:3354-9
- Louie TJ, Cannon K, Byrne B, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin reexpression and recurrence of CDI. Clin Inf Dis 2012;55(Suppl 2):S132-S42
- Cornely OA, Crook DW, Esposito R, et al.; OPT-80-004 Clinical Study Group. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12:281-9
- Cornely OA, Nathwani D, Ivanescu C, et al. Clinical efficacy of fidaxomicin compared with vancomycin and metronidazole in Clostridium difficile infections: a meta-analysis and indirect treatment comparison. J Antimicrob Chemother 2014;69:2892-2900
- L’Assurance Maladie 2015. Base des médicaments à code UCD, February 1, 2012. http://www.ameli.fr/professionnels-de-sante/directeurs-d-etablissements-de-sante/codage/medicaments/base-des-medicaments-a-code-ucd.php. Accessed June 15, 2015
- ISPOR Global Health Care Systems Road Map 2009. http://www.ispor.org/htaroadmaps/france.asp#1. Accessed June 15, 2015
- Haute autorité de santé. Choix méthodologiques pour l’évaluation économique à la HAS. http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf. Accessed June 15, 2015
- Inspection Générale des Affaires Sociales. Evaluation médico-économique en santé. 2014. http://www.igas.gouv.fr/IMG/pdf/2014-066R_-_Rapport_DEF.pdf. Accessed June 15, 2015
- Bartsch SM, Umscheid CA, Fishman N, et al. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis 2013;57:555-61
- Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health 2013;16:297-304
- Nathwani D, Cornely OA, Van Engen AK, et al. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother 2014;69:2901-12
- Watt M, McCrea C, Johal S, et al. A cost-effectiveness and budget impact analysis of first-line fidaxomicin for patients with Clostridium difficile infection (CDI) in Germany. Infection 2016;44:599-606
- Konijeti GG, Sauk J, Shrime MG, et al. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis 2014;58:1507-14
- Crook DW, Walker SA, Kean Y, et al. Fidaxomicin versus vancomycin for clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Inf Dis 2012;55(Suppl 2):S93-S103
- Briggs A, University of Oxford. Parametric survival models and decision models: relating continuous hazards to discrete-time transition probabilities. 2004. http://www.herc.ox.ac.uk/conferences-and-presentations/presentationfilelinks/abw151004. Accessed June 15, 2015
- French National Life Tables. French national life tables Eurostat. 2014. http://ec.europa.eu/eurostat/web/main/home. Accessed March 16, 2015
- Coignard B, Hébert M, Eckert C, et al. Epidemiological and microbiological characteristics of Clostridium difficile infections, France, 2009: a national, multicentre, prospective survey. Abstracts 20th European Congress of Clinical Microbiology and Infectious Diseases; Vienna, Austria; 10-13 April 2010
- Karas JA, Enoch DA, Aliyu SH. A review of mortality due to Clostridium difficile infection. J Infect 2010;61:1-8
- Yoon YK, Kim MJ, Sohn JW, et al. Predictors of mortality attributable to Clostridium difficile infection in patients with underlying malignancy. Support Care Cancer 2014;22:2039-48
- Le Monnier A, Duburcq A, Zahar J-R, et al., on behalf of the GMC study Group. Hospital cost of Clostridium difficile infection in French acute-care hospitals: the impact of recurrences. J Hosp Infect 2015;91:117-22
- L’Assurance Maladie. Tarifs conventionnels des médecins généralistes en France métropolitaine, 27 April 2015. http://www.ameli.fr/professionnels-de-sante/medecins/votre-convention/tarifs/tarifs-conventionnels-des-medecins-generalistes/tarifs-des-medecins-generalistes-en-metropole.php. Accessed June 15, 2015
- Guillemin I, Marrel A, Lambert J, et al. Patients’ experience and perception of hospital-treated Clostridium difficile infections: a qualitative study. Patient 2014;7:97-105
- Hautmann MG, Hipp M, Kölbl O. Clostridium difficile-associated diarrhea in radiooncology: an underestimated problem for the feasibility of the radiooncological treatment? Radiat Oncol 2011;6:89
- Di Bella S, Capone A, Musso M, et al. Clostridium difficile infection in the elderly. Infez Med 2013;21:93-102
- Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol 2016;50:631-7
- Le F, Arora V, Shah DN, et al. A real-world evaluation of oral vancomycin for severe Clostridium difficile infection: implications for antibiotic stewardship programs. Pharmacotherapy 2012;32:129-34
- Vardakas KZ, Polyzos KA, Patouni K, et al. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. Int J Antimicrob Agents 2012;40:1-8
- Johnson S, Louie TJ, Gerding DN. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014;59:345-54
- Public Health England. Updated guidance on the management and treatment of Clostridium difficile infection. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/321891/Clostridium_difficile_management_and_treatment.pdf. Accessed July 17, 2015
- Figueroa I, Johnson S, Sambol SP, et al. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin Inf Dis 2012;55(Suppl 2):S104-S9