Abstract
Aims: This study assessed the cost-effectiveness of ezetimibe with statin therapy vs statin monotherapy from a US payer perspective, assuming the impending patent expiration of ezetimibe.
Methods: A Markov-like economic model consisting of 28 distinct health states was used. Model population data were obtained from US linked claims and electronic medical records, with inclusion criteria based on diagnostic guidelines. Inputs came from recent clinical trials, meta-analyses, and cost-effectiveness analyses. The base-case scenario was used to evaluate the cost-effectiveness of adding ezetimibe 10 mg to statin in patients aged 35–74 years with a history of coronary heart disease (CHD) and/or stroke, and with low-density lipoprotein cholesterol (LDL-C) levels ≥70 mg/dL over a lifetime horizon, assuming a 90% price reduction of ezetimibe after 1 year to take into account the impending patent expiration in the second quarter of 2017. Sub-group analyses included patients with LDL-C levels ≥100 mg/dL and patients with diabetes with LDL-C levels ≥70 mg/dL.
Results: The lifetime discounted incremental cost-effectiveness ratio (ICER) for ezetimibe added to statin was $9,149 per quality-adjusted life year (QALY) for the base-case scenario. For patients with LDL-C levels ≥100 mg/dL, the ICER was $839/QALY; for those with diabetes and LDL-C levels ≥70 mg/dL, it was $560/QALY. One-way sensitivity analyses showed that the model was sensitive to changes in cost of ezetimibe, rate reduction of non-fatal CHD, and utility weight for non-fatal CHD in the base-case and sub-group analyses.
Limitations: Indirect costs or treatment discontinuation estimation were not included.
Conclusions: Compared with statin monotherapy, ezetimibe with statin therapy was cost-effective for secondary prevention of CHD and stroke and for primary prevention of these conditions in patients whose LDL-C levels are ≥100 mg/dL and in patients with diabetes, taking into account a 90% cost reduction for ezetimibe.
Introduction
In the US, coronary heart disease (CHD) and stroke represent a significant healthcare burdenCitation1,Citation2. As two of the leading causes of disability and mortality, CHD and stroke account for nearly 31% and 5% of deaths each year, respectivelyCitation1,Citation3,Citation4. It has been estimated that ∼85.6 million adult Americans over 20 years of age (35%) had at least one type of cardiovascular disease (CVD) in 2012Citation1, and nearly 795,000 people in the US experience a new or recurrent stroke every yearCitation1. Once an individual has had a myocardial infarction (MI) or stroke, he or she is more likely to have a recurrent event; each year, there are 305,000 recurrent CHD events (MI or CHD death) and 185,000 recurrent stroke events (ischemic and hemorrhagic)Citation1.
CHD and stroke also result in a substantial economic burden; together they accounted for 15% of total healthcare expenditures in the US from 2011–2012Citation1. In those same years, the average annual combined direct and indirect costs for CHD and stroke were estimated to be $320.1 billion, with $33.6 billion related to stroke aloneCitation1. By the year 2030, total direct medical costs associated with CVD are projected to increase to ∼$818–$918 billionCitation1,Citation3, and costs associated with stroke alone are expected to rise to ∼$184.1 billionCitation1,Citation4.
Treatment guidelines from the American College of Cardiology and American Heart Association (ACC/AHA)Citation5 and the National Lipid Association (NLA)Citation6 recommend lipid-lowering therapies as secondary prevention for patients with a history of CHD or stroke, or other cardiovascular risk factors. The 2013 ACC/AHA guideline recommends that high-intensity statin therapy be used (unless contraindicated) for secondary prevention of CVD among women and men aged ≤75 years with clinical atherosclerotic CVD (ASCVD)Citation5. Moderate-intensity statin therapy is recommended for those with diabetes and low-density lipoprotein cholesterol (LDL-C) level of 70–189 mg/dLCitation5. No specific LDL-C targets are recommended by ACC/AHA for primary or secondary prevention of ASCVDCitation5; however, the NLA guideline does recommend treatment goals for LDL-C according to risk categoryCitation6. The very high-risk category relates to secondary prevention goals for individuals with preexisting ASCVD or diabetes mellitus (type 1 or type 2) and ≥2 risk factors for ASCVD. The NLA recommends that those with ASCVD be treated with moderate- or high-intensity statin therapy to LDL-C levels <100 mg/dL, and that those with diabetes and ASCVD risk factors be treated to levels <70 mg/dLCitation6.
Ezetimibe, a cholesterol-absorption inhibitor, can be prescribed in addition to a statin, and this combination has shown greater efficacy than statins alone for lowering LDL-C levels and improving other lipid parametersCitation7–9. Clinical evidence from the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) has demonstrated that ezetimibe added to simvastatin results in a lower rate of cardiovascular events (a composite endpoint of death from cardiovascular causes, major coronary event, or non-fatal stroke) than simvastatin alone (hazard ratio = 0.936; 95% confidence interval = 0.89–0.99; p = 0.016)Citation7. The ACC updated its guidelines in 2016 in response to these findings and emerging data for other non-statin therapiesCitation10. For secondary prevention in patients who are unable to attain sufficient LDL-C reduction despite taking a high-intensity statin, the ACC recommends that clinicians consider ezetimibe as a first-line, optional, non-statin therapy, in addition to a statinCitation10. Statin-associated muscle symptoms have been reported to affect 7–29% of patients in real-world studiesCitation11; ezetimibe may also be considered in these patients or those who otherwise cannot tolerate a high-intensity statin and require further LDL-C reductionCitation10.
Economic analyses have shown that guideline-based use of lipid-lowering therapies is cost-effective for secondary prevention. A multi-technology assessment of statin monotherapies published by the National Institute for Health and Care Excellence (NICE) in the UK concluded that high-intensity statin therapy is both clinically efficacious and cost-effective compared with low- or moderate-intensity statin therapy or no treatment in patients with CVDCitation12. For secondary prevention of CHD, the incremental cost-effectiveness ratio (ICER) ranged from £2,959 per quality-adjusted life year (QALY) for atorvastatin 20 mg vs no treatment to £3,275 per QALY for atorvastatin 80 mg vs no treatment in the base-case scenarioCitation12. Similarly, a US-based cost-effectiveness study showed that high-dose statin therapy is cost-effective in comparison to conventional-dose therapy in patients with acute coronary syndrome (ACS; ICER of $12,900 per QALY); however, the gains in QALYs were smaller and the ICER was more sensitive to changes in efficacy and cost for stable coronary artery disease (CAD; ICER of $33,400 per QALY)Citation13.
The cost-effectiveness of ezetimibe monotherapy compared with no treatment resulted in an ICER of £23,026 for ezetimibe in the base-case model of patients with a CVD history and high LDL-C levels who had statin intolerance or contraindication. In a univariate sensitivity analysis, the majority of parameter changes (e.g. varying cohorts by age and sex, effectiveness of ezetimibe in lowering LDL-C, relative risk for LDL-C lowering) resulted in an ICER below £30,000 per QALYCitation14 (the maximum cost-effectiveness threshold used by NICE is between £20,000–30,000Citation15). In a separate study, when co-administered with statin therapy, the cost-effectiveness ratios for ezetimibe therapy vs statin-only strategies were below €18,000 per life year gained (LYG) in patients with CHD, and below €30,000 per LYG in patients with diabetes (without CHD) in three European countries (Norway, Spain, and Germany)Citation16.
In a recent comparative economic study by the Institute for Clinical and Economic Review, the impact of adding ezetimibe or the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors alirocumab or evolocumab to statin therapyCitation17 was examined. In the model for secondary prevention among patients with a CVD history and LDL-C level ≥70 mg/dL on background statin therapy, the ICER was $154,000 per QALY for ezetimibe and $414,000 per QALY for PCSK9 inhibitorsCitation17, with drug cost inputs based on the lifetime branded cost of ezetimibe and PCSK9 inhibitors. However, the Institute for Clinical and Economic Review study did not consider the likely cost reductions expected from the patent expiration of ezetimibe in the second quarter of 2017. Therefore, the objective of this study was to model the cost-effectiveness of this combination vs statin monotherapy from a US payer perspective while taking these cost reductions into account.
Methods
Model overview
To compare the cost-effectiveness of an ezetimibe-statin combination vs that of statin monotherapy, an analysis was performed using a Markov-like modeling approach. The analysis took a US payer perspective, and, therefore, did not include indirect costs.
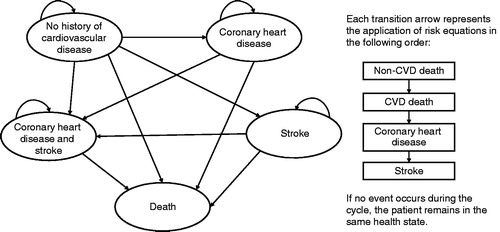
The model consisted of 28 distinct health states within four separate modules based on CVD history (no previous events, prior CHD only, prior stroke only, or prior CVD [both CHD and stroke]). Patients began in one of these modules and would progress to another module(s) if events occurred. Once a patient had a history of CHD and stroke, he or she could not return to any previous module; movement through the model is illustrated in . Within each module, patients could be alive in the acute stage of disease, alive with stable disease, or deceased due to CVD or non-CVD causes. This type of modeling approach was chosen because it allows for the assessment of disease progression over time, enabling simultaneous analysis of treatment cost and efficacy.
Patient-level data used in this model were linked data from IMS Health’s PharMetrics Plus Health Plan Claims database (PMTX+) and IMS’s Electronic Medical Record (EMR) database, which comprises adjudicated claims for more than 150 million unique patients across the US, with diverse representation of geography, employers, payers, providers, and therapeutic areas. Data were extracted for January 1, 2007 through June 30, 2014. To be included in the analysis, patients’ diagnoses, procedures, and lipid profiles had to meet the criteria for one of the three patient cohorts defined by the ACC/AHA clinical guidelinesCitation5, detailed in Supplemental Table 1. Baseline patient-level data are shown in .
Table 1. Characteristics of patients identified from PMTX + claims and EMR databases.
Model inputs
Risk equations for the CHD and CVD modules were derived from the simvastatin arm of the IMPROVE-IT studyCitation7. Piecewise exponential models were generated to account for the differing rates of CVD events in the first year (acute disease) and in subsequent years (stable disease). Variable selection was based on the CVD risk factors that have been identified as significantly impacting CVD risk in the Reduction of Atherothrombosis for Continued Health (REACH) registryCitation18. Major risk factors included diabetes, prior ischemic event, and polyvascular disease; the complete list of risk factors is reported by Bhatt et al.Citation19. Risk equations were derived for CHD events, including non-fatal MI, hospitalization for unstable angina, or percutaneous coronary intervention (PCI)/coronary artery bypass surgery (CABG) greater than 30 days post-randomization, non-fatal stroke, and cardiovascular death. Mortality rates were based on data from IMPROVE-IT, which provided long-term data on cardiovascular outcomes in patients treated with ezetimibeCitation7. The risk for both non-fatal and fatal stroke events among patients with only a history of stroke was derived from the standard antiplatelet arm of the stroke cohort within the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2P)–Thrombolysis in Myocardial Infarction (TIMI) 50 (TRA2P-TIMI 50) study, a large, multinational, randomized clinical trial for vorapaxar for secondary stroke prevention in which the majority of patients were also on lipid-lowering therapiesCitation20. The probability of each event was applied in a hierarchical manner to patients who were “at risk”, in the following order: non-CVD death, CVD death, non-fatal CHD, and non-fatal stroke.
Transition probabilities to the non-CVD death state for each module were taken from age- and sex-adjusted US mortality dataCitation21 for all causes except cardiovascular death (defined by International Classification of Diseases, 10th revision codes I00–I99)Citation22. To calculate the transition probabilities for CHD associated with each treatment, the following equation was applied:
where u = {log(t) – Xb}/σ and Xb = b0 + x1*b1 +… xn*bn, has length of t = 1 year.
RRi represents the rate reduction (RR) for the ith patient, based on the relationship of CVD risk reduction per mmol/L change in LDL-C derived from the Cholesterol Treatment Trialists’ (CTT) Collaboration meta-analysisCitation23. Findings from the IMPROVE-IT studyCitation7 have been shown to conform to the results of this meta-analysis: the hazard ratio for clinical benefit per mmol/L reduction in LDL-C levels with ezetimibe added to statin therapy was 0.80 in the IMPROVE-IT trialCitation7, which was similar to the ratio of 0.78 observed with statins in the CTT meta-analysisCitation23. The level of risk reduction per mmol/L LDL-C reduction in the CTT meta-analysis was not found to be dependent on baseline LDL-C levelCitation23.
Cost inputs for health states were derived from a cost-effectiveness analysis using a Markov model to project the lifetime health outcomes and CVD-related costs of 1 million hypothetical US adults aged 40–75 years who received statin treatmentCitation24. Data from this analysis included direct medical costs, and were applied to each health state in the model on an annual basis (). The price of ezetimibe was set at the current wholesale acquisition cost (WAC) of $7.74 per dose, and was reduced by 90% after the first year of therapyCitation25. In a similar cost-effectiveness analysis of lipid-lowering therapy, Mullins et al.Citation26 estimated the cost-effectiveness of atorvastatin following loss of exclusivity. In this case, it was assumed that atorvastatin would become available at the price of generic simvastatin, a difference in price of ∼85%Citation26. The WAC of the statin therapies is shown in .
Table 2. Model parameters for base-case scenario and one-way sensitivity analyses.
Table 3. Costs of statin treatments.
The default utility weights for those with no history of CHD or stroke were derived from a cost-effectiveness analysis of ezetimibe monotherapy vs no treatment in a cohort of 1,000 men aged 55 years with a history of CVD and a baseline LDL-C level of 4.0 mmol/LCitation14. Utility weights in this analysis were based on the EuroQoL 5 Dimensions Questionnaire (EQ-5D), a standardized instrument for measuring health outcomesCitation14. The age-specific utility was calculated based on utility weights for respective disease statesCitation14. All death states received a utility weight of zero.
The life-table method was applied for half-cycle corrections implemented in the calculations for costs, life years, and QALYs. Costs of CVD outcomes were in 2013 US dollarsCitation24. A discount rate of 3%, based on guidance of the International Society for Pharmacoeconomics and Outcomes Research, was used for the analysisCitation27,Citation28. Treatment discontinuation and costs associated with adverse events were not considered in the model.
Statistical analyses
This US-based payer perspective model evaluated the cost-effectiveness of ezetimibe 10 mg addition to statin therapy in patients aged 35–74 years with a history of CHD (with or without prior stroke) and with LDL-C levels ≥70 mg/dL. The model used a lifetime horizon wherein each patient’s costs and benefits were accrued to age 100 years. The base-case scenario assumptions are listed in . The cumulative percentage of patients experiencing fatal CVD events and the cumulative number of fatal and non-fatal CVD events were projected for both treatment groups; however, significance testing was not conducted on these projections. In addition, two sub-group analyses were performed to evaluate the cost-effectiveness of adding ezetimibe to statin therapy for those with LDL-C levels ≥100 mg/dL (n = 179) and those with diabetes mellitus plus LDL-C levels ≥70 mg/dL (n = 195).
A one-way sensitivity analysis was undertaken in which the parameters of the base-case model, including utility weights and LDL-C level lowering efficacy, were varied across their plausible ranges. One-way sensitivity analyses also were performed for the two sub-group models.
Results
CVD outcomes
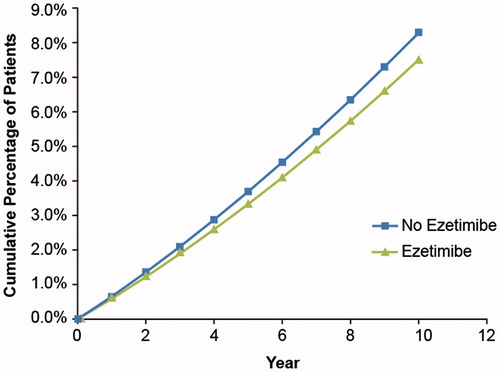
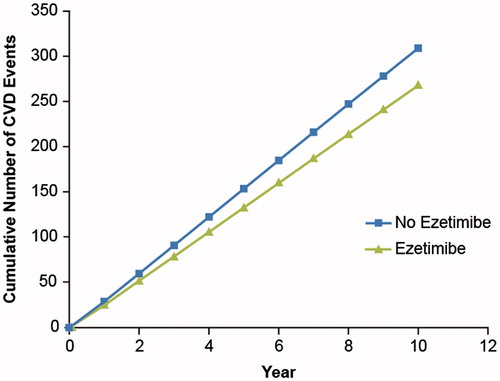
Clinical outcomes were projected as the total number of fatal and non-fatal CVD events experienced by patients in the model for the base case and sub-groups. The number of fatal and combined (fatal and non-fatal) CVD events was higher in the statin monotherapy group than in the ezetimibe-statin combination group. The model projected that, at the 10-year mark, fatal CVD events would affect 7.5% of patients treated with ezetimibe plus statin and 8.3% of patients who received statin monotherapy (). The Markov transition probability model projected that patients treated with ezetimibe plus statin would have a total of 268 CVD events (fatal and non-fatal) compared with 309 events for the statin monotherapy group ().
Base-case analysis
In the base-case analysis, incremental costs and incremental QALYs were evaluated for all patients with a history of ASCVD and LDL-C levels ≥70 mg/dL, and were compared for the ezetimibe–statin combination and statin monotherapy, accounting for a 90% reduction in the cost of ezetimibe after 1 year. Treatment with ezetimibe added to statin resulted in 15.87 LYG and 11.80 QALYs, compared with 15.67 LYG and 11.63 QALYs for statin monotherapy. The discounted ICER for ezetimibe added to statin therapy was $9,149 for a lifetime of treatment. Results of the base-case analysis are shown in . CVD events were the primary source of costs.
Table 4. ICER summary for base-case analysis of patients with a history of ASCVD and LDL-C levels ≥70 mg/dL.
One-way sensitivity analysis of the base case for variations in LDL-C level lowering efficacy, CVD event rate reductions, utility weights, baseline risk, allocation of CVD death, and percent price reduction of ezetimibe is shown in . The incremental cost-effectiveness of treatment with ezetimibe plus statin was impacted most by varying the amount of the ezetimibe cost reduction from 95% to 80%, resulting in an ICER range of –$3,645 to $34,735. Altering the rate reduction for non-fatal CHD and the utility weights for non-fatal CHD and non-fatal stroke also influenced the ICER.
Table 5. One-way sensitivity analysis for patients with a history of ASCVD and LDL-C levels ≥70 mg/dL (n = 548).
Sub-group analyses
The sub-group analyses for patients with LDL-C levels ≥100 mg/dL and patients with diabetes mellitus and LDL-C levels ≥70 mg/dL are shown in . The discounted ICER for ezetimibe added to statin therapy among patients with LDL-C levels ≥100 mg/dL was $839. For patients with diabetes mellitus and LDL-C levels ≥70 mg/dL, the discounted ICER was $560, accounting for a 90% reduction in the price of ezetimibe after 1 year. One-way sensitivity analyses for the sub-groups are shown in . Similar to the base case, ezetimibe price reduction was the greatest driver of changes in the ICER, resulting in a range from −$9,497 to $21,510 for patients with LDL-C ≥100 mg/dL and a range of –$8,471 to $18,622 for patients with diabetes and LDL-C ≥70 mg/dL. In both sub-groups, altering the rate reduction and baseline risk adjustment for non-fatal CHD also influenced the ICER.
Table 6. ICER summary for sub-group analyses of patients with LDL-C levels ≥100 mg/dL and patients diagnosed with diabetes mellitus with LDL-C levels ≥70 mg/dL.
Table 7. One-way sensitivity analyses of patient sub-groups.
Discussion
In this study, the cost-effectiveness of ezetimibe in combination with statin therapy for secondary prevention of fatal and non-fatal CHD and stroke was examined from a US payer perspective. The model included a 90% reduction in the cost of ezetimibe at the 1-year mark, reflective of the impending patent expiration in the second quarter of 2017. The discounted ICER for the base-case scenario (patients with a history of ASCVD and LDL-C levels ≥70 mg/dL treated with ezetimibe-statin combination vs statin monotherapy) was $9,149 for a lifetime of treatment. The model also was used to examine the cost-effectiveness of this treatment strategy for primary or secondary prevention of fatal and non-fatal CHD and stroke in sub-populations of patients with certain risk factors for ASCVD. In the two sub-groups analyzed (patients with LDL-C levels ≥100 mg/dL and those with diabetes and LDL-C levels ≥70 mg/dL), the discounted ICERs were $839 and $560 per QALY, respectively.
In one-way sensitivity analyses of both the base case and sub-group scenarios, cost reduction of ezetimibe, rate reduction of non-fatal CHD, and utility weight for non-fatal CHD were the most influential drivers of incremental cost. The utility weight for non-fatal stroke also was influential in the base case. Because non-fatal CHD and non-fatal stroke were the costliest events in the model, it is logical that changes in rate reduction and utility weight for these events would influence the ICER.
Several recent cost-effectiveness models for ezetimibe-statin combination therapy have been employed in settings within Europe and the UKCitation29–31. Two such models demonstrated that ezetimibe in combination with statin therapy is cost-effective at the willingness-to-pay thresholds of €30,000 per QALY (The Netherlands; 2011)Citation31 and <£14,000 per QALY (the UK; 2010)Citation29. An additional study showed this treatment combination to be cost-effective in specific sub-populations. In a 2010 study of patients with and without diabetes, Soini et al.Citation30 reported ICERs under a threshold of €30,000 per QALY for men with and without diabetes, as well as women with diabetes (Finland).
A recent US-based cost-effectiveness analysis performed by the Institute for Clinical and Economic Review showed that, for secondary prevention of cardiovascular events, the addition of ezetimibe to statin therapy was associated with a lower incremental cost per QALY than the addition of PCSK9 inhibitors to statins ($154,000 per QALY for ezetimibe vs $414,000 per QALY for PCSK9 inhibitors)Citation17. Although the ICER reported for ezetimibe in that analysis is substantially higher than in the present study ($154,000 vs $9149 per QALY, respectively), the Institute’s analysis did not account for patent expiration when calculating costs for ezetimibeCitation17. In addition, this model lacked long-term data on CV outcomes for PCSK9 inhibitorsCitation17, whereas the present model included long-term data on CV outcomes from IMPROVE-IT, which had a median follow-up time of 6 yearsCitation7. While the PCSK9 inhibitors have shown dramatic reductions in LDL-C in clinical trials, they have not yet gained support from ACC/AHA and NLA for widespread use among a broad range of dyslipidemia patientsCitation6,Citation10.
All of the above-mentioned cost-effectiveness models of the ezetimibe-statin combination assumed no change in the lifetime cost of ezetimibe, whereas the present model includes the impact of anticipated reductions in the drug’s cost following loss of patent exclusivity. A similar model was developed to explore the cost-effectiveness of atorvastatin for secondary CVD prevention following loss of patent exclusivity, compared with other generic statinsCitation26. Several cost scenarios were tested, ranging from atorvastatin remaining at its branded price to variation of co-pay schemes. In that analysis, the ICER was $10,344 per CVD endpoint avoided over a 7-year time horizon for atorvastatin at its branded price compared with generic statinsCitation26. When branded atorvastatin was switched to generic atorvastatin after 3 years, the ICER was dominant (–$951)Citation26. Although the present model did not entail testing as many scenarios as the atorvastatin model, both studies demonstrated, as expected, that price reductions owing to loss of patent exclusivity result in greater cost-effectiveness.
The Markov-like modeling approach used in the present analysis has several limitations. Because a payer perspective was examined, only direct costs were captured. In addition, the model did not account for treatment discontinuation. Recent cohort analyses have shown that high levels of adherence to statin therapy occur in approximately half of the patients prescribed these therapies for primary or secondary prevention of CVDCitation32–34. Model inputs in the present study also assumed strict adherence to recent ACC/AHA treatment guidelines for CVD, yet real-world evidence has demonstrated that many patients who fulfil the criteria for statin therapy do not receive treatmentCitation35.
The present cost-effectiveness model used proportional reduction in CVD risk per mmol/L change in LDL-C, derived from the CTT meta-analysis of statin therapyCitation23. Any change in this assumption would impact the rate reductions of cardiovascular risk, and, in turn, the number of projected CVD events, and, therefore, the overall cost-effectiveness, as CVD events are the greatest source of costs in the model. However, results of the IMPROVE-IT study are consistent with those of the CTT meta-analysis, which provides a degree of confidence that this assumption is appropriate. IMPROVE-IT is the first major clinical trial to demonstrate the ability of ezetimibe in combination with statin therapy to further reduce the risk of CVD events, relative to statin monotherapyCitation7. The alignment of findings from IMPROVE-IT with those from the CTT meta-analysis therefore reduces uncertainty surrounding the ability of ezetimibe to reduce the risk of CVD events in patients who participated in the present analysis.
Conclusions
The model used in the present study is unique in this treatment setting because it accounts for the future availability of generic ezetimibe, an important factor in the assessment of the treatment algorithm for cardiovascular conditions. Findings suggest that generic ezetimibe will be a cost-effective option when added to statin therapy for secondary prevention of CHD and stroke, and for primary prevention of these conditions in patients with LDL-C levels ≥100 mg/dL and in patients with diabetes.
Transparency
Declaration of funding
This study was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other relationships
GD is a full-time employee and holds shares in Merck & Co., Inc. (Kenilworth, NJ, USA), with no other interests. AV was a full-time employee of Rutgers University, which received funding from Merck & Co., Inc. at the time this work was conducted. AV is currently employed by the University of Rhode Island. CB is a full-time employee of MSD Ltd, a subsidiary of Merck & Co., Inc. and holds shares in Merck & Co., Inc., with no other interests. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary Material.pdf
Download PDF (80.1 KB)Acknowledgments
The authors take full responsibility for the content of the paper. They thank Jacob Willet and Karen Kurtyka (Oxford PharmaGenesis, Inc., Newtown, PA) for medical writing support, editorial assistance, and collation and incorporation of comments from all authors. These services were funded by Merck & Co., Inc., Kenilworth, NJ.
References
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38-e360
- Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591-608
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933-44
- Ovbiagele B, Goldstein LB, Higashida RT, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke 2013;44:2361-75
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl 2):S1-S45
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015;9:129-69
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97
- Mikhailidis DP, Sibbring GC, Ballantyne CM, et al. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin 2007;23:2009-26
- Morrone D, Weintraub WS, Toth PP, et al. Lipid-altering efficacy of ezetimibe plus statin and statin monotherapy and identification of factors associated with treatment response: a pooled analysis of over 21,000 subjects from 27 clinical trials. Atherosclerosis 2012;223:251-61
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2016;68:92-125
- Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015;36:1012-22
- National Institute for Health and Care Excellence (NICE). Cardiovascular disease: risk assessment and reduction, including lipid modification. Clinical guideline [CG181]. July 2014. www.nice.org.uk/guidance/cg181. Accessed December 12, 2016
- Chan PS, Nallamothu BK, Gurm HS, et al. Incremental benefit and cost-effectiveness of high-dose statin therapy in high-risk patients with coronary artery disease. Circulation 2007;115:2398-409
- Ara R, Pandor A, Tumur I, et al. Cost effectiveness of ezetimibe in patients with cardiovascular disease and statin intolerance or contraindications: a Markov model. Am J Cardiovasc Drugs 2008;8:419-27
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed March 28, 2017
- Cook JR, Yin D, Alemao E, et al. Cost-effectiveness of ezetimibe coadministration in statin-treated patients not at cholesterol goal: application to Germany, Spain and Norway. Pharmacoeconomics 2004;22(Suppl3):49-61
- Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743-53
- Ohman EM, Bhatt DL, Steg PG, et al. The REduction of Atherothrombosis for Continued Health (REACH) Registry: an international, prospective, observational investigation in subjects at risk for atherothrombotic events-study design. Am Heart J 2006;151:786.e1-10
- Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350-7
- Morrow DA, Alberts MJ, Mohr JP, et al. Efficacy and safety of vorapaxar in patients with prior ischemic stroke. Stroke 2013;44:691-8
- Kochanek KD, Murphy SL, Xu J, et al. Deaths: final data for 2014. Natl Vital Stat Rep 2016;65:1-122
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems. ICD-10 2010. http://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf. Accessed December 9, 2016
- Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81
- Pandya A, Sy S, Cho S, et al. Cost-effectiveness of 10-year risk thresholds for initiation of statin therapy for primary prevention of cardiovascular disease. JAMA 2015;314:142-50
- Wholesale Acquisition Cost. First DataBank Inc. 2015 www.analysource.com. Accessed October 5, 2015
- Mullins CD, Rattinger GB, Kuznik A, et al. Cost-effectiveness of intensive atorvastatin treatment in high-risk patients compared with usual care in a postgeneric statin market: economic analysis of the aggressive lipid-lowering initiation abates new cardiac events (ALLIANCE) study. Clin Ther 2008;30:2204-16
- Severens JL, Milne RJ. Discounting health outcomes in economic evaluation: the ongoing debate. Value Health 2004;7:397-401
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA 1996;276:1253-8
- Reckless J, Davies G, Tunceli K, et al. Projected cost-effectiveness of ezetimibe/simvastatin compared with doubling the statin dose in the United Kingdom: findings from the INFORCE study. Value Health 2010;13:726-34
- Soini EJ, Davies G, Martikainen JA, et al. Population-based health-economic evaluation of the secondary prevention of coronary heart disease in Finland. Curr Med Res Opin 2010;26:25-36
- van Nooten F, Davies GM, Jukema JW, et al. Economic evaluation of ezetimibe combined with simvastatin for the treatment of primary hypercholesterolaemia. Neth Heart J 2011;19:61-7
- Monaldi B, Bologna G, Costa GG, et al. Adherence to statin treatment following a myocardial infarction: an Italian population-based survey. Clinicoecon Outcomes Res 2015;7:273-80
- O’Brien EC, McCoy LA, Thomas L, et al. Patient adherence to generic versus brand statin therapy after acute myocardial infarction: Insights from the Can Rapid Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the American College of Cardiology/American Heart Association Guidelines Registry. Am Heart J 2015;170:55-61
- Rannanheimo PK, Tiittanen P, Hartikainen J, et al. Impact of statin adherence on cardiovascular morbidity and all-cause mortality in the primary prevention of cardiovascular disease: a population-based cohort study in Finland. Value Health 2015;18:896-905
- Johansen ME, Green LA, Sen A, et al. Cardiovascular risk and statin use in the United States. Ann Fam Med 2014;12:215-23
- Ara R, Pandor A, Stevens J, et al. Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation. Health Technol Assess 2009;13:1-74, 75-118