Abstract
Aims: The objective of this study was to quantify the current and to project future patient and insurer costs for the care of patients with non-small cell lung cancer in the US.
Materials and methods: An analysis of administrative claims data among patients diagnosed with non-small cell lung cancer from 2007–2015 was conducted. Future costs were projected through 2040 based on these data using autoregressive models.
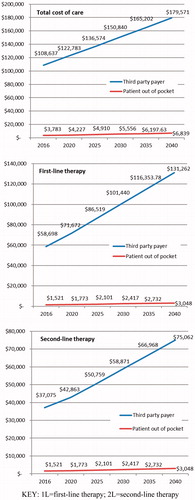
Results: Analysis of claims data found the average total cost of care during first- and second-line therapy was $1,161.70 and $561.80 for patients, and $45,175.70 and $26,201.40 for insurers, respectively. By 2040, the average total patient out-of-pocket costs are projected to reach $3,047.67 for first-line and $2,211.33 for second-line therapy, and insurance will pay an average of $131,262.39 for first-line and $75,062.23 for second-line therapy.
Limitations: Claims data are not collected for research purposes; therefore, there may be errors in entry and coding. Additionally, claims data do not contain important clinical factors, such as stage of disease at diagnosis, tumor histology, or data on disease progression, which may have important implications on the cost of care.
Conclusions: The trajectory of the cost of lung cancer care is growing. This study estimates that the cost of care may double by 2040, with the greatest proportion of increase in patient out-of-pocket costs. Despite the average cost projections, these results suggest that a small sub-set of patients with very high costs could be at even greater risk in the future.
Introduction
Lung cancer is diagnosed in ∼224,390 individuals each year, and is the leading cause of cancer-related death among both men and women in the USCitation1. The most common form of lung cancer is non-small cell lung cancer (NSCLC), and the majority of patients will be diagnosed with stage IV or metastatic disease. A diagnosis of NSCLC is a serious condition, with only 17.7% surviving 5 years or longerCitation2. However, the sooner NSCLC is diagnosed, the better the prognosis. For patients diagnosed with disease that has not spread, more than 55% will live 5 years or longer. Unfortunately, only 4.3% diagnosed with metastatic disease will live this longCitation2.
The treatment of patients diagnosed with advanced or metastatic NSCLC generally involves chemotherapy treatment with or without biologic therapy, and most recently includes immune checkpoint inhibitors as a novel immunotherapy treatment for the treatment of disease that demonstrates high PD-L1 expressionCitation3. For patients with advanced or metastatic disease, treatment will often continue through multiple lines of therapy until the patient no longer responds to the treatment or until the patient is no longer willing or able to tolerate therapy. Few patients will experience long periods where the disease is in remissionCitation4. Due to the continual need for active therapy to control the disease, similar to other cancers, as new and more active agents are becoming available, cost considerations are increasingly becoming important in the treatment of NSCLC. Patients are living longer and, therefore, treated for longer periods of time; since 2000, there has been a clinically small but significant improvement in survival for patients with NSCLC (hazard ratio, HR = 0.78, 95% confidence interval, CI = 0.73–0.84)Citation5.
A retrospective study found that the annual Medicare cost of total care for a patient with NSCLC was $85,087 in 2011Citation3. A commercial claims study of data from 2006–2010 found that the cost of first-line therapy varied based on the regimen used, from $19,182 (for erlotinib monotherapy) to $167,847 for regimens containing two or more branded agentsCitation6. They found that the cost of drugs accounted for 38% of the total cost of lung cancer care, whereas hospitalizations accounted for 19% of the total costCitation6. Other studies have estimated the cost of care but have done so using modeling approaches from clinical trial dataCitation7–10, which do not reflect the actual costs of routine patient care in an uncontrolled setting. None of these retrospective studies measured the patient portion of costs, and economic models typically are designed from a third-party payer perspective (e.g. insurer), which also does not account for patient out-of-pocket costs.
Patient out-of-pocket costs in cancer were estimated by a 2008 survey, which included an item asking cancer patients to estimate what they had spent for care in the last yearCitation11. While limited due to the collection of self-reported data alone, 25% of lung cancer patients reported out-of-pocket expenses in excess of $5,000 for the past year, whereas 36% reported spending less than $1,000. In 2015, the American Cancer Society published a report evaluating insurance coverage for cancer patientsCitation12. In this report, they found that coverage for intravenous medications was unclear, and the impact on patients could not be determined due to lack of clarity in the healthcare plans they evaluated. Concern about the costs of care to the cancer patient has also drawn more recent media attention; however, the focus in research has been on the overall costs, and less attention has been given to what patients actually payCitation3,Citation13,Citation14.
The overall objective of this study was to fill this gap in knowledge by using real world data to determine the current costs and to project the future financial impact of lung cancer on patients and insurers, respectively. While the commercial insurance industry in the US is able to diffuse the costs of care across a large group of those they insure, patients must absorb all the costs of their own care that are not covered by their insurance policies. As stated above, there is increasing evidence related to the total cost of care, but little remains known about the actual costs paid by patients with NSCLC for their own care. This study investigates the costs incurred by patients who are diagnosed with NSCLC, the cancer that claims more lives than any other cancer in the US each year.
Methods
Data source
Claims data from January 1, 2007 through December 31, 2015 from the Truven MarketScan (Ann Arbor, MI) database were used for this study. Truven MarketScan is a HIPAA-compliant, patient-level database containing inpatient, outpatient, drug, and laboratory data from commercial, Medicaid, and Medicare supplemental sources. The data are collected from ∼350 different insurance companies and third-party administrators, and include longitudinal data from more than 65 million covered lives.
Study population
Patients were eligible for this study if they were 18 years of age or older at the time of initial diagnosis of lung cancer (ICD-9-CM codes of 162.2–162.9). The first diagnostic code for lung cancer was required to be on or after July 1, 2007. Patients were required to have received anti-cancer therapy (e.g. chemotherapy, biologic therapy) after the index diagnosis. Patients were excluded who had a prior cancer to ensure that the cancer-related costs were specific to lung cancer care. To ensure the cohort was primarily NSCLC, patients receiving agents used for the treatment of small-cell lung cancer were excluded from the study. The index date was defined as the first claim with a lung cancer ICD-9-CM code, and the pre-index period included the 6 months of data prior to the cancer diagnosis. Follow-up of each patient continued to the date of death, disenrollment from the health insurance plan, or end of database, whichever occurred first. The 6-month pre-index period was used to assess baseline comorbidities using the Carlson Comorbidity Index (CCI)Citation15, and to calculate baseline pre-cancer healthcare costs.
Statistical analysis
All analyses were conducted using SAS version 9.2. All variables were summarized descriptively through the tabular and graphical display of mean, median, minimum/maximum values, and standard deviation for continuous variables, and frequency count and percentage for categorical variables. The average monthly and total all-cause, treatment-related, and hospitalization-related patient out of pocket costs and insurer costs were described by line of therapy and over the entire patient life time (e.g. index through the duration of follow-up). Treatment-related costs included any aspect of care that was billed at the time of infusion, including but not limited to infusion costs, supportive care costs, anti-cancer drug costs (including chemotherapy, biologic, and/or targeted agents), and pre-medications. Hospitalization-related costs included all costs within the bill for overnight stay for any cause during the treatment period, including surgical procedures. Actual paid amounts, not charges, were used for this study for both patient and payer costs. Costs were adjusted for inflation and reported in 2014 US dollars using the medical care component of the Consumer Price Index (CPI).
Propensity score matching was used to compare costs in patient groups by age (<70 vs ≥70), metastatic disease status (metastatic vs non-metastatic), insurance type (commercial vs Medicare), and method of infusion (intravenous vs oral chemotherapy), separately. For each comparison, the propensity scores were calculated using a logistic regression model, with the comparative patient groups as dependent variable and patient demographic, baseline clinical, and prior treatment characteristics as independent variables. A greedy propensity score matching was performed on the logit of the propensity score against the caliper of 0.2-times the pooled standard deviation of the logit of the propensity score. Covariate balance between the comparative patient groups was assessed by using absolute normalized differences before and after matching. P-value for differences in costs between comparative groups from the propensity score matching was calculated using a generalized linear model (GLM) with log link function and Gamma distribution.
Logistic regression (using the highest and lowest cost quartiles, which were used to identify factors that were thought to be most meaningful to insurers and patients) was used to identify predictors of patient out-of-pocket costs and insurer costs, respectively, for all-cause costs, treatment-related costs, and hospitalization-related costs. Due to potential differences in duration of line of therapy which could influence the predictive analyses, average monthly costs were used. The potential predictor variables in each of the models included demographics (age, gender, geographic region, insurance type), baseline clinical characteristics (metastatic vs non-metastatic disease, comorbidities, prior medication use), and baseline cost variables (patient out of pocket and insurer costs during the 6-month pre-index period).
Autoregressive error models were used to correct for autocorrelation of the time series healthcare costs data over years. Using the SAS AUTOREG procedure, models with different orders of the autoregressive error (NLAG = 1, 2, 3, …) and different types of estimates to be computed (METHOD = options, i.e. maximum likelihood estimates, unconditional least squares estimates, Yule-Walker estimates, or iterative Yule-Walker estimates) were explored to fit the total yearly all-cause, treatment-related, and hospitalization-related costs to patients and insurers, respectively. The cost data is typically skewed, but the departure from the normal distribution becomes insignificant under the large sample size. The best models were determined based on the goodness-of-fit statistics (the model with the highest R2 was selected). These models were further used to forecast the costs in future years. The anticipated costs with 95% confidence intervals in 2016, 2020, 2025, 2030, 2035, and 2040 were reported, based on the extrapolation of the best fitting models.
Results
Baseline clinical and demographic characteristics of the eligible patient cohort (n = 47,207) are presented in . The median age was 64.9 years and the majority (54.3%) was male. Half of the patients (51.1%) had evidence ICD-9-CM codes reflecting metastatic disease at diagnosis.
Table 1. Eligible patient characteristics.
Total costs and by line of therapy
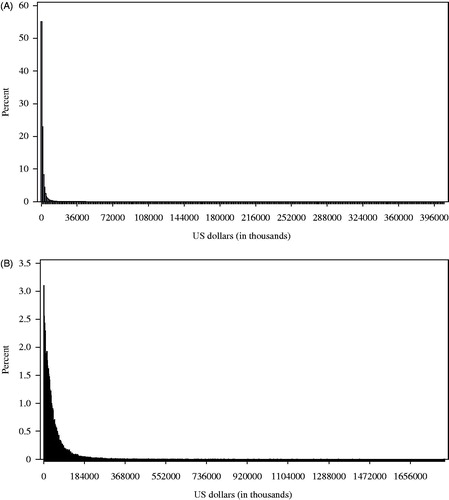
Costs were not normally distributed and were skewed with long right tail (); due to high cost outliers, mean costs were higher than the median values for both patient out-of-pocket and payer costs (). The average all-cause total cost of care for insurers for the care of patients diagnosed with lung cancer from the time of diagnosis through the end of follow-up was $159,301.60. Within these total costs, the average cost of chemotherapy, biologic agents, targeted, and radiation therapy was $18,849.40, $56,931.00, $36,849.70, and $25,095.50 to the insurer, respectively, and the average cost of supportive care was $13,779.60. The average patient out-of-pocket costs from diagnosis through the end of follow-up was $4,913.00. Treatment with chemotherapy, biologic agents, targeted therapy, radiation therapy, and supportive care resulted in average out-of-pocket costs of $308.00, $653.90, $670.00, $555.90, and $694.60, respectively. Similar to overall costs, each of these cost categories demonstrated a similar skewed distribution and large standard deviations ().
Table 2. Summary of cost categories.
For first-line therapy, after propensity score matching, there were significant differences in mean total all-cause patient out-of-pocket costs by metastatic vs non-metastatic disease ($1,280.30 vs $1,095.50, p = .01, ), age <70 vs ≥70 years ($988.70 vs $1,424.40, p < .0001), and commercial plans vs Medicare patients ($852.00 vs $1,046.60, p = .02) (). First-line all-cause costs paid by insurers were also significantly different for patients with metastatic vs non-metastatic disease, as shown in ($47,587.20 vs $37,461.20, p = .003), by age (<70 years $43,138.90 vs ≥70 years $32,449.70, p < .0001), and for those with commercial plans vs Medicare plans ($46,779.40 vs $36,169.40, p < .0001). Since the propensity score matching was done for each comparative group of interest (metastasis status, age group, healthcare plan type), the inference applies to each individual comparative group of interest only.
Table 3. Factors associated with patient out-of-pocket and insurer costs.
In the second-line setting, there were significant differences in a number of factors for both the insurer and the patient out-of-pocket costs for treatment-related costs. There were significant differences by patient age for the average all-cause costs paid by the insurer (<70 years $25,661.80 vs ≥70 years $18,286.60, p < .0001) and for commercial vs Medicare (commercial $23,332.10 vs Medicare $22,205.30, p = .01) in the second-line setting. Patient out-of-pocket costs were not significantly different by age group or for Medicare vs commercial plans in the second-line setting.
Patient out-of-pocket costs
As presented in , the median total patient out-of-pocket costs for cancer care from the time of diagnosis through end of follow-up was $2,964.30. The median amount paid by patients was $0 for chemotherapy, biologic, and targeted therapy, and $305 for supportive care. The mean values are higher due to high cost outliers. In the first-line setting, the median overall patient out-of-pocket cost was $349. The median hospital and chemotherapy, biologic, and targeted therapy-related out-of-pocket costs per patient in this setting were both $0, while the mean costs were $282 and $497, respectively. In the second-line setting, the median overall patient out-of-pocket cost was $83. The hospital and chemotherapy, biologic, and targeted therapy-related out-of-pocket costs per patient in this setting were $0 and $37, respectively, while the mean costs were $197 and $797, respectively.
Factors associated with costs
Factors associated with monthly patient out-of-pocket costs and with costs paid by insurers are presented in (factors with lower costs have an odd ratio of less than 1.0, and factors with higher costs demonstrate an odds ratio of greater than 1.0). Factors significantly associated with lower all-cause monthly patient out-of-pocket costs during first-line therapy with an odds ratio of ≤0.80 included year of index diagnosis (p < .05; 2008 was also statistically significant with an odds ratio of 0.81). Factors significantly associated with higher monthly patient out-of-pocket costs with an odds ratio of ≥1.2 included all health plan types (other than consumer-driven plans) vs high-deductible health plans (p < .0001). Factors significantly associated with lower costs with an odds ratio of ≤0.80 for chemotherapy, biologic, or targeted therapy-related patient out-of-pocket costs included year of index diagnosis and having a POS, PPO, or EPO plan vs high deductible health plan (all p < .001).
For the insurer, factors significantly associated with lower monthly total costs during first-line treatment with an odds ratio ≤0.80 included a comprehensive or HMO plan vs a high-deductible plan (both p < .0001). The factors significantly associated with higher monthly total costs with an odds ratio (OR) of ≥1.2 included year of diagnosis after 2009 (more recent years had higher costs) (all p < .01). For chemotherapy, biologic or targeted therapy-related costs during first-line therapy to the insurer, patients without metastatic disease, patients with EPO, comprehensive, consumer-driven or HMO plans vs those with high-deductible plans has significantly lower costs with an odds ratio of less than 0.80 (all p ≤ .01). Male patients have significantly higher treatment-related costs than female patients to the insurer (OR = 1.27, p < .0001, ).
Projected total future costs
Projected future all-cause and treatment-related costs are presented in by line of therapy for the patient and for the insurer. The total cost of care from initial diagnosis of lung cancer to the end of follow-up is expected to reach $6,839.25 for patients and $179,571.13 for insurers by 2040. If the current trend in costs continues in the first-line setting, all-cause patient out-of-pocket costs are projected to be $1,772.93 in 2020, rising to $3,047.67 by 2040. This is estimated to result in average monthly costs of $253.98 by 2040, double the monthly out-of-pocket costs in 2016 (estimated to be $126.79). For the insurer, the all-cause costs of first-line therapy are projected to rise from $71,672.10 in 2020 to $131,262.39 by 2040. Of these costs, more than half are expected to be costs related to chemotherapy, biologic, and targeted therapy. These treatment-related patient and insurer costs are projected to reach $1,150.76 and $69,286.98 (first-line) and $868.33 and $33,786.05 (second-line), respectively, by 2040. The details of the cost projection are provided in .
Table 4. Mean (95% confidence interval) future projected patient out-of-pocket and insurer costs through 2040, based on data from 2007–2015.
Discussion
This study evaluated the costs paid by patients and insurers following a diagnosis of NSCLC in a representative claims database, and used these data to estimate the potential future financial impact of care for patients with NSCLC and payers in the US. The total cost of care for patients with lung cancer is growing, and is estimated to rise from $3,783.02 for patients and from $108,636.65 insurers in 2016 to reach $6,839.25 for patients and $179,571.13 for insurers by the year 2040. The estimated future costs of care for patients with NSCLC are sobering.
The magnitude of difference between the mean and median values demonstrates that, while at least half of patients pay no out-of-pocket costs for their care, there is a small sub-set of patients who pay very high costs that have the potential to cause financial devastation. While published case reports and media reports highlighting these issues have been published for yearsCitation16,Citation17, this study demonstrates that the majority of patients do not experience high out-of-pocket costs, and, for most patients with insurance, the healthcare system and payment structures protect them from financial burdens. However, based on the distribution, there is a small group of insured patients who pay very high costs, such as those found from this study. While insurance has a growing total cost the rate of patient out-of-pocket costs appears to be rising more rapidly than that of the insurer. This suggests that the sub-set of patients with high costs could be at even greater risk as costs continue to escalate. Structures should be put in place to prevent financial devastation following a cancer diagnosis. Future research should be conducted to not only evaluate the problem of rising cancer costs, but also to develop solutions for those individuals at greatest risk of high out-of-pocket expenses. One goal of future work could be to help identify those individuals at greatest risk of extremely high financial costs in order to develop strategies to help minimize potential financial impact associated with a diagnosis of NSCLC.
This study included analyses to better understand which patients may be at highest risk of incurring greater out-of-pocket and insurance costs. Out-of-pocket costs for patients with metastatic disease, patients aged 70 and over, and those with Medicare plans were higher across lines of therapy; however, insurers paid more costs for younger patients, and those with commercial plans in the first-line setting specifically. This could be due to a number of factors, such as the longer duration of treatment, or the possibility that younger patients may receive more aggressive therapy than older patients after initial diagnosis. There were fewer cost differences in the total cost of second line therapy, which could be related with the shorter duration of treatment and less time for those costs to be incurred. Patient support programs may be important to have in place for patients who are more likely to incur a larger financial burden for their care.
The finding that high deductible health plans were associated with higher costs to the patient was expected, yet these plans were also associated with higher costs to the insurer. These plans are designed to be more affordable to patients by offering lower premiums, but with the trade-off that benefits do not generally become available until the patient has reached a relatively high out-of-pocket expense, often in the high thousands to tens of thousands per year. With NSCLC, this deductible may be reached at the time of diagnosis; but, even at that time, these plans still do not cover 100% of the cost of care, and patients are still obligated to cover a portion of the cost through co-payments or other structures. Further research could further evaluate patients with high-deductible plans to determine if they may forgo healthcare visits at the time when a patient may first suspect a health problem, and if diagnosis and treatment at later stages of disease may contribute to the higher cost of care to the patient and insurer. Previous work has suggested that those with lower income who have high-deductible plans are likely to delay or avoid healthcare visits to control costsCitation18. Further investigation is warranted as it relates to the risk of delayed cancer diagnosis and subsequent care.
While this study quantified a number of aspects of the cost of care to insurers and patients, retrospective claims database studies are not without limitations. Administrative claims data are not collected for research purposes; therefore, there may be errors in entry and coding that cannot be accounted for. Claims data do not contain important clinical factors, such as stage of disease at diagnosis, tumor histology, or data on disease progression, which may have important implications on the cost of care. The approval of new agents in the second-line setting in 2015 (i.e. pembrolizumab and nivolumab) may have influenced the sharp increase in projected costs in the second-line setting in this study. As new biologic or targeted agents begin to be incorporated in the first-line setting, the projected costs may become higher than measured in this study in the first-line setting as well. Patients may also have had dual primary tumors, and no attribution to NSCLC-specific costs could be made in the claims data. Despite these limitations, the cost of care for patients with NSCLC is increasing, and there is a need for further study and the development of solutions to address this increasing cost trend.
Transparency
Declaration of funding
This was a research project conducted by employees of Eli Lilly and Company and InVentiv Health Clinical. There was no designated research funding provided for this study.
Declaration of financial/other interests
LMH, ZCL, PJG, and ABO are employees of Eli Lilly and Company. YW and YF are employees of InVentiv Health Clinical, which receives funding from Eli Lilly and Company for analytical and programming support of research projects conducted at Eli Lilly.
Previous presentation
This work was submitted as an abstract and presented as a poster to the International Association for the Study of Lung Cancer (ASLC) in Chicago, IL, 2016.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30
- Howlander N, Noone AM, Krapcho M, et al. SEER Statistics Review, 1975–2013. Bethesda, MD: National Cancer Institute; 2016
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines) Version 2.2017. 2016. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed June 5, 2017
- Yu B, Tiwari RC, Cronin KA, et al. Cure fraction estimation from the mixture cure models for grouped survival data. Stat Med 2004;23:1733-47
- Bradley CJ, Yabroff KR, Mariotto AB, et al. Antineoplastic treatment of advanced-stage non-small-cell lung cancer: treatment, survival, and spending (2000 to 2011). J Clin Oncol 2017;35:529-35
- Henk HJ, Ray S. Treatment patterns and healthcare costs among patients with advanced non-small-cell lung cancer. Lung Cancer Manag 2013;2:189-97
- Hess LM, Rajan N, Winfree K, et al. Cost Analyses in the US and Japan: a cross-country comparative analysis applied to the PRONOUNCE trial in non-squamous non-small cell lung cancer. Adv Ther 2015;32:1248-62
- Hess LM, Cinfio FN, Wetmore S, et al. Enhancing the budget impact model for institutional use: a tool with practical applications for the hospital oncology pharmacy. Hosp Pharm 2016;51:452-60
- Ting J, Tien Ho P, Xiang P, et al. Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health J Int Soc Pharmacoecon Outcomes Res 2015;18:774-82
- Kumar G, Woods B, Hess LM, et al. Cost-effectiveness of first-line induction and maintenance treatment sequences in non-squamous non-small cell lung cancer (NSCLC) in the U.S. Lung Cancer 2015;89:294-300
- Markman M, Luce R. Impact of the cost of cancer treatment: an internet-based survey. J Oncol Pract Am Soc Clin Oncol 2010;6:69-73
- ACS-CAN. ACS CAN examination of cancer drug coverage and transparency in the health insurance marketplaces. ACS-CAN; 2015. https://www.acscan.org/policy-resources/acs-can-examination-cancer-drug-coverage-and-transparency-health-insurance. Accessed June 5, 2017
- Appleby J. Cancer meds often bring big out-of-pocket costs for patients, report finds. Kaiser Health News 2015 http://khn.org/news/cancer-meds-often-bring-big-out-of-pocket-costs-for-patients-report-finds/ Accessed June 5, 2017
- Marsa L. The high cost of cancer care: Your money or your life? Newsweek 2015. http://www.newsweek.com/2015/07/31/high-cost-cancer-care-your-money-or-your-life-356369.html. Accessed June 5, 2017
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9
- Johnson CY. The burden of cancer isn't just cancer. The Washington Post [Internet]. 2016. www.washingtonpost.com/news/wonk/wp/2016/04/08/cancers-sinister-side-effect-financial-toxicity. Accessed September 29, 2016
- DeLuna R. Seattle woman experiences financial devastation following cancer diagnosis. KUOWORG [Internet]. 2015. kuow.org/post/seattle-woman-experiences-financial-devastation-following-cancer-diagnosis. Accessed September 29, 2016
- Kullgren JT, Galbraith AA, Hinrichsen VL, et al. Health care use and decision making among lower-income families in high-deductible health plans. Arch Intern Med 2010;170:1918-25