Abstract
Aims: To quantify healthcare costs in patients with psoriasis overall and in psoriasis patient sub-groups, by level of disease severity, presence or absence of psoriatic arthritis, or use of biologics.
Methods: Administrative data from Truven Health Analytics MarketScan Research Database were used to select adult patients with psoriasis from January 2009 to January 2014. The first psoriasis diagnosis was set as the index date. Patients were required to have ≥6 months of continuous enrollment with medical and pharmacy benefits pre-index and ≥12 months post-index. Patients were followed from index until the earliest of loss to follow-up or study end. All-cause healthcare costs and outpatient pharmacy costs were calculated for the overall psoriasis cohort and for the six different psoriasis patient sub-groups: (a) patients with moderate-to-severe disease and mild disease, (b) patients with psoriatic arthritis and those without, and (c) patients on biologics and those who are not. Costs are presented per-patient-per-year (PPPY) and by years 1, 2, 3, 4, and 5 of follow-up, expressed in 2014 US dollars.
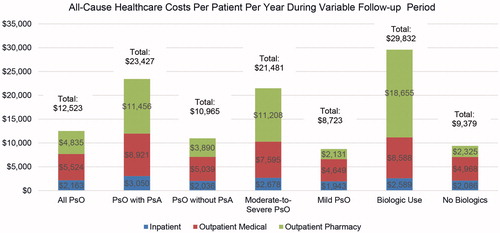
Results: A total of 108,790 psoriasis patients were selected, with a mean age of 46.0 years (52.7% females). Average follow-up was 962 days. All-cause healthcare costs were $12,523 PPPY. Outpatient pharmacy costs accounted for 38.6% of total costs. All-cause healthcare costs were highest for patients on biologics ($29,832), then for patients with psoriatic arthritis ($23,427) and those with moderate-to-severe disease ($21,481). Overall, all-cause healthcare costs and outpatient pharmacy costs presented an upward trend over a 5-year period.
Conclusions: Psoriasis is associated with significant economic burden, which increases over time as the disease progresses. Patients with moderate-to-severe psoriasis, those with psoriatic arthritis, or use of biologics contributes to higher healthcare costs. Psoriasis-related pharmacy expenditure is the largest driver of healthcare costs in patients with psoriasis.
Introduction
The prevalence rate of psoriasis in the US is estimated to be 2–3%, affecting ∼7.5 million peopleCitation1–4. Although psoriasis can develop at any age, most cases occur in young adults of working ageCitation5,Citation6. Plaque psoriasis is the most common type of psoriasis, occurring in 90% of patients, and is characterized by silvery-white or red scaly patchesCitation3,Citation7. Plaques often occur on the scalp, elbows, knees, and lower back, and are associated with symptoms of itching and burningCitation6,Citation8,Citation9. Psoriasis is considered mild, moderate, or severe if less than 3%, 3–10%, or more than 10% of the body is affected, respectivelyCitation9. Approximately 21–33% of patients with psoriasis have moderate-to-severe diseaseCitation10,Citation11, and 15–30% may develop psoriatic arthritisCitation6,Citation12. Patients with both psoriasis and psoriatic arthritis have an increased risk of developing other chronic conditions, including cardiovascular disease, Crohn’s disease, depression, diabetes, metabolic syndrome, osteoporosis, uveitis, and liver diseaseCitation9.
Psoriasis severity is a factor in treatment choiceCitation4,Citation6,Citation13. Topical therapies including corticosteroids, anthralin, synthetic vitamin D3, vitamin A, and coal tar are typically used for mild psoriasisCitation6,Citation14,Citation15. Localized phototherapy may be used in mild cases, while more extensive disease can be treated with whole body phototherapy. Systemic therapy is used for moderate-to-severe psoriasisCitation6,Citation9,Citation14. Systemic therapy includes drugs that affect the immune system in general, such as methotrexate and cyclosporine, but also includes biologics, which allow for more effective and safer treatmentsCitation16,Citation17. Furthermore, the presence or absence of psoriatic arthritis may impact the choice of treatment in psoriasisCitation18.
The economic burden of psoriasis is substantial. Expressed in 2013 US dollars, the US annual direct psoriasis costs were estimated to be between $51.7–$63.2 billion, based on studies published between 2008–2013Citation19. Estimating the 2013 population size of adult patients with psoriasis in the US to be 7.4 million, the annual direct costs would range between $6,985–$8,536 per patientCitation19. Patients with moderate-to-severe psoriasis were reported to have higher annual healthcare costs ($10,593 in 2007 dollars) compared to patients with mild disease ($5,011)Citation14, and the majority of psoriasis-related costs for patients with moderate-to-severe disease were attributed to systemic therapy including biologics, non-biologics systemic therapy, and phototherapiesCitation20.
To our knowledge, the majority of cost studies in psoriasis in the US have estimated healthcare costs in the short-term, i.e. over 12 months of follow-up. Also, many of the cost studies have evaluated healthcare costs prior to and after treatment initiation, not taking into account the natural progression of the disease. In this study, we characterize adult patients with psoriasis from their initial diagnosis up to year 5 of follow-up in a large, commercially insured population in the US. We further estimate all-cause healthcare costs over time and in years 1, 2, 3, 4 and 5 following initial psoriasis diagnosis. Lastly, we examine the cost trends over time by psoriasis severity, presence of psoriatic arthritis, and use of biologics.
Methods
Data sources
Patients were identified from the Truven Health MarketScan Commercial Claims and Encounters databases. The database consists of employer- and health plan-sourced data containing medical and drug data for ∼120.3 million employees and their dependents, with 35 million lives in the 2013 database. Individuals are covered under a variety of fee-for-service and managed care plans. The database provides detailed cost, use, and outcomes data for healthcare services performed in both inpatient and outpatient settings. Medical claims are linked to outpatient prescription drug claims and person-level enrollment information.
Study populations
Patients were selected based on the presence of at least one inpatient or two or more non-diagnostic outpatient claims at least 30 days apart, with a primary or secondary psoriasis diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 696.1x) between January 1, 2009 and January 31, 2014. The date of the first psoriasis diagnosis was assigned as the index date. Patients were required to be age 18 or older as of the index date and have at least 6 months of continuous enrollment with medical and pharmacy benefits pre-index and at least 12 months post-index. The follow-up period was variable in length for each patient, starting from the index date and ending at the earliest of inpatient death, end of continuous enrollment with medical and pharmacy benefits, or end of the study (January 31, 2015).
Psoriasis patients were further stratified post-index based on disease severity, the presence of psoriatic arthritis, or use of biologics. Patients were considered to have moderate-to-severe disease if they had at least one claim of systemic therapy, including biologics, indicated for psoriasis and/or psoriatic arthritis (adalimumab, etanercept, infliximab, ustekinumab, golimumab, and certolizumab) or at least one claim of phototherapy during the follow-up period. Patients on no systemic treatment, or biologic treatment, or phototherapy during follow-up, were considered to have mild disease. Patients with a diagnosis code of psoriatic arthritis (ICD9-CM 696.0x) during the pre-index or follow-up period were flagged as having psoriatic arthritis. Patients who had at least one biologic therapy claim during the follow-up period were flagged as biologic treated.
Demographic and clinical variables
Patient demographic characteristics (i.e. age, gender, insurance plan type, and geographic region) were captured as of the index date. Patient clinical characteristics were measured during the 6-month pre-index and years 1, 2, 3 4, and 5 post-index. Clinical characteristics included the Deyo Charlson Comorbidity Index (DCI)Citation21 and pre-defined comorbidities such as anxiety, depression, hypertension, hyperlipidemia, coronary heart disease, overweight/obesity, psoriatic arthritis, ankylosing spondylitis, among others.
Study outcomes
All-cause healthcare utilization and costs were reported during baseline and follow-up for overall healthcare services and by individual service category (i.e. inpatient, outpatient emergency room visits, office visits, lab test, radiology, hospital outpatient, other outpatient services, and outpatient pharmacy). In addition, psoriasis-specific pharmacy costs and the number of prescriptions and claims were captured. Psoriasis-specific medication included biologic agents approved for treating psoriasis and/or psoriatic arthritis (etanercept, adalimumab, ustekinumab, infliximab, golimumab, and certolizumab), non-biologic systemic agents, phototherapy, and topical prescription therapy. Outpatient prescriptions billed by National Drug Codes (NDCs) and office-administered medications billed by the Healthcare Common Procedure Coding System (HCPCS) codes were included in psoriasis-specific pharmacy reporting.
Costs were based on paid amounts of adjudicated claims, including insurer and health plan payments, as well as patient cost sharing in the form of copayment, deductible, and coinsurance. Costs for services provided under capitated arrangements were estimated using payment proxies that were computed based on paid claims at the procedure level using the Commercial database. All dollar estimates were inflated to 2014 dollars using the Medical Care Component of the Consumer Price Index. Costs were estimated during the variable follow-up and reported as per-patient per-year (PPPY) as well as for discrete years 1–5.
Analyses
Bivariate analyses were conducted. Categorical variables were presented as the count and percentage of patients in each category; continuous variables were summarized by the mean and standard deviation. Comparisons were conducted for the following sub-groups: (a) patients with moderate-to-severe disease vs mild disease, (b) patients with psoriatic arthritis vs those without, and (c) patients on biologics vs those who are not. Statistical tests of significance for differences across these groups were conducted. Chi-square tests were used for categorical variables; t-tests or non-parametric tests were used for continuous variables. A critical value of 0.05 was specified a priori as the threshold for statistical significance.
Results
Study samples
A total of 347,886 psoriasis patients were eligible for entry into the study between January 1, 2009 and January 31, 2015, and 108,790 (31.3%) of them met the study selection criteria and were included in the final study sample. Overall, patients were followed for an average of 962 days. Psoriasis patients with moderate-to-severe disease, with comorbid psoriatic arthritis and treated with biologics, represented 29.6%, 12.5%, and 15.4% of the overall psoriasis study sample, respectively.
Patient demographic and clinical characteristics
presents the demographic characteristics of the overall psoriasis study sample and defined patient sub-groups. The mean age of psoriasis patients was 46.0 (SD = 11.8) years at index, and the majority of psoriasis patients were women (52.7%). Psoriasis patients with psoriatic arthritis were older than those without psoriatic arthritis (47.4 vs 45.7 years, p < .001), while psoriasis patients treated with a biologic were younger than those untreated (45.1 vs 46.1 years, p < .001). More male patients were treated with biologics than patients without biologics (50.8% vs 46.7%, p < .001).
Table 1. Demographics and pre-period clinical characteristics of psoriasis patients.
Hypertension (16.2%) and hyperlipidemia (13.4%) were the most common comorbid conditions in the overall psoriasis study sample. About 4% of the psoriasis patients had a diagnosis of psoriatic arthritis during the 6-month pre-period (). Rates of comorbid conditions were generally higher in psoriasis patients with moderate-to-severe disease, in patients with psoriasis and psoriatic arthritis, and in psoriasis patients treated with biologics compared to patients with psoriasis with mild disease, with no psoriatic arthritis and on no biologics, respectively.
For the overall psoriasis study sample, cumulative rates of comorbid conditions increased over time, with the rates of hypertension, hyperlipidemia, and psoriatic arthritis being 25.9%, 23.4%, and 8.4%, respectively, during the first year following psoriasis diagnosis (i.e. 12-month post-index). The cumulative rate of psoriatic arthritis among psoriasis patients more than tripled by end of year 4 of follow-up from baseline (13.6%, n = 16,853) ().
Table 2. Cumulative rates of comorbid conditions at baseline and following psoriasis diagnosis of psoriasis patients.
Healthcare utilization and costs
presents all-cause healthcare utilization during the variable length of follow-up for all study cohorts. For the overall psoriasis study sample, 14.3% had at least one hospitalization. The mean number of emergency room visits and physician office visits were 0.3 and 7.1 per year, respectively. Psoriasis patients received, on average, 15.1 outpatient prescriptions per year. The utilization rates were generally higher for psoriasis patients with moderate-to-severe disease, for psoriasis patients with psoriatic arthritis and psoriasis patients treated with biologics compared to those with mild disease, with no psoriatic arthritis, and on no biologics. Numerically, the utilization rates were highest for psoriasis patients with psoriatic arthritis: 18.8% had at least one hospitalization during follow-up, their mean number of emergency room visits and physician office visits were 0.4 and 9.7 per year, respectively, and they received on average 22.2 outpatient prescriptions per year.
Table 3. All-cause healthcare utilization during variable follow-up period.
All-cause healthcare costs for psoriasis patients amounted to an average of $12,523 (median = $5,626) PPPY during the variable length of follow-up. Outpatient services and outpatient pharmacy were the main cost drivers, accounting for 44.1% and 38.6% of total costs, respectively. Psoriasis patients with psoriatic arthritis incurred more than double the costs of psoriasis patients with no comorbid psoriatic arthritis ($23,427 vs $10,965, p < .001; median $17,443 vs $4,981), Furthermore, all-cause healthcare costs for psoriasis patients with moderate-to-severe disease and psoriasis patients treated with biologics were $21,481 (median = $14,872) and $29,832 (median = $25,641), respectively, 2.5- and 3.2-times the all-cause healthcare costs of those with mild disease and those who did not receive biologics (p < .001 in both comparisons) (). The cost differences were primarily driven by outpatient pharmacy, which accounted for 48.9%, 52.2%, and 62.5% of total costs in psoriasis patients with psoriatic arthritis, in psoriasis patients with moderate-to-severe disease, and in psoriasis patients treated with biologics, respectively.
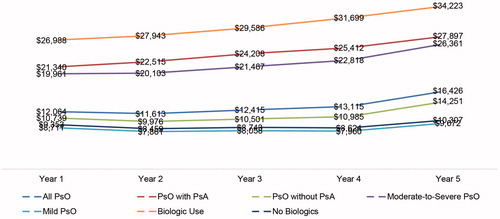
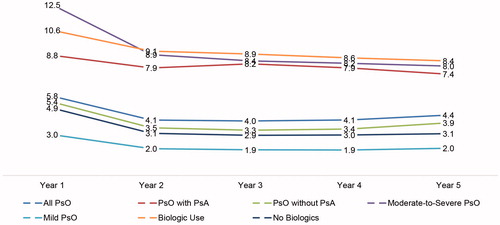
presents the average all-cause healthcare costs from discrete year 1 to year 5. The all-cause healthcare costs for the overall psoriasis study sample increased steadily over time, from $12,064 in the first year of psoriasis diagnosis to $16,426 in year 5. The upward trend was far more pronounced among the sub-groups of psoriasis patients with psoriatic arthritis, psoriasis patients with moderate-to-severe disease, and psoriasis patients treated with biologics. Compared to year 1, the annual all-cause healthcare costs increased by $6,557, $6,400, and $7,235 in year 5 for psoriasis patients with psoriatic arthritis, psoriasis patients with moderate-to-severe disease, and psoriasis patients treated with biologics, respectively.
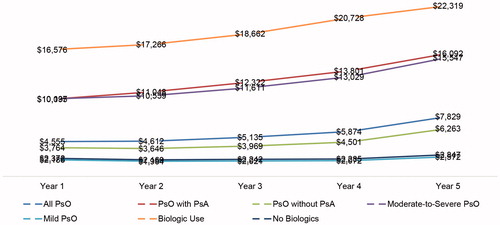
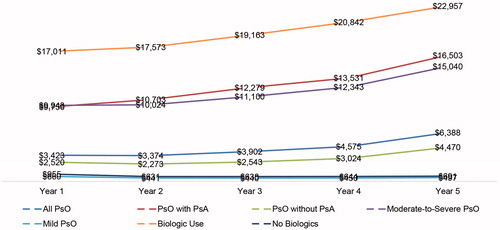
All-cause outpatient pharmacy and psoriasis-specific pharmacy costs followed the same upward trend, as shown in and . Among the overall psoriasis study sample, all-cause outpatient pharmacy costs rose from $4,555 in year 1 to $7,829 in year 5, with the largest increase observed in psoriasis patients with psoriatic arthritis ($5,996), in psoriasis patients treated with biologics ($5,744), and in psoriasis patients with moderate-to-severe disease ($5,411). Psoriasis patients with no comorbid psoriatic arthritis ($2,499), with mild disease ($387), or not treated with biologics ($475) also had increases during the same period (). Similarly, psoriasis-related pharmacy costs increased from $3,423 in year 1 to $6,388 in year 5 for the overall psoriasis study sample. The increase was highest among psoriasis patients with psoriatic arthritis ($6,753), in patients with moderate-to-severe disease ($5,098), and in those treated with biologics ($5,946) (), despite a decline in the number of prescriptions/claims over time ().
Discussion
Based on a large national representative claims database for a commercially insured population, this study provided up-to-date healthcare costs, both short- and long-term, for patients with psoriasis overall, as well as for sub-groups of psoriasis patients stratified by disease severity, presence of psoriatic arthritis comorbidity, and biologic use. Psoriasis patients ever treated with a biologic incurred the highest all-cause healthcare costs over time ($29,832 PPPY), ∼ 3.5-times the PPPY costs of psoriasis patients with mild disease ($8,723). Patients with psoriasis who also have psoriatic arthritis had the second highest all-cause healthcare costs estimated at $23,427 PPPY; 48.9% of which came from outpatient pharmacy. The current study further contributes to the understanding of the economic burden of psoriasis by showing the progression of all-cause healthcare costs over time, and showcasing the 5-year cost and utilization trends for psoriasis patients overall and sub-groups of psoriasis patients of interest. The findings of this study consistently showed the year-over-year upward trend for all-cause healthcare costs, all-cause outpatient pharmacy costs, and psoriasis-specific pharmacy costs in all psoriasis patient groups. The percentage increase due to more biologic treatment over time was not examined.
In a study of 543,231 patients with psoriasis based on the 2007 Medical Expenditure Panel Survey (MEPS) database, Lin et al.Citation22 found mean psoriasis-specific annual drug costs to be $1,309 per patient, as compared to our findings of $4,829 for all psoriasis patients. The difference in cost may be attributed to older data (2007), a smaller percentage of patients on biologic or systemic therapy (9.7% vs 15.4% in our cohort), or changes in drug prices over timeCitation22. Similar to our findings, the use of biologic or systemic therapy was associated with a 2.5-times increase in annual medication spendingCitation22. Kimball et al.Citation23 compared healthcare utilization and costs among psoriasis patients with and without comorbidities and estimated 6-month average costs to be $1,980 for psoriasis patients without comorbidities and $6,103 for patients with psoriatic arthritis. Our findings are higher, but our study presents annual, rather than 6-month costs and newer data when the use of biologics has increased and associated biologic prices may have gone higher. Additionally, their study found that patients with psoriatic arthritis used more inpatient, outpatient, and emergency care services as compared to psoriasis patients without psoriatic arthritisCitation23. These findings are consistent with our results that show higher inpatient hospitalizations, outpatient office visits, and emergency room visits among psoriasis patients with psoriatic arthritis. Finally, a more recent study by Wu et al.Citation24 examined healthcare resource utilization and costs among psoriasis patients treated with two biologics between 2009–2012 and found that, similar to our study, pharmacy costs made up the largest cost component. Total healthcare costs were estimated at $48,423 and $33,421 for patients treated with ustekinumab and adalimumab, respectivelyCitation24. These estimates are higher, but similar to our findings for psoriasis patients with biologic use ($34,186 per patient in year 5 of follow-up).
Findings from this study are subject to a number of limitations. First, inherent in the use of administrative claims data is the potential for misclassification of psoriasis and comorbidities due to miscoding of claims. Clinical information could not be verified through patient records or chart review. Second, there are no readily available diagnoses to capture psoriasis severity. As a proxy, we used treatment as an indicator, which may have resulted in misclassification of disease severity. Third, patients with psoriasis but no healthcare services such as physician office visits or treatment for longer than 6 months pre-index were excluded, which may have biased cost estimates. Fourth, this study examined a commercially insured population, and, thus, the findings may not be generalizable to patients enrolled in Medicare, Medicaid, other insurance policies, or the uninsured. Lastly, this study is descriptive in nature and has not applied adjustments to account for differences in the comparison patient sub-groups.
Conclusions
Psoriasis is associated with significant economic burden, which increases over time as the disease progresses. Overall, psoriasis patients with moderate-to-severe disease, those who have co-existing psoriatic arthritis, or are being treated with biologics contribute to higher healthcare costs than psoriasis patients with mild disease, those who do not have psoriatic arthritis or are not treated with biologics, respectively. Psoriasis-related pharmacy expenditure is the largest driver of overall healthcare costs among all psoriasis patients.
Transparency
Declaration of funding
Funding for the study was provided by Eli Lilly and Company.
Declaration of financial/other relationships
SAS, SAF, OMG, WNM, and BZ are employees and stockholders of Eli Lilly and Company. NS and XS are employees of Truven Health Analytics. Truven Health Analytics received funding from Eli Lilly and Company to conduct this analysis. SRF is an employee of The Wake Forest University School of Medicine and serves as a clinical advisor for Eli Lilly. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Caroline Henriques performed programming for this study. Ms Janna Manjelievskaia assisted with the development of the manuscript.
References
- Gunnarsson C, Chen J, Rizzo JA, et al. The direct healthcare insurer and out-of-pocket expenditures of psoriasis: evidence from a United States national survey. J Dermatolog Treat 2012;23:240-54
- Raval K, Lofland JH, Waters H, et al. Disease and treatment burden of psoriasis: examining the impact of biologics. J Drugs Dermatol 2011;10:189-96
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc 2004;9:136-9
- Disorders, National Institute of Arthritis and Muscoloskeletal and Skin. Questions and answers about psoriasis. 2015. https://www.niams.nih.gov/health_info/psoriasis/. Accessed July 26, 2015
- Farber EM, Nall ML. The natural history of psoriasis in 5,600 patients. Dermatologica 1974;148:1-18
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol 2008;59:772-80
- Lewis-Beck C, Abouzaid S, Xie L, et al. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer Adherence 2013;7:199-205
- Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis 2005;64(Suppl2):ii18-23
- Foundation, National Psoriasis. About psoriasis. 2015. https://www.psoriasis.org/about-psoriasis. Accessed July 26, 2015
- Crown WH. The cost of psoriasis. Manag Care 2013;12:10-13; discussion 20–1
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol 2012;66:e67-76
- Zachariae H, Zachariae R, Blomqvist K, et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108-13
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008;58:826-50
- Yu AP, Tang J, Xie J, et al. Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin 2009;25:2429-38
- Foundation, National Psoriasis. Topical treatments. 2015. https://www.psoriasis.org/about-psoriasis/treatments/topicals. July 26, 2015
- Foundation, National Psoriasis. Moderate to severe psoriasis and psoriatic arthritis: Biologic drugs. 2015. https://www.psoriasis.org/about-psoriasis/treatments/biologics. Accessed July 26, 2015
- Foundation, National Psoriasis. Traditional systemic medications. 2015. https://www.psoriasis.org/about-psoriasis/treatments/systemics. Accessed July 26, 2015
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol 2009;61:451-85
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol 2015;151:651-8
- Feldman SR, Zhao Y, Shi L, et al. Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J Manag Care Spec Pharm 2015;21:874-88
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- Lin H, Lucas PT, Feldman SR, et al. Medication use and associated health care outcomes and costs for patients with psoriasis in the United States. J Dermatol Treat 2012;23:196-202
- Kimball AB, Guérin A, Tsaneva M, et al. Economic burden of comorbidities in patients with psoriasis is substantial. J Eur Acad Dermatol Venereol 2011;25:157-63
- Wu, JJ, Guérin A, Gauthier G, et al. Healthcare resource utilization, healthcare costs and dose escalation in psoriasis patients initiated on ustekinumab versus adalimumab: a retrospective claim study. J Dermatol Treat 2016;15:1-9