Abstract
Aims: Data highlighting the cost drivers for non-valvular atrial fibrillation (NVAF) patients in terms of vitamin K antagonist (VKA) treatment and monitoring are lacking in France. This study aimed to evaluate the real-life daily cost of VKA treatment in 2013, in French patients suffering from NVAF.
Methods: This longitudinal observational study was performed using the EGB (Echantillon Généraliste des Bénéficiaires) database, a random sample of the French national insurance (NHI) database, which covers 80% of the population. All adult patients whose first NVAF anticoagulant treatment in 2013 was a VKA were analyzed. Costs were calculated for the duration of follow-up and then divided by the number of days of therapy. The analysis was performed both from the French NHI perspective (amount reimbursed by the NHI) and from a collective perspective.
Results: In this study, 3,254 NVAF patients treated with VKA in 2013 were included, and this sample comprised 52.6% males. The mean daily cost of VKA treatment was €1.13 (±1.18) according to the collective perspective (89.4% of this cost was associated to INR measurement) and €1.05 (±1.16) according to the NHI perspective.
Limitations: As diagnoses associated with procedures are not available in the EGB database, proxies were used, and an algorithm was created to define the AF population.
Conclusions: This analysis is the first to consider an exhaustive spectrum of the costs of VKA treatment in France using EGB data. VKA medication requires exhaustive follow-up, and, thus, associated costs are important. The results of the present study confirmed this close follow-up for VKA patients, making the cost of treatment by VKA nearly 10-times more expensive than the cost of medication itself.
Introduction
Atrial Fibrillation (AF) is the most common cardiac arrhythmia. In France, its prevalence is estimated between 600,000 and one million people, two thirds of whom are over 75 years, and annual incidence is estimated between 110,000–230,000 new casesCitation1. AF is associated with a 5-fold increased risk of ischemic stroke, considered as its principal complication. AF is one of the major risk factors for stroke and thromboembolism, and, when stroke occurs in patients with AF, there is a higher mortality, disability, and longer hospital stays, resulting in higher associated healthcare costsCitation2–6.
The leading anticoagulation treatment options for the prevention of stroke in AF patients are Vitamin K antagonists (VKAs)Citation7. VKAs were introduced on the market ∼60 years ago and were, up until recently, the only oral anticoagulant treatmentsCitation8. These molecules have demonstrated their effectiveness in different indicationsCitation9,Citation10, but have a therapeutic balance that is difficult to maintain due to their narrow therapeutic window, their variable inter- and intra-individual response, and their drug and food interactionsCitation11,Citation12. For these reasons, treatment with VKA requires very regular laboratory tests of coagulation with International Normalised Ratio (INR) measurementCitation13. Owing to the necessity of maintaining patients in a narrow therapeutic window by repeated laboratory tests, it has been shown that the total cost of anticoagulant therapy with VKA entails much more than the cost of the drug. To date, few data are available in France concerning the main cost drivers for NVAF patients in terms of VKA treatment and monitoring. In this context, it is important to further understand the overall cost of medical therapy with VKA including real-life cost of laboratory test follow-up associated to adequately controlling patients. Therefore, the main objective of this study was to evaluate the real-life daily cost of VKA treatment (medication and INR testing) in 2013, in French patients suffering from NVAF.
Methods
Data sources
This was a population-based study of a cohort of patients identified from Echantillon généraliste de bénéficiaires (EGB database; French permanent representative sample of health insurance beneficiaries), which comprises a representative 1/97th random sample of the population covered by the French national health insurance (NHI) system, Système national d'information inter-régimes de l'Assurance maladie (SNIIR-AM)Citation14,Citation15. The EGB database currently includes more than 600,000 beneficiaries, and has been used widely for public health and pharmacoepidemiological purposesCitation14–16 and medico-economic studiesCitation17. Since 2005, the EGB database includes basic demographic data and prospectively collects all claims for visits to physicians and reimbursed drugs dispensed in retail pharmacies (including quantity delivered, dates of prescription, and date of dispensing). No direct information on the medical indication is linked with each reimbursement, but the SNIIR-AM includes information on long-term disease (LTD) status coded in International Classification of Diseases 10th version (ICD-10 codes). LTD status allows patients to receive treatment for severe and costly conditions without out-of-pocket payment. SNIIR-AM also contains information on free-access-to-care status, which enables patients of lower socioeconomic status to receive free medical care. The EGB database also contains data collected by the Programme de médicalisation des systèmes d’information (PMSI; program for medicalization of information systems) in healthcare institutions (medical and surgical departments); thus, during the patient’s stay, principal diagnoses and associated diagnoses are available, coded according to the International classification of diseases 10th edition (ICD-10). Associated diagnoses represent a proxy for comorbidity assessment and identification of triggering factors. All medical procedures performed during each stay are identified with their specific codes from the common classification of medical procedures. The EGB database also includes registration of the date of death, recorded automatically from the Institut national de la statistique et des études économiques (INSEE; National institute for statistics and economic studies), independent of the use or not of healthcare resources.
Study design
Using the EGB database, we conducted a longitudinal observational study and included patients treated with VKA for Non-Valvular Atrial Fibrillation (NVAF) in 2013. This study was conducted on anonymized data, and the National Informatics and Liberty Committee (CNIL) has delivered an overall authorization to use EGB data for research purposes. This study was performed after approval by the French Institute for Health Data (Institut des Données de Santé, approval n° 117, March 10, 2015). NVAF patients were followed for 1 year, starting from index date, defined as the first dispensation date of VKA in 2013. VKA discontinuation was defined as neither VKA delivered nor INR performed for at least 60 days. The following variables were considered: patient demographics (age, sex), stroke risk quantified using the CHA2DS2-VASc (Cardiac failure or dysfunction, Hypertension, Age 65–74 or, Age ≥75 [Doubled], Diabetes, Stroke [Doubled]-Vascular disease, and Sex category [Female]) score, VKA treatment type, number of INR tests performed to assess coagulation level (INR analysis, blood sample collection by the clinical laboratory, possible patient’s transportation), and number of Generalist Practitioners (GPs) and specialists visits related to VKA renewal and INR follow-up.
Study population
All adult patients treated for NVAF and whose first NVAF treatment in 2013 was a VKA identified through reimbursement were extracted from the database.
Adult patients belonging to the General French Social Security scheme (Régime Général de la Sécurité Sociale) with a reimbursement of VKA in 2013 and meeting at least one of the following inclusion criteria were considered as suffering from NVAF and included in the study:
In LTD, under the heading of the 5th exonerating disorder entitled “Severe heart failure, severe cardiac dysrhythmia, severe valvular heart disease, severe congenital heart disease” with an AF diagnosis (ICD-10 I48 code) in the 36 months prior to index date; or
A diagnosis of AF (ICD-10 code I48) in hospitalization data (PMSI) in the 36 months prior to index date; or
A reimbursement of an anti-arrhythmia treatment (C01B class) in the 40 days prior to or after the index date; or
An act of cardioversion in hospitalization data (PMSI) in the 36 months prior to index date; or
An act of AF ablation in hospitalization data (PMSI) in the 36 months prior to index date.
Conversely, patients with Valvular Atrial Fibrillation considered with at least one medical code for cardiac valve surgery were identified in the 3 years prior and up to the index date (i.e. patients with a procedure related to a valvular cardiac surgery or a diagnosis related to a valvular disease (ICD-10 codes I05, I08, and Z95) in hospitalization data were excluded from the analyses).
Furthermore, in order to identify patients that initiated VKA treatment from patients that were already under treatment and followed-up regularly, three sub-populations of patients were derived from the study population and defined as follows ():
Figure 1. Definition of sub-groups of patients: strictly incident, strictly prevalent, and non-incident non-prevalent patients.

Strictly incident patients: patients who did not receive any deliveries of VKA during the 6 months prior to index date;
Strictly prevalent patients: patients with at least two deliveries of VKA before index date, with less than 60 days between these deliveries; and
Non-incident non-prevalent patients: all patients that did not comply with definitions of strictly incident and prevalent above.
The rationale of these sub-populations was to describe the cost of VKA initiation and the cost of patients regularly treated, after the dose adjustment period following the VKA initiation.
Cost estimation
The analysis was performed both from the French NHI perspective (amount reimbursed by the National Health Insurance) and from a collective perspective. Costs estimated from the collective perspective include all the costs reimbursed by the basic universal public health insurance system and all the costs not reimbursed, such as extra fees applied by doctors usually reimbursed by a complementary private insurance. Costs were calculated for the duration of follow-up and then divided by the number of days of therapy.
For patients without discontinuation of treatment, the number of days of therapy was 365 days.
A patient was considered as having discontinued treatment with VKA if no VKA nor any INR was reimbursed during 2 months or more. In this case, the number of days of therapy was calculated by taking into account the date of the first and of the last delivery of VKA, to which was added 30 days. A sensitivity analysis extending the therapeutic window to 4 months without VKA nor INR reimbursement to assess treatment discontinuation was performed.
The cost was expressed as a mean cost per day per patient, in euros. This daily cost was calculated by summing the following items:
VKA medication costs, which consisted of all VKA treatments dispensed during the follow-up; and
INR measurements costs in outpatient practice (blood sample collection, analysis and transportation) comprised all INRs performed during the follow-up. The blood sample collection referred to a clinical laboratory or a nurse coming at the patient’s home to collect the sample. Possible transportation costs related to INRs were those generated at the same date as an INR. They were estimated as follows:
– If there was a blood sample collection registered the same day as the INR test, it was considered that a clinical laboratory performed the blood sample collection. In this case, INR total cost was calculated as the sum of INR analysis, blood sample collection by the clinical laboratory, and possible patient’s transportation if they occurred the same day.
– If there was no blood sample collection registered and there was a nursing care registered the same day as the INR test, it was considered that a nurse performed the blood sample collection, and the INR total cost was calculated as the sum of INR analysis, nurse care, any nurse transportation, any specific extra fees for Sunday or night act, and/or additional transportation fees if they occurred the same day.
Furthermore, the daily cost of doctor visits related to INRs and to VKA medication renewal was estimated in order to assess the overall cost of NVAF anticoagulant treatment management. These daily costs were calculated by summing the following items:
the daily cost generated by all doctors’ visits (GPs and specialists) related to follow-up of INRs defined as follows:
– the visit immediately following the conduct of an INR with or without prescription of VKA at this date within a limit of 10 days, and
– the cost of the visit immediately prior to the conduct of an INR with or without prescription of VKA at this date within a limit of 1 month; and
the daily cost generated by all doctors’ (GPs and specialists) visits related to VKA medication renewal, excluding visits already considered as related to the follow-up of INRs. The latest consultation within the 7 days prior to VKA delivery—including day of delivery—was considered as related to a VKA prescription
Statistical analysis
All analyses were descriptive. The number of data provided and missing data were systematically indicated. Quantitative variables were described using mean, standard deviation, median, minimum, maximum, 1st and 3rdrd quartiles. The costs between “strictly incident patients” and “strictly prevalent patients” were compared using the t-Student test for normally distributed variables, and 95% confidence intervals were calculated for the study endpoints, to have a better vision of uncertainty. Qualitative variables were described by sample size (data provided, missing values) within each modality and relative percentages. Missing values were excluded from the calculation of percentages.
All the analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
In 2013, 572,778 patients were extracted from the EGB database. The overall population enrolled in the present study consisted of 3,254 patients treated with VKA for NVAF in 2013 and compliant with the inclusion/exclusion criteria. Among these patients, 52.6% were male, and the mean age was 76.1 ± 10.3 years. According to the definitions used for the sub-populations, 15.4% of the patients (n = 500) were defined as strictly incident, 42.3% (n = 1,376) strictly prevalent, and 42.3% (n = 1,378) did not respect the definitions of strictly incident or strictly prevalent patients.
There were 47.2% of males among strictly incident patients, with a mean age of 77.2 ± 11.0 years, 57.8% of males among strictly prevalent patients, with a mean age of 74.7 ± 10.4 years, and 49.3% of males among non-incident non-prevalent patients with a mean age of 77.1 ± 9.7 years. Demographic characteristics of patients are summarized in .
Table 1. Demographic characteristics and health information of NVAF patients treated with VKA in 2013.
Furthermore, as described in , the mean CHA2DS2-VASc score was 3.5 ± 1.4 in the entire study population, with a score ≥2 observed for over 90% of the entire study population, as well as each sub-population of analysis.
On average, the total cost of VKA treatment in collective perspective was €1.13 ± €1.18 per day per patient and €1.05 (±1.16) (92.9%) was reimbursed by the NHI. This cost can be broken down into VKA medication, representing 10.6% (€0.12 ± €0.06), and INR measurements, representing 89.4% (€1.01 ± €1.18), which includes the cost of blood sample collection (38.0%), the cost of analyses related to INR (55.0%), and the cost of transportation related to INR testing (7.0%) (). The results estimated with the sensitivity analysis were similar to the primary analysis, with a total daily cost of VKA treatment of €1.10 (€1.13). Furthermore, the costs observed for non-incident non-prevalent patients showed the same tendencies as compared to the costs obtained for prevalent patients, with a total mean daily cost of €1.07 (±1.35).
Table 2. Overall VKA treatment daily cost (€) per NVAF patient treated with VKA in 2013.
The analyses performed according to sub-groups of patients showed a higher cost among incident patients with total mean ± SD daily cost of VKA treatment of €1.66 ± €1.51, as opposed to a cost of €1.00 ± €0.73 for prevalent patients (p < 0.001) (). The NHI reimbursement rate was ∼89% for incident patients, and ∼94% for prevalent patients.
Table 3. Overall VKA treatment daily cost (€) per incident and prevalent patient treated with VKA in 2013.
Furthermore, the overall daily cost of NVAF anticoagulant management in 2013 was €2.07 (€1.63). This cost comprised 55% of VKA treatment, 43% of doctor visits related to INR follow-up, and 2% of doctor visits related to VKA renewal. The amount reimbursed by the NHI represented 89.9% of the total NVAF anticoagulant management cost ().
Table 4. NVAF anticoagulant management costs (€) per patient treated with VKA in 2013.
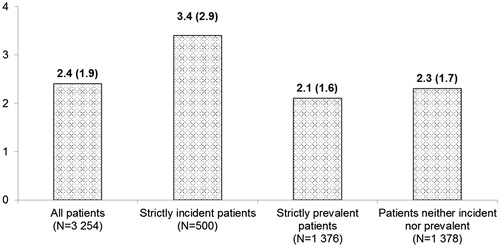
Finally, the number of INRs per month was estimated, and the results showed that the average number of INRs per month was higher among strictly incident patients, as opposed to the entire population or to the strictly prevalent patients ().
Discussion
Using comprehensive data from the French NHI, the present study provides detailed information on daily cost of VKA treatment in French patients with NVAF in 2013.
To the best of our knowledge, this is the first study that takes into consideration all the costs related to VKA treatment and INR follow-up in AF patients, using NHI data in France. The costs estimated in the present study were higher than recent published estimations for FranceCitation18–20, as they did not consider patient transportation and/or blood sample collection costs. The daily cost reimbursed by the NHI in the present study was evaluated to €1.05, compared to €0.42 according to an analysis performed by the CNAMTSCitation18 and to the €0.77 observed in the de PouvourvilleCitation19 assessment, highlighting the differences in the methods used, the populations analyzed, and the costs considered in the INRs measurements.
Furthermore, in order to evaluate the most cost driving period during the entire follow-up, the total cost of VKA treatment was estimated in three different sub-cohorts of patients, i.e. strictly incident patients referred to patients with no evidence of anticoagulant treatment immediately before index prescription (6 months), strictly prevalent patients corresponded to patients with clear evidence of anticoagulant treatment before index prescription (two prescriptions), and non-incident non-prevalent patients corresponded to patients who did not received two prescriptions of VKA before the index day. These analyses highlighted that the cost of VKA treatment was higher in incident patients, and main explanation for these results is related to the number of INRs performed by incident and prevalent patients. The results showed that the average number of INRs measurements per month was 3.4 (2.9) for incident patients, and 2.1 (1.6) for prevalent patients. This being probably related to the need for a closer management during the titration period immediately after initiation, as mentioned in the ANSM recommendationCitation21.
Finally, accounting for the possible costs related to physicians visits, the estimated cost of NVAF anticoagulant management was estimated at €2.07 per day. However, as the reasons for physician visits were not available in the database, the link between a VKA delivery or INR follow-up was considered using time window proxies (based on expert definitions). A recent French study reported that a large proportion of patients treated with VKA are followed-up by their GP through phone calls (54% during initiation phase and 64% during maintenance phase); estimation of the cost related to physicians’ visit is, thus, possibly over-estimated. Therefore, the real cost of NVAF anticoagulant management is probably between €1.15 and €2.07 per day.
Strengths and limitations
Our study provides novel evidence about the costs of VKA treatment among a large population of AF patients. We used EGB data that were extracted from the French NHI reimbursement database. These data have been extensively used and validated in previous researchCitation14,Citation22, highlighting that the results of this study could be extrapolated to the other NVAF patients in France. An exhaustive analysis of the costs of VKA treatment in real life was performed considering the cost of medication, as well as the total cost of INRs measurements, including blood sample collection, analysis, transportation, and all the related extra fees. The sensitivity analysis extending the therapeutic window to 4 months without VKA or INR to assess treatment discontinuation confirmed that the original assumption can be taken into consideration when evaluating treatment duration. However, the EGB database presents some limitations, such as the lack of diagnoses associated with procedures, as well as the unavailability of a comprehensive medical profile of AF patients that are not all recorded in LTD. In order to overcome the lack of diagnoses, proxies were used and an algorithm was created to define the AF population. Moreover, as the reasons for physician visits were not available in the database, the link between a VKA delivery or INR follow-up was considered using time window proxies (based on expert definitions), possibly over-estimating the costs of doctor visits in the present study. A recent French studyCitation23 estimated, from a collective perspective, a daily cost related to INR of €0.10, as opposed to the €0.89 observed in our study, and €0.09, as opposed to €0.77, from the NHI perspective. Adding these INR-related costs to those of VKA treatment would result in a cost of NVAF management of €1.23, as opposed to the €2.02 observed in the present study. Additionally, this database registers only reimbursed medical care. Neither self-medication nor consumption of prescribed but non-reimbursed medications appear in the database. Finally, coagulation related events such as major bleeding or stroke, VKA pharmaceutical interviews, as well as anticoagulation self-monitoring were not taken into consideration in the analyses. These costs could lead to higher economic burden.
Conclusions
Based on data from the French NHI and considering an exhaustive spectrum of the costs of VKA treatment, this analysis showed that, in real life, the costs of VKA medication in patients with AF cannot be appreciated by considering the drug costs alone, as these accounted for only 11% of the cost estimated in this study.
VKA medication requires exhaustive follow-up and, thus, associated costs are important. The results of the present study showed that the cost of VKA treatment is nearly 10-times more expensive than the cost of medication itself.
Transparency
Declaration of funding
The study was sponsored by Bayer HealthCare SAS.
Declaration of financial/other relationships
JD and JYLH have received honoraria and research support from Bayer HealthCare. XA, NK, and FM are employed by QuintilesIMS. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
Some of the data presented in this manuscript was presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) conference in 2017, in Boston.
References
- Charlemagne A, Blacher J, Cohen A, et al. Epidemiology of atrial fibrillation in France: extrapolation of international epidemiological data to France and analysis of French hospitalization data. Arch Cardiovasc Dis 2011;104:115-24
- Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke J Cereb Circ 2005;36:1115-19
- Brüggenjürgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the berlin acute stroke study. Value Health J Int Soc Pharmacoeconomics Outcomes Res 2007;10:137-43
- Ghatnekar O, Glader E-L. The effect of atrial fibrillation on stroke-related inpatient costs in Sweden: a 3-year analysis of registry incidence data from 2001. Value Health J Int Soc Pharmacoeconomics Outcomes Res 2008;11:862-8
- Lamassa M, Di Carlo A, Pracucci G, et al. Characteristics, outcome, and care of stroke associated with atrial fibrillation in Europe: data from a multicenter multinational hospital-based registry (The European Community Stroke Project). Stroke J Cereb Circ 2001;32:392-8
- Le Heuzey J-Y, Paziaud O, Piot O, et al. Cost of care distribution in atrial fibrillation patients: the COCAF study. Am Heart J 2004;147:121-6
- Le Heuzey J-Y, Ammentorp B, Darius H, et al. Differences among western European countries in anticoagulation management of atrial fibrillation. Data from the PREFER IN AF registry. Thromb Haemost 2014;111:833-41
- Tendera M, Syzdół M, Parma Z. ARISTOTLE RE-LYs on the ROCKET. What’s new in stroke prevention in patients with atrial fibrillation? Cardiol J 2012;19:4-10
- Bushnell CD, Matchar DB. Pharmacoeconomics of atrial fibrillation and stroke prevention. Am J Manag Care 2004;10(3Suppl):S66-S71
- Szucs TD, Bramkamp M. Pharmacoeconomics of anticoagulation therapy for stroke prevention in atrial fibrillation: a review. J Thromb Haemost JTH 2006;4:1180-5
- Boulanger L, Kim J, Friedman M, et al. Patterns of use of antithrombotic therapy and quality of anticoagulation among patients with non-valvular atrial fibrillation in clinical practice. Int J Clin Pract 2006;60:258-64
- Beinema M, Brouwers JRBJ, Schalekamp T, et al. Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. Thromb Haemost 2008;100:1052-7
- Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy. Chest 2012;141(2Suppl):e152S-e184S
- Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Dépidémiologie Santé Publique 2010;58:286-90
- Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: What interest for medical research? Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne 2015;36:411-17
- Martin-Latry K, Bégaud B. Pharmacoepidemiological research using French reimbursement databases: yes we can! Pharmacoepidemiol Drug Saf 2010;19:256-65
- Belhassen M, Demoly P, Bloch-Morot E, et al. Costs of perennial allergic rhinitis and allergic asthma increase with severity and poor disease control. Allergy 2016;72:948-58
- Caisse Nationale d’Assurance Maladie. Nouveaux anti-coagulants oraux: une étude de l’Assurance Maladie souligne la dynamique forte de ces nouveaux médicaments et la nécessité d’une vigilance accrue dans leur utilisation; CNAM, France: Etude de l’Assurance Maladie sur l’utilisation des nouveaux anti-coagulants orau. 2013
- de Pouvourville G. Comment améliorer le suivi des AVK et pour quel coût? Arch Cardiovasc Dis Suppl 2016;8:169-73
- Detournay B. Coût direct des AVK en France. Arch Cardiovasc Dis Suppl 2016;8:174-9
- ANSM AN de la S. Bon usage des médicaments antivitamine K (AVK): actualisation-Juillet; ANSM, France: Bon usage des médicaments antivitamine K (AVKI) : Actualisation - Juillet 2012. 2012
- Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: What interest for medical research? Rev Med Interne 2015;36:411-17
- Ansolabehere X, Augier G. Cost of visits associated with management of international normalized ratio (INR) results in atrial fibrillation (AF) patients treated with Vitamin K Antagonist (VKA). Value in Health 2017