Abstract
Aim: This analysis assessed the direct medical costs of newly-diagnosed, temozolomide (TMZ)-treated glioblastoma (GBM) from the perspective of a US commercial setting.
Materials and methods: The analysis included subjects identified from the IMS PharMetrics LifeLink Plus™ claims database from January 1, 2008 to August 31, 2014 who were ≥18 years of age, had ≥1 malignant brain cancer diagnosis, had brain surgery ≤90 days prior to TMZ initiation, had TMZ treatment, and were continuously enrolled for ≥12 months pre-diagnosis and ≥1 month post-diagnosis. Per-patient per-month (PPPM) and cumulative costs from 3 months pre-diagnosis to various post-diagnosis follow-up time points were calculated. Multivariable analyses were used to estimate adjusted mean cost and identify contributors of cost.
Results: The study included 2,921 subjects (median age = 56 years; 60% male). After diagnosis, the median (interquartile range, IQR) number of inpatient, emergency department, and outpatient visits were 2 (1–4), 1 (1–3), and 19 (13–27); median (IQR) length of stay per hospitalization was 5 (3–9) days. Mean total cumulative costs per patient from 3 months pre-diagnosis to 12 months and to 5 years post-diagnosis were $201,749 (197,490–206,024) and $268,031 (262,877–274,416). Mean (SD) PPPM costs were $818 (1,128) and $7,394 (8,676) pre- and post-GBM diagnosis, respectively. The variables most predictive of cumulative costs included radiation therapy (+$81,732), ≥2 weeks of hospitalization (+$49,629), and ≥7 MRI scans (+$40,105).
Conclusions: The direct medical costs of newly-diagnosed, TMZ-treated GBM in commercially insured patients are substantial, with estimated total cumulative costs of $268,031.
Introduction
Glioblastoma (GBM) is an aggressive, high-grade brain tumor associated with a significant clinical burdenCitation1. Nearly half of adult primary malignant brain tumors are GBM. According to the Central Brain Tumor Registry of the US, the average annual age-adjusted incidence of GBM is 3.2 cases per 100,000 peopleCitation2. In the US, ∼12,000 people were diagnosed with GBM in 2013Citation3. These tumors tend to occur in adults between the ages of 45–70 years, with a median age in the mid-60s. The number of cases is expected to increase as the population ages.
Patients are often severely affected by symptoms prior to and after GBM diagnosis. Common symptoms include headaches, seizures, cognitive or personality changes, and various focal neurological deficits that reflect the location of the lesion within the brainCitation4. Treatment for newly-diagnosed GBM is aggressive and typically includes surgical resection, radiation therapy, and chemotherapy with temozolomide (TMZ), but, despite advances in these therapies, outcomes remain poor in most patients, with a 5-year survival rate of ∼5%Citation2. Other FDA - approved treatments include carmustine (to treat metastatic brain tumors) and bevacizumab (to treat recurrent glioblastoma).
As the use of TMZ and bevacizumab increased, it has resulted in an increase of overall cost in glioblastomaCitation5. A recently published analysis evaluated the direct medical costs of GBM in a US commercial insurance setting, including patients who receive Medicare benefits through employer-sponsored commercial health plansCitation6. In this analysis, which included patients with a mean (SD) age of 56 (14.5) (29.9% were ≥65 years old), the average total costs (including both patient paid amount and plan paid amount) per patient in the 6 and 12 months following a GBM-related surgery were $138,767 and $184,107, respectively. Although this study provided total and per-patient per-month (PPPM) costs within 12 months after brain surgery, it did not provide a longitudinal assessment of total cumulative costs, including from prior to the time of surgery, when GBM-related costs may already accrue.
The objectives of our analysis were: (1) to summarize patient characteristics including clinical and demographic profiles of newly-diagnosed, TMZ-treated US GBM patients who are commercially insured; (2) to estimate the cumulative healthcare resource utilization (HCRU) and costs from as far as 12 months prior to a GBM diagnosis until 5 years post-diagnosis; and (3) to provide further insight into covariates that may be independently predictive of total cost of GBM care at 1 year post-diagnosis.
Methods
Database and study design
This retrospective database analysis utilized IMS LifeLink PharMetrics Plus™ commercial claims, which consists of adjudicated claims of more than 150 million unique enrollees across the US since 2006, including more than 95 million individuals with both medical and pharmacy benefits. The key variables included in this dataset are patient demographics, payer types, inpatient/outpatient diagnoses and procedures, prescription records, pharmacy and medical benefits, inpatient stay and provider details, and enrollment information. Costs were calculated based on the allowable charges, which includes paid amount to provider and patient co-pay. All data were anonymized to ensure Health Insurance Portability and Accountability Act (HIPAA) compliance.
Patient selection and follow-up
Because GBM lacks a specific International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis code, a previously described algorithm was adapted to identify GBM patients based on types of treatment receivedCitation6. This analysis included subjects aged ≥18 years, identified from January 1, 2008 to August 31, 2014, who had ≥1 malignant brain cancer diagnosis (ICD-9-CM, 191.xx), treated with TMZ, had brain surgery 90 days prior to TMZ initiation, and were continuously enrolled for ≥12 months pre-diagnosis and a minimum of 1 month post-diagnosis. Patients were followed until they dropped out of the data set, or the data cut-off at August 2014. As a result, different patients would have different lengths of follow-up. Patients with other primary cancers or secondary brain metastases in the 12-month pre-diagnosis timeframe were excluded. Patients with pre-diagnosis chemotherapy or radiation were also excluded, to reduce the number of secondary GBM.
Data was included for each patient over time until no longer available. Because this dataset does not report death information, if cost information for a patient ceased to be tracked (other than due to administrative censoring at the end of study period; i.e. August 31, 2014), there was no way to ascertain with certainty whether this was due to death or a change of health plan. As a result, if a patient was administratively censored at the end of study period, s/he was considered an incomplete case, and was censored. However, if a patient was lost for another reason besides this administrative censoring, s/he was considered a complete case and was not censored. Because this study measured the direct medical costs incurred under commercial insurance, through plans included in the IMS LifeLink PharMetrics Plus database, this approach was considered appropriate. Therefore, costs incurred by patients covered through insurance outside of these plans, due to switching coverage, were considered outside of the scope of this study and, hence, were ignored.
The study was separated into two main time periods: pre-GBM period and post-GBM period. The GBM activity start date is defined as 3 months before the documented date of GBM diagnosis, because initial data inspection determined that patients experience GBM-related events and care (e.g. seizure-related activities and diagnosis activities) before the first GBM diagnosis date. Thus, the HCRU and medication (other than chemotherapy) costs have been reported from 12 to 3 months pre-diagnosis (pre-GBM period) and 3 months pre-diagnosis to end of the follow-up (post-GBM period).
Study outcomes and covariates
This study had two primary outcome metrics. First, several HCRU measures, including hospitalizations, lengths of stay, emergency room department (ED) visits, and outpatient visits were examined during the pre- and post-GBM periods. Second, direct medical costs for each claim based on the average allowed reimbursable amount (including amount paid by payers and patient’s co-pay) by the health plan were examined for the pre- and post-GBM periods. Both GBM-related costs and GBM non-related costs were calculated. The cumulative per-patient costs included key cost items, such as inpatient visits, radiation, chemotherapy, and ED visits. Total costs included inpatient visits, chemotherapy, radiation, ED visits, outpatient visit, and non-TMZ drug costs, including anti-microbials, analgesics, anti-hypertensives, psychoactive drugs, hypnotics, anti-coagulants, anti-convulsants, anti-diabetics, anti-emetics, anti-hyperlipidemics, corticosteroids, and gastric acid-reducing drugs. Independent variables of interest included patient age, sex, geographic region, payer type, and Charlson Comorbidity Index (CCI).
Statistical analysis
Descriptive statistics were generated for patient demographics, health plan characteristics, and comorbidity scores. HCRU was reported as (1) the proportion of patients with an HCRU event, and (2) the total number of events per patient-year. Costs were reported as (1) PPPM costs, and (2) cumulative costs over time. PPPM costs were calculated as average allowable charges for both GBM and non-GBM claims per patient-month. Patients had varying lengths of follow-up, so PPPM costs were averaged on the actual number of follow-up months for each patient. Cumulative costs were calculated from 12 months pre-diagnosis (i.e. first mention of ICD9-CM code 191.xx) to the maximum post-diagnosis follow-up (5.7 years), and reported for total costs, and separately for inpatient visits, chemotherapy, radiation, and ED visits. As this analysis was conducted from a payer’s perspective, costs were adjusted for administrative censoring (i.e. time until data cut-off) using the inverse probability weighting (IPW) methodology for dealing with censored costsCitation7,Citation8. Bootstrapping with replacement was performed to provide 95% confidence intervals (CIs) of the cumulative mean cost.
Partitioned inverse probability-weighted generalized linear regression models (known as Lin’s regression) were performed to estimate the association between patient demographic, clinical, and treatment factors and cumulative costs in the post-GBM period, while adjusting for censoringCitation8–11. Adjustment for censoring was used because patients who were administratively censored and were still incurring costs to the plan after August 31, 2014 are not observed, and, hence, not adjusting for censoring will under-estimate overall costs. Lin’s regression adjusts for censoring in the following manner: (1) the post-GBM period is divided into equal, monthly, parts; (2) for each of these, average costs associated with each covariate, for patients who are alive at the beginning of the month, are estimated using a generalized linear model with a log link and gamma distribution; (3) the estimated cost-coefficients for each month are multiplied by the Kaplan-Meier survival probability of being alive at the beginning of the month, and (4) all monthly weighted coefficients are summed over the post-GBM period for each covariate.
Two key points related to the model need to be emphasized. First, we wanted to report cost estimates (in $US) for ease of interpretation. Since the log link was found to be the most appropriate for the models, the method of recycled predictions was used to estimate the predicted mean costs in $US from the models. Second, only cumulative costs from 3 months prior to diagnosis to 12 months post-diagnosis were considered. There were two reasons for this approach: (1) this was the timeframe during which the majority of costs were incurred, and (2) not all monthly models converged in the period following these 15 months (and, hence, resulted in biased estimation). Statistical analyses were performed in SAS 9.4 and Stata 14.
Results
The database included 62,197 patients with a malignant brain cancer diagnosis (ICD-9-CM, 191.xx). Among these patients, 6,279 were treated for TMZ and had continuous enrolment for 12 months pre- and 1 month post-diagnosis. After applying the brain surgery and age criteria, 2,921 were identified as the final analytic cohort. After applying the inclusion and exclusion criteria, 2,921 unique patients were identified as the final analytic cohort. The median (interquartile range, IQR) length of follow-up was 14 (8–24) months, with the longest follow-up of 68 months.
The majority of patients were male (60%), with a mean and median patient age of 54 and 56 years, respectively, and median CCI score of 3.0. The majority (97%) of patients were covered by commercial insurance (67% commercial and 30% commercial-based self-insured) with Medicare/Medicaid patients comprising a very small percentage ().
Table 1. Demographic characteristics and annual healthcare resource utilization pre- and post-GBM.
During the pre-GBM period, the per-patient median number of inpatient and outpatient visits was 1 and 4, respectively. Inpatient and outpatient visits increased to 2 and 19, respectively, during the post-GBM period. The median length of hospital stay per inpatient visit during the pre-GBM period and post-GBM period was 3 and 5 days, respectively ().
The post-GBM period event rates across all variables were higher than the pre-GBM period event rates across all variables. We reported the cost of drug classes that were most relevant to GBM. Excluding TMZ, the three most costly co-medication classes prescribed (on a PPPM basis) during the post-GBM period were anti-coagulants ($396), anti-diabetic agents ($72), and anti-convulsants ($60).
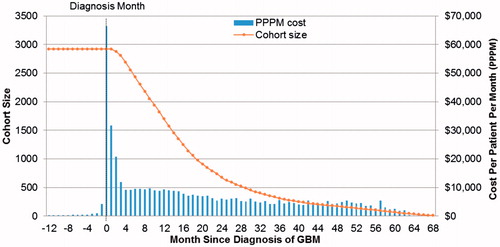
During the pre-GBM period, the mean (SD) PPPM cost was $818 ($1,128). PPPM mean (SD) costs increased to $7,394 ($8,676) during the post-GBM period. After 1 year of follow-up, the cohort size contracted to 1,696 patients (62% of the initial cohort) ().
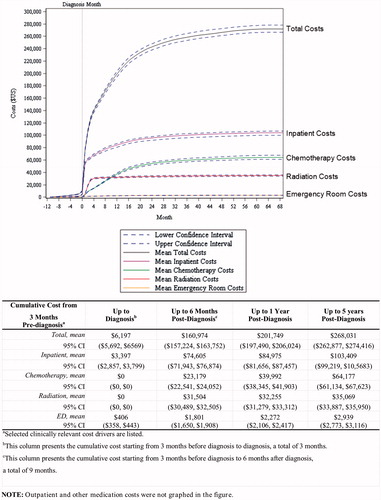
Cumulative cost started to increase from 3 months prior to GBM diagnosis. Specifically, mean total cumulative costs per patient from 3 months prior to diagnosis were $6,197. Mean total cumulative cost per patient in the post-GBM period ranged from $160,974 for 6 months post-diagnosis, to $201,749 for 12 months post-diagnosis, and to $268,031 for up to 5 years post-diagnosis (). Inpatient services accounted for the majority of costs, followed by radiation therapy and chemotherapy.
Using multivariable analysis, characteristics that were found to be predictive of cumulative cost during the post-GBM period up to 1 year post-diagnosis included male sex, residence in the West and Midwest census regions, a CCI score of ≥3, receipt of radiation therapy, re-admission ≤7 days after discharge, ≥2 ED visits, ≥2 weeks of hospitalization, having ≥7 MRI scans, and a commercial payer coverage (). The strongest contributor of costs in our model was treatment with radiation therapy; patients who received radiation in the 12 months post-diagnosis contributed costs of $81,732 more than patients who did not. Hospitalization-related variables were also highly predictive of 1-year costs. For example, patients who were hospitalized for ≥2 weeks contributed $49,629 more to the costs than patients who were hospitalized for less than 2 weeks. In addition, patients who had been readmitted within 7 days of any hospitalization discharge contributed $23,416 more to the costs than patients who were not re-admitted within 7 days. Moreover, patients with a CCI score of ≥3 at diagnosis spent $28,824 more than patients with a CCI score of ≤0 at diagnosis. Lastly, patients who had seven or more MRI scans cost $40,105 more than those who had zero MRI scans.
Table 2. Multivariate contributors of 1 year cumulative costs ($US).
Discussion
GBM is a devastating disease with a 5-year survival rate of ∼5%, one of the lowest among solid tumorsCitation2. Advances in surgical resection, radiation therapy, and chemotherapy with TMZ have improved outcomes, but most patients continue to fare poorlyCitation11. The recently approved Optune device confers an additional survival benefit, with an overall survival of 20.5 months over 15.6 months of survival using TMZ alone, with an increase in cost by $180,000 per patientCitation12,Citation13. The relatively low incidence rate of GBM (3.2 cases per 100,000 people)Citation2 makes retrospective cohort analyses investigating resource utilization and cost in real-world databases more difficult than for more prevalent cancers. A recent search of the literature uncovered only one retrospective database analysis investigating the resource utilization and associated costs in GBM in a US commercial databaseCitation6. The study provided a useful approach to identifying GBM from claims data, given that a GBM-specific ICD-9 or ICD-10 code does not exist. It also offered a perspective on the HCRU and direct medical costs associated with commercially insured US patients in the year after GBM diagnosis. Another French study looked at GBM cost from 2004–2008 and 2011 and detected a cost increase along with the introduction of chemotherapy and improvement of overall survivalCitation14. Similar findings were observed in additional studies, where cost increase was observed and incremental cost-effectiveness ratios were found to be within acceptable rangesCitation5,Citation15,Citation16. Our study adds to this prior valuable body of work in two fundamental ways. First, it quantifies overall and direct medical cumulative costs over time. While it was expected that the cost curve would look like a typical cumulative distribution function, our analysis demonstrated that costs begin to accrue at least 3 months prior to GBM diagnosis, an observation that would not have been possible with conventional reporting methods. Second, our analysis provides insight into the covariates that are independently associated with the cost of care in GBM patients using a method that adjusts for censoring.
In the current study, we used the LifeLink PharMetrics Plus database to analyze real-world HCRU and the associated cumulative cost of care for commercially insured US patients with newly-diagnosed GBM. Various clinically relevant time points and resource utilization categories were examined in an effort to fully characterize pre- and post-diagnosis-related resource utilization. We found that the overall cost of GBM care during the disease course was $268,031 in the post-GBM period, defined as 3 months pre-diagnosis to up to 5 years post-diagnosis. Key cost drivers included inpatient care and radiation therapy. The aforementioned prior studyCitation6 used a different database (namely, the Truven MarketScan commercial database) than the one used herein, with 29.9% of the patients being ≥65 years old. This previous analysis used the time of surgery instead of the 3 months prior to the initial GBM diagnosis date as the starting point for quantifying costs, and estimated the 1-year cost of GBM (in patients identified in a similar manner as the present study) at $184,107Citation6. The 1-year cost estimate including the 3 months prior to GBM diagnosis in the present analysis was comparable ($201,749). However, our analysis consisted of slightly younger patients (mean age of 54 vs 56 years old), and we also calculated cumulative costs over the entire timeframe from 3 months pre-diagnosis to up to 5 years post-diagnosis, which more comprehensively estimates the direct medical cost impact of GBM.
A search of the literature failed to identify other independent contributors of the overall cost of GBM care. In the current analysis, radiation therapy, MRI scans, and hospitalizations (both in terms of frequency and duration of stay) were found to be key cost drivers. In addition, our analysis showed that patients with a higher number of comorbid conditions (CCI score ≥3) experienced greater HCRU and incurred higher costs than patients with a CCI score of ≤2. It was somewhat surprising to observe that age was not a statistically significant independent contributor of costs. However, the effect of age on costs may have been minimized because, by definition (given the inclusion criteria), all patients in the analysis received some form of brain surgery and TMZ, whereas, in reality, age and tumor location may predict receipt of surgical resection and TMZ therapy. This is also the first analysis to verify the intuitive finding that TMZ-treated GBM patients with more hospitalizations, longer hospitalizations, more MRI scans, and more comorbidities prove costlier to the healthcare system. Importantly, these data provide a clearer picture of the relevant categories of HCRU in this population, over time, and identify the primary drivers of costs for GBM care. With the increasing focus on value within the US healthcare system, including the introduction of the recent American Society of Clinical Oncology (ASCO) value frameworkCitation17–19, the data presented here allow for a more refined understanding of the direct medical costs of treating patients with GBM in a commercially insured US population. Furthermore, understanding independent contributors of cost may guide future efforts to optimize the efficiency of GBM care.
Claims data analysis of this type has a number of inherent limitations. First, we used proxy criteria to identify GBM patients, because there is no specific diagnosis code for GBM. As reported previously, we identified patients with brain cancer, recent brain surgery, receipt of TMZ, and without evidence of other primary cancers to define a cohort likely to have GBMCitation6. Because patients with other types of high-grade gliomas could meet these criteria, the cohort is unlikely to comprise GBM patients exclusively. However, because GBM is far more common than other types of brain tumors for which TMZ is typically prescribed, the majority of the patients included here are likely GBM patients. In addition, as a result of the selection criteria, the analysis could not consider GBM patients who were not treated with TMZ or did not have brain surgery ≤90 days prior to TMZ initiation. Hence, the analysis pertains specifically to patients who received surgery and TMZ, which could be expected to incur more costs (both as a result of more intensive care and longer survival) than patients who did not receive surgery and TMZ. The retrospective nature of this analysis and the lack of available medical record information introduce a number of potential biases that are challenging to mitigate in a study of this type. Additionally, indirect costs are not captured in the database studied here; hence, the overall societal impact cannot be measured. Finally, the generalizability of the results to non-commercially insured patients is uncertain. Future studies using data sources covering patients with insurance coverage through Medicare or Medicaid can shed light on potential differences in HCRU and cost patterns among GBM patients, whose care is funded by non-commercial payers.
Conclusions
In conclusion, the present analysis showed that the overall cost of GBM care for US commercially-insured, newly-diagnosed, TMZ-treated patients during the disease course is high, with a total cumulative cost of $268,031 from 3 months pre-diagnosis to 5 years post-diagnosis. The costs of GBM in this population start to accrue before a formal diagnosis is confirmed via biopsy or surgery. Key cost drivers included inpatient care, radiation therapy, and higher comorbidity scores.
Transparency
Declaration of funding
This analysis was supported by Celldex Therapeutics Inc.
Declaration of financial/other interests
SJ, DP, and AA are consultants to Pharmerit International. MB is a shareholder and employee of Pharmerit International. Pharmerit International received financial support from Celldex Therapeutics to conduct this research. KH and ARW are employees of Celldex Therapeutics and have stock options in the company. AN is a professor/physician in Dana-Farber/Brigham and Women’s Cancer Center and he is a paid consultant of Celldex. Peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentation
A portion of the results have been presented as an oral presentation at the 2016 American Society of Clinical Oncology (ASCO) conference.
Acknowledgments
The authors are grateful to Sarah Ronnebaum and Youngmin Kwon for their editorial support and helpful comments. Dr Kate Worthington is thanked for review of earlier versions of results described in this manuscript. Dr Richard White is thanked for expert review of the manuscript.
References
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA 2013;310:1842-50
- Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 2013;15:ii1-56
- Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2006;2:494-503
- Yuile P, Dent O, Cook R, et al. Survival of glioblastoma patients related to presenting symptoms, brain site and treatment variables. J Clin Neurosci 2006;13:747-51
- Raizer JJ, Fitzner KA, Jacobs DI, et al. Economics of malignant gliomas: a critical review. J Oncol Pract 2014;11:e59-e65
- Ray S, Bonafede MM, Mohile NA. Treatment patterns, survival, and healthcare costs of patients with malignant gliomas in a large US commercially insured population. Am Health Drug Benefits 2014;7:140-9
- Griffiths RI, Gleeson ML, Danese MD, et al. Inverse probability weighted least squares regression in the analysis of time-censored cost data: an evaluation of the approach using SEER-Medicare. Value Health 2012;15:656-63
- Zheng Z, Onukwugha E, Hanna N, et al. Cost-effectiveness of second-line chemotherapy/biologics among elderly metastatic colon cancer patients. Adv Ther 2014;31:724-34
- Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics 2005;6:93-109
- Lin D. Linear regression analysis of censored medical costs. Biostatistics 2000;1:35-47
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus Temozolomide vs Temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 2015;314:2535-43
- Bernard-Arnoux F, Lamure M, Ducray F, et al. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol 2016;18:1129-36
- Henaine A, Paubel N, Ducray F, et al. Current trends in the management of glioblastoma in a French University Hospital and associated direct costs. J Clin Pharm Ther 2016;41:47-53
- Lamers LM, Stupp R, van den Bent MJ, et al. Cost‐effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme. Cancer 2008;112:1337-44
- Uyl-de Groot CA, Stupp R, Bent Mvd. Cost-effectiveness of temozolomide for the treatment of newly diagnosed glioblastoma multiforme. Expert Rev Pharmacoecon Outcomes Res 2009;9:235-41
- Campion FX, Larson LR, Kadlubek PJ, et al. Advancing performance measurement in oncology: quality oncology practice initiative participation and quality outcomes. J Oncol Pract 2011;7:31s-5s
- Newcomer LN, Gould B, Page RD, et al. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract 2014;10:322-6
- Schnipper LE, Davidson NE, Wollins DS, et al. Updating the American Society of Clinical Oncology value framework: revisions and reflections in response to comments received. J Oncol Pract 2016;34:2925-34