Abstract
Objectives: To describe the clinical characteristics, treatment pattern, and medical costs associated with metastatic castration-resistant prostate cancer (mCRPC) in Chinese patients.
Methods: This retrospective study identified a group of patients with newly-developed mCRPC from 2012 to 2016 to extract information from their medical and billing records in two tertiary care hospitals. Descriptive statistical methods were used to summarize patient clinical characteristics, treatment pattern, and monthly medical costs. Conventional regression analyses explored the predictors for the utilization of chemotherapy and monthly medical costs.
Results: Seventy-eight identified mCRPC patients were characterized with a high proportion of stage IV tumors at diagnosis of prostate cancer (75.0%) and high prevalence of bone metastasis (91.0%). Sixty-six percent of the identified patients only received one treatment episode. Among the patients receiving the first treatment episode, hormone therapy, chemotherapy, and best supportive care (BSC) accounted for 75.7%, 21.4%, and 2.9%, respectively; the previous orchiectomy was significantly associated with chemotherapy (odds ratio = 5.325, p = 0.034); and chemotherapy was associated with comparable drug costs and higher non-drug costs (coefficient = 0.657, p = 0.056) when compared to hormone therapy after the adjustment of patient characteristics.
Conclusions: Chinese mCRPC patients were characterized with delayed diagnosis of prostate cancer and high prevalence of bone metastasis. The treatment options for mCRPC were limited in Chinese patients as the conventional hormone therapy was still heavily used after the development of mCRPC. The medical costs associated with mCRPC were mainly driven by chemotherapy in Chinese patients.
Introduction
In the 2015 National Central Cancer Registry of China, prostate cancer in Chinese males ranked sixth, with a yearly incidence of 60,000 new casesCitation1. As prostate cancer screening was not required in China, people with prostate cancer were often diagnosed late. The 5-year survival rate of people with prostate cancer in China was about half of that in the US (53.8% vs 99%)Citation2,Citation3. With the rapid increase of prostate cancer incidence in the last 10 years and growing aged population in China, prostate cancer has become a major health problem.
Because of the relatively slow progression of prostate cancer and the high cure rate after radical surgeryCitation4, prostate cancer in its early stage has a very limited impact on life expectancy and utilization of healthcare resources. On the other hand, when prostate cancer is in its advanced stage, androgen deprivation therapy (ADT) would only delay the disease progression by about 2 yearsCitation5. Then, metastatic castration-resistant prostate cancer (mCRPC) would develop and become the leading cause of mortality and health resources utilizationCitation6–8. The treatment options for mCRPC in China are highly limited. Chemotherapy was the main recommended treatment option for mCRPC in ChinaCitation9 before the market approvals of enzalutamide and abiraterone acetate, a steroidal CYP17A1 inhibitor that may further suppress the synthesis of androgens in testicular, adrenal, and prostatic tumor tissuesCitation10. Sipuleucel-T immunotherapy and radium-223 haven’t been approved in China. The economic burden associated with prostate cancer in China remains unclarified because of the lack of real-world studies for treatment patterns and utilization of health-resources associated with mCRPC in Chinese patients. Hence, we conducted this retrospective study to generate the evidence indicating the unmet medical needs and the economic burden in Chinese patients with mCRPC.
Patients and methods
This retrospective study was conducted using data sources in the two Chinese tertiary care hospitals. The study protocol was reviewed and approved by the ethics review boards of the two study sites.
Study patients
The ICD-10 code for prostate cancer (C61) was used to screen hospital records on admission of patients diagnosed with prostate cancer between January 1, 2012 and December 31, 2016 in the two tertiary care hospitals. The hospital medical records of the patients identified with prostate cancer were reviewed further to assess their mCRPC status according to a combination of the following criteria: increase of the prostate-specific antigen (PSA) by more than 50% on three consecutive and weekly tests, reduction of the serum testosterone to less than 50 ng/dl or 1.7 nmol/L after ADT through orchiectomy or chemical method, and documented imaging tests proving the development of metastatic prostate cancerCitation11. To guarantee the inclusion of the patients with newly-diagnosed mCRPC during the observation time (January 1, 2012 to December 31, 2016), our study excluded patients with mCRPC, diagnosed before January 2012.
Data extraction
We used the data sources of the Hospital Information Systems of the two tertiary care hospitals to extract data. These information systems included the medical and billing records associated with both hospitalization and hospital-based outpatient clinics. The typical medical information associated with hospitalization included patient demographics, social economic status, disease diagnosis, treatment history, comorbidities, and disease management (laboratory tests, exams, surgical procedure, and drug treatment) during the hospitalization. The medical information associated with outpatient clinical visits mainly included prescription information, laboratory tests, and medical exams. This study defined the follow-up time for data extraction from the first hospitalization establishing the diagnosis of mCRPC to December 31, 2016. An electronic data collection form was developed using Microsoft Access to facilitate the manual extraction of non-standardized information in the medical records. The medical records associated with the hospitalization establishing the diagnosis of mCRPC were the data source for patient baseline characteristics, which included demographics, social economic status, the diagnosis of prostate cancer (date of diagnosis and tumor stage at diagnosis), metastasis site, complications, PSA, previous treatments, and comorbidities. The prescription records associated with hospitalizations and outpatient clinics during the defined follow-up time were scrutinized for the prostate cancer-related treatments, as classified by clinical practices guidelines [hormone therapy, chemotherapy, and best supportive care (BSC)]. This study tracked the prescription records of the included patients to define the treatment episode using the date of first treatment prescription and the estimated date of last treatment, based on the last treatment prescription date and dose for the same treatment category. The billing records, associated with hospitalizations and visits at the outpatient clinics during the follow-up, were extracted and aggregated by drug costs and non-drug costs for each included patient. The collected costs were adjusted to the Chinese currency in 2017 using the officially reported historical inflation index from 2012 to 2017Citation12.
Statistical data analysis
The collected data were converted to a study database consisting of standardized data for patient clinical characteristics, treatment pattern, and medical costs. Descriptive statistical methods were used to summarize the patient clinical characteristics at the diagnosis of mCRPC, using the mean and standard deviation for continuous variables and percentage for categorical variables. The patient baseline characteristics were summarized using the collected information. The included patients with missing specific variable were excluded for the summary of this specific variable. The treatment pattern was described by a treatment episode, defined as the time when patients received the same category of treatment, classified as hormone therapy, chemotherapy therapy, or BSC, before treatment switch or treatment discontinuation. In accordance with the treatment classifications defined in the Clinical Practice Guidelines, treatment types in the data analysis included hormone therapy, chemotherapy, and BSC. The patients having one and the same treatment episode were grouped to calculate the distribution of the treatment types and to summarize hospitalization, outpatient clinic visits, and medical costs. To demonstrate the differences in monthly medical costs (the aggregated costs divided by the follow-up time) associated with the treatment types included in the same treatment episode, Wilcoxon rank sum test for cost outcomes was used for the unadjusted comparisons of those costs between hormone therapy and chemotherapy. Best supportive care was not included in the comparisons because of small sample size. Because chemotherapy was the main treatment option for mCRPC before the launch of abiraterone in January 2016, we conducted both simple and multiple logistic regression analyses to explore any patient baseline characteristics that could significantly be associated with the utilization of chemotherapy in the patients classified by treatment episodes after the diagnosis of mCRPC. The independent variables in this multiple logistic regression analysis included age, residence city, smoking lifestyle, and previous treatments such as orchiectomy, radiation therapy, endocrine therapy, and chemotherapy. Additionally, a log-linked gamma generalized linear model was used to explore the impact of patient baseline characteristics and treatment types on the monthly medical costs for drugs, non-drug medical care, and all medical care in the patients in the same treatment episode. The independent variables taken in the multivariable generalized linear regression analyses for medical costs included tumor stage III at diagnosis of prostate cancer, time to mCRPC (years), total PSA (ng/ml), infection at diagnosis of mCRPC, anemia at diagnosis of mCRPC, urological diseases, and chemotherapy for mCRPC. The data analysis described above was conducted using statistical software R. The statistical significance was defined as a two-sided p-value less than 0.05.
Results
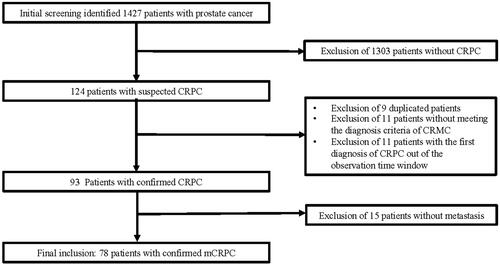
The initial prostate cancer screening at the two tertiary care hospitals identified 1,427 hospitalized patients with a diagnosis of prostate cancer. After the review of their latest medical records, 124 patients with suspected CRPC were included for further assessment. After exclusion of nine duplicated patients, 11 patients without meeting diagnostic criteria for CRPC, 11 patients developing CRPC beyond our defined observation time, and 15 patients without documented image tests supporting the metastatic status, 78 patients with confirmed mCRPC were included for full data extraction and the data analyses in this study ().
Clinical characteristics of the included mCRPC patients
summarizes the baseline characteristics of the patients. The mCRPC patients were characterized with old age (70.7 ± 9.9 years), high proportion of urban patients (84.6%), stage IV prostate cancer at the first diagnosis of prostate cancer (75%), and with bone metastasis at the diagnosis of mCRPC (91%). In our study cohort, the total PSA was 76.9 ± 194.1 ng/ml, and the time to the diagnosis of mCRPC from the diagnosis of prostate cancer was 2.0 ± 2.0 years. In our study group, the previous treatment information, before the diagnosis of mCRPC, mainly included hormone therapy only (74.4%), orchiectomy (23.1%), the combination of hormone therapy and chemotherapy (2.6%), chemotherapy only (23.1%), and radiation therapy (15.4%).
Table 1. Summary of patient baseline characteristics of the included mCRPC patients.
Treatment pattern in the included mCRPC patients
Tracking the treatments associated with the included patients during the time of follow-up, we found that 90% of the patients received at least one treatment episode, 24% of the patients received at least two treatment episodes, and 4% of the patients received three treatment episodes. Then we ranked the treatment episodes by treatment sequence, and below follows the description of the treatment pattern.
Among the 70 patients receiving the first treatment episode, hormone therapy, chemotherapy, and BSC accounted for 75.7%, 21.4%, and 2.9%, respectively. The combination of androgen receptor antagonist and luteinizing hormone-releasing hormone (LHRH) agonists was the most used hormone therapy (66.0%). Docetaxel was contained in all chemotherapies, and the most used chemotherapy was the combination of docetaxel and prednisone (46.7%). Other chemotherapies were the combination of docetaxel and androgen receptor antagonist and/or LHRH agonists. The distribution of medications used in the hormone therapy indicated that bicalutamide was ranked as the most utilized medication (71.7%), followed by goserelin (60.4%) and leuprolide (24.5%). Among the 19 patients receiving the second treatment episode, seven patients received hormone therapy and 12 received chemotherapy. Three patients received the third treatment episode. summarizes the treatment pattern associated with the three treatment episodes.
Table 2. Summary of the treatment patterns associated with treatment episodes after the diagnosis of mCRPC.
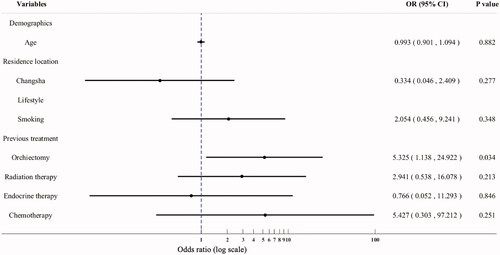
The patients receiving the first treatment episode were included to identify the patient baseline characteristics that are significantly associated with chemotherapy. Univariate logistic regression analysis identified significant associations between chemotherapy and the following patient characteristics: age at diagnosis of mCRPC [odds ratio (OR) = 0.938, p = 0.036], patients living in rural area (OR = 4.083, p = 0.044), non-smoker (OR = 0.286, p = 0.042), and previous treatments including orchiectomy (OR = 5.841, p = 0.005), hormone therapy (OR = 0.130, p = 0.001), chemotherapy (OR = 14.056, p < 0.001), and radiation therapy (OR = 5.444, p = 0.013). Multiple logistic regression analysis () confirmed that the utilization of chemotherapy was only significantly associated with previous treatment with orchiectomy (OR = 5.325, p = 0.034).
Medical costs associated with included mCRPC patients
Unadjusted comparisons of the monthly medical costs associated with the three treatment types during the first treatment episode indicated that chemotherapy was associated with significantly higher median medical costs for drugs (¥3,255 vs ¥6,298, p = 0.028, ¥1 = US$0.14 as of November 20, 2018) and non-drug care (median: ¥5,218 vs ¥1,693, p = 0.001) when compared to hormone therapy (). BSC was associated with the lowest monthly medical costs for drugs (median = ¥130) only. When both drug costs and non-drug costs aggregated for total medical costs, the chemotherapy was associated with doubled costs per month for all medical care when compared to hormone therapy (median = ¥12,151 vs ¥5,173, p = 0.002). Nevertheless, the multiple generalized linear regression analysis with adjustment for patient baseline characteristics indicated comparable drug costs per month, associated with chemotherapy (coefficient = 0.021, p = 0.932); when compared to hormone therapy, a trend, showing higher non-drug costs associated with chemotherapy (coefficient = 0.463, p = 0.174), is observed. The cost of the hormone therapy in the second treatment episode was significantly higher for drugs per month than chemotherapy, which is most likely due to the cost of abiraterone (median = ¥35,870 vs ¥4,942, p = 0.002). However, the hormone therapy was associated with ¥1,000 lower non-drug costs than chemotherapy (median = ¥1,705 vs ¥2,827, p = 0.068). summarizes the results of the multivariate generalized linear regression analysis for drug costs, non-drug costs, and total costs per month. Because of the small sample size of patients in the second treatment episode, we did not conduct regression analysis to confirm the differences in medical costs between hormone therapy and chemotherapy. For the same reason, we did not conduct the cost analysis for the third treatment episode, which only included three patients; two received hormone therapy and one received chemotherapy.
Table 3. Summary of health resources utilization and medical costs associated with mCRPC treatments in the first treatment episode.
Table 4. Results of multivariate generalized linear regression analysis exploring the predictors for drug costs, non-drug costs, and total costs per month in the first treatment episode.
Discussion
Even though our study had a small sample size, the previous clinical research on the natural history of prostate cancer in Chinese patients report comparable patient characteristics as our included patients. For example, a retrospective cohort study assessed the treatment effects of chemotherapy for mCRPC in 115 patients at a tertiary care settingCitation13. The patient characteristics, including age (68 years vs 70.7 years), duration of treatment response to ADT (23 months vs 24 months), PSA level (90.5 ng/ml vs 76.9 ng/ml), and the prevalence of bone metastasis (100% vs 90%) were highly aligned with the baseline characteristics of our study patients. Additionally, the high proportion of patients with stage IV at the diagnosis of prostate cancer in our study group confirmed that the delayed diagnosis was responsible for the poor prognosis of prostate cancer in Chinese patients. Thus, our study further demonstrated the need of organized prostate screening for the control of the disease burden of prostate cancer in China. According to a well-recognized cost-effectiveness analysis assessing prostate cancer screening in the US male populationCitation14, prostate cancer screening could be cost-effective when limited to two or three screens of people from 55–59 years old. Because the cost-effectiveness analysis assessing prostate cancer screening in Chinese males will need local evidence to estimate model variables for disease progression and medical costs associated with advanced prostate cancer under current treatment patterns, the generated evidence associated with mCRPC in our study could be crucial evidence sources for future health economic evaluation related to prostate cancer in China, where health service research associated with mCRPC was rarely conducted. Finally, the identified high medical costs associated with mCRPC patients for the first treatment episode after the diagnosis of mCRPC in the two tertiary care hospitals confirmed the observed impact of mCRPC on the economic burden of prostate cancer in high income countries, and also the low or moderate income countries.
Our study only identified three treatment episodes associated with mCRPC. The distribution of these three treatment episodes in our study group indicated that most patients only received one treatment episode after the development of mCRPC. As the terminal disease, mCRPC was associated with a life expectancy less than 2 years with docetaxel-based chemotherapyCitation15. Even though our study was unable to identify the survival status of the included patients, the short life expectancy associated with mCRPC might be responsible for the observed distribution of the three treatment episodes. However, the duration of the first treatment episode was about half a year, which was much shorter than the expected overall survival of mCRPC. Additonally, over 70% of patients still took ADT after the development of mCRPC. It was obvious that the treatment options were highly limited in these included mCRPC patients.
Even though chemotherapy is recommended as first-line therapy for mCRPC, our study only identified ∼20% of mCRPC patients receiving docetaxel-based chemotherapy. The low utilization of chemotherapy in our study cohort might factor the treatment limitations associated with chemotherapy, such as limited effects, significant toxicity, and inconvenient administration methods. According to a large observational study prospectively following up 403 patients receiving docetaxel-based chemotherapy for mCRPC at 32 tertiary care hospitals across ChinaCitation16, only one-third of the enrolled patients completed the planned chemotherapy due to lack of treatment response (14.1%), hospitalization required for administration (22.6%), and other reasons (23.3%) that were probably linked with the treatment toxicity, which occurred in 20.8% of the study patients. Additionally, our study only identified a significant association between previous orchiectomy and the utilization of docetaxel-based chemotherapy. This finding may suggest that the physicians were unlikely to use ADT in patients with orchiectomy, which was supposed to have the comparable treatment response as ADT through androgen receptor antagonist and/or LHRH agonistsCitation17. With increasing patient preference to medication-based ADT, orchiectomy will be conducted less frequently in the future. Thus, the preference of chemotherapy for mCRPC could be further reduced in the future. Additionally, the arriving new treatment option with superior treatment effects and more convenient administration method, such as abirateroneCitation18,Citation19, could further reduce the utilization of chemotherapy for mCRPC.
Another purpose of our study was to demonstrate the economic burden of mCRPC in Chinese patients by estimating the medical costs associated with mCRPC. Because the sample size associated with second and third treatment episodes were too small for a robust cost analysis, our cost analysis mainly focused on the patients receiving the first treatment episode. As the hormone therapy in the first treatment episode mainly used androgen receptor antagonist and LHRH agonists, such as bicalutamide, goserelin, and leuprolide, which had comparable drug acquisition costs as docetaxel, the observed slightly higher drug costs associated with chemotherapy could be due to the management of toxicity caused by chemotherapy. Different from the oral administration route of hormone therapy, docetaxel chemotherapy is usually administrated through intravenous route in hospital. Thus, the chemotherapy was also associated with higher non-drug costs than hormone therapy. To further address the utility of chemotherapy for mCRPC in Chinese patients, future studies should clarify the real-world health benefits associated with mCRPC and conduct a cost-effectiveness analysis to confirm the appropriateness of using chemotherapy for mCRPC in Chinese patients.
Even though our study was unable to estimate the overall economic burden of prostate cancer in China, the monthly medical costs associated with hormone therapy and chemotherapy in the first treatment episode after the diagnosis of mCRPC in our patients were over 40 times the average of monthly medical expenditure (¥279) in the Chinese general population in 2016Citation20. Thus, mCRPC was likely to be the major contributor to the economic burden of prostate cancer in China. The substantial impact of mCRPC on the economic burden of prostate cancer has been reported in other country settings. One Canadian study reported 48,428 Canadian dollars per patient with mCRPC over an average period of 28.1 monthsCitation21. The annual medical cost associated with mCRPC in Canada was 20,755 Canadian dollars per patient, about 5-times the annual medical expenditure per capita in Canada. In the US, the medical costs in the last year of life in patients with late stage prostate cancer were over 3-times the medical costs associated with prostate cancer in the first year after diagnosis ($33,691 vs $10,612)Citation22. Thus, prevention and the control of mCRPC could be the key to reduce the economic burden of prostate cancer. Thus, our study could be used to further support the economic values of prostate cancer screening, which could save substantial medical costs associated with mCRPC through appropriate treatments after the diagnosis.
As a retrospective study, our study was associated with several limitations that could introduce bias and damage the quality of the generated evidence. For example, our study cohort could be associated with selection bias, as 84.6% of our included mCRPC patients lived in urban areas. Even though the epidemiological studies showed a higher prevalence of prostate cancer patients living in urban cities, the highly skewed distribution of patient residence could reflect the limited access to tertiary care in patients who lived in rural areas. Additionally, prostate cancer patients were usually managed in outpatient settings due to the chronic nature of prostate cancer. The risk of missing information associated with our study was considered high, as our study only collected medical information of the included patients in the two tertiary care hospitals, which usually didn’t provide palliative care for patients with late stage cancer. Additionally, the distance and poor economic status associated with patients living in the rural areas could further reduce the patient access to tertiary care hospitals in urban cities. Finally, the data sources in our study didn’t collect health outcomes and quality-of-life, which were the direct outcome measures to demonstrate unmet medical needs associated mCRPC in Chinese patients under current treatment patterns.
In summary, this retrospective cohort study confirmed the substantially delayed diagnosis of prostate cancer in the Chinese patients with developed mCRPC and supported the potential needs of prostate cancer screening to address the rising disease burden of prostate cancer associated with the aged population in China. Additionally, the observed heavy use of conventional hormone therapy for mCRPC and low rate of docetaxel-based chemotherapy in our study cohort demonstrated the needs of new treatments with superior treatment effects and more convenient treatment administration method to address the unmet medical needs in Chinese patients with mCRPC. Finally, the observed substantial medical costs associated with mCRPC in our study suggested that the economic burden of prostate cancer was mainly driven by mCRPC and could be controlled through preventing and managing mCRPC.
Transparency
Declaration of funding
This study was funded by Xian Janssen. Xian Janssen was not involved with the development of the study protocol, data collection, data analysis, or manuscript development.
Declaration of financial/other relationships
WC is the founder of Normin Health, a consulting firm receiving industry funds to conduct health economics and outcomes research. The authors and JME peer reviewers have no other relevant financial or other relationships to disclose
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Zeng H, Zheng R, Guo Y, et al. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136:1921–1930.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–1717.
- Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244.
- Halabi S, Lin CY, Kelly WK, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. JCO. 2014;32:671–677.
- Valderrama A, Tangirala K, Babajanyan S, et al. Treatment, healthcare resource utilization, and costs associated with non-metastatic and metastatic castration-resistant prostate cancer: A claims analysis. J Clin Oncol. 2017;35:e18341.
- Kovačević A, Dragojević-Simić V, Rančić N, et al. End-of-life costs of medical care for advanced stage cancer patients. Vojnosanit Pregl. 2015;72:334–341.
- Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479.
- Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. JCO. 2009;27:3742–3748.
- Cookson MS, Roth BJ, Dahm P, et al. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190:429–438.
- https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG?locations=CN
- Huo YY, Dai B, Kong YY, et al. Risk factors associated with overall survival in mCRPC patients receiving docetaxel-based chemotherapy. CJC. 2012;10:779–783.
- Heijnsdijk EA, De Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst. 2014;107(1):366.
- Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245.
- He D, Sun Z, Guo J, et al. 813P Real-world use of docetaxel for metastatic castration-resistant prostate cancer in China: Results from a large observational study. Ann Oncol. 2017;28(suppl_5).
- Prostate Cancer Trialists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. The Lancet. 1995;346:265–269.
- De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
- Ryan CJ, Smith MR, De Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148.
- The annual national report of China health development in 2016. http://www.nhfpc.gov.cn/guihuaxxs/s10748/201708/d82fa7141696407abb4ef764f3edf095.shtml
- Dragomir A, Dinea D, Vanhuyse M, et al. Drug costs in the management of metastatic castration-resistant prostate cancer in Canada. BMC Health Serv Res. 2014;14:252.
- Roehrborn CG, Black LK. The economic burden of prostate cancer. BJU Int. 2011;108:806–813.