 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aims: Non-vitamin K antagonist oral anticoagulants (NOACs) and vitamin K antagonists (VKAs) are used to prevent stroke in patients with atrial fibrillation (AF). This paper aimed to evaluate the clinical efficacy and safety of NOACs when compared to VKAs by calculating the number needed to treat (NNT) at 2 years using incidence rates and hazard ratios (HRs) derived from a meta-analysis of studies conducted in real-world settings.
Materials and methods: HRs were sourced from a published systematic literature review and a meta-analysis of real-world evidence on the use of NOACs vs VKAs. Rivaroxaban, dabigatran, and apixaban vs VKAs were investigated. The efficacy outcomes included: a composite of ischaemic stroke and systemic embolism (IS/SE), ischaemic stroke (IS), and all-cause mortality. The safety analysis assessed major bleeding and intracranial haemorrhage (ICH).
Results: Superiority of NOACs vs VKAs was observed in 10/15 comparisons. Treating patients with rivaroxaban and dabigatran was associated with a reduced risk of IS and all-cause mortality compared to VKAs, with one death prevented every 22 and 32 patients, respectively, and one IS prevented every 206 and 166 patients, respectively. Rivaroxaban was significantly associated with a reduced risk of IS/SE compared to VKA (NNT: 107). No significant differences were observed between apixaban and VKAs. Dabigatran and apixaban were associated with a reduced risk of major bleeding compared to VKA (NNT: 59 and 38, respectively). No significant difference was observed between rivaroxaban and VKAs regarding major bleeding. Rivaroxaban, dabigatran, and apixaban were significantly associated with a reduced risk of ICH (NNT: 205, 115, and 108, respectively).
Limitations: Heterogeneity in definitions of major bleeding across studies.
Conclusions: The NNT calculation, when approached and interpreted properly, is a practical measure of the effectiveness of a treatment. The calculation based on HRs showed that NOACs are safe and effective alternatives to VKAs in real life.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmiaCitation1,Citation2. It affects between 0.4–1% of the general population, and 10% of the population above 80 years oldCitation1,Citation3. Patients with AF experience symptoms that interfere with their quality-of-life, including dizziness, syncope, reduced exercise tolerance, and chronic fatigueCitation1.
Among the complications of AF, the most serious one is thromboembolic strokeCitation2. AF increases the risk of strokeCitation1, and, in patients with non-valvular AF, this risk is increased by 4–5-timesCitation4. Furthermore, it is calculated that ∼15% of strokes are due to AFCitation1,Citation5. The risk of AF-related stroke mortality is 1.5% in patients aged 50–59 years and 24% in patients aged 80–89 yearsCitation4. AF also appears to increase the severity of strokes independently of age and other risk factorsCitation2.
Evidently, stroke prevention is essential in the management of AF. In particular, the use of oral anticoagulants has been shown to be effective in preventing strokes due to AFCitation6. Vitamin K antagonists (VKAs), including warfarin, were the first anticoagulation agents prescribed for stroke prevention in AF. They are effective in preventing stroke compared to aspirin and control, reducing the risk of stroke by two-thirds and mortality by one-quarterCitation6,Citation7.
Due to their efficacy and relative safety, VKAs have become the standard of careCitation6. However, their use is not without its drawbacks. They require careful monitoring and dose-adjusting, and have several food and drug interactionsCitation6,Citation8. Anticoagulation therapy is often discontinued for these reasons, or not prescribed at allCitation6,Citation9.
Non-vitamin K antagonist oral anticoagulants (NOACs) are a more recent alternative to VKAs. Evidence from numerous clinical trials has shown that NOACs have an improved or similar efficacy to VKAsCitation9–12. Furthermore, unlike VKAs, they do not require regular monitoring of coagulation parametersCitation6. As a result, current guidelines from the European Society of Cardiology (ESC) for stroke prevention in AF recommend initiation of anticoagulant treatment with NOACs over VKAs in eligible patientsCitation6.
We previously conducted a systematic literature review (SLR)Citation13 and meta-analysis (MA)Citation14 of real-world evidence (RWE) on the use of NOACs vs VKAs. In particular, we analysed the evidence on the use of rivaroxaban, dabigatran, and apixaban vs VKAs in stroke prevention in AF. Our findings confirmed that, in real life, NOACs are a suitable alternative for VKAs, and are at least as safe and effective as VKAs in routine clinical practice. In practice, however, warfarin is commonly prescribedCitation6,Citation15.
The number needed to treat (NNT) is a useful measure of the effectiveness of a treatment, with a straightforward interpretation reflecting the absolute benefit of the treatment. It represents the number of patients who must be treated with a given intervention in order to prevent one negative event or gain one desired outcome. Therefore, a properly estimated NNT comparing the effectiveness and safety of NOACs vs VKAs will provide doctors and drug regulators with valuable information to help them make more informed decisions.
Several methods for calculating the NNT have been described in the literature, and the selection of appropriate methodology should take into account the study design, the type of outcome variables, and the type of reported estimatesCitation16. The use of adequate methodology for NNT calculation ensures that the appropriate conclusions are drawnCitation16–18.
The classical method of NNT calculation, taking the inverse of the absolute risk reductionCitation16,Citation19, is not adequate for time-to-event endpoints because it does not account for varying follow-up time and premature study drop-outsCitation16. For studies with time-to-event outcomes, such as the ones analysed in this paper, the NNT can be calculated using incidence rates and HRs from the survival analysisCitation20. Therefore, the aim of this paper was to estimate the clinical efficacy and safety of NOACs vs VKAs by calculating the NNT at 2 years (NNT2y) using incidence rates and HRs identified from a previously performed MA of available RWE.
Methods
Systematic literature review and MA
A SLR and MA of the available RWE on the use of NOACs vs VKAs in patients with non-valvular AF were previously carried out and have been published elsewhereCitation13,Citation14. Studies were considered relevant regardless of geographical region, source of financing and other potential confounding factors, in order to provide estimates for real-world effectiveness and safety that were as representative as possible. To avoid the bias associated with multiplication of the same clinical data, only the estimate with the best precision (narrowest confidence intervals [CIs]) was considered. The identified body of clinical evidence allowed to assess the relative effectiveness and safety of rivaroxaban, apixaban and dabigatran separately versus VKA. On the contrary, we assumed a common effect for all VKA compounds, including warfarin and phenprocoumon. Thus the studies, in which patients received different VKA compounds in the reference arms were pooled together. All relevant HRs with 95% CIs were pooled in a MA using the inverse variance-weighting method.
NNT calculation method
The NNT2y was calculated based on data extracted from the previously described MACitation14 for selected efficacy and safety endpoints. The following pairwise comparisons were investigated: rivaroxaban vs VKAs, dabigatran vs VKAs, and apixaban vs VKAs. The efficacy outcomes included a composite of ischaemic stroke and systemic embolism (IS/SE), ischaemic stroke (IS), and all-cause mortality. The safety analysis assessed major bleeding and intracranial haemorrhage (ICH).
The NNT2y was calculated according to the method previously described by Altman and AndersenCitation20, based on HRs and the proportion of patients with events in the VKA group (PVKA_2y), using Equationequation (1)(1)
(1) . The CIs were calculated based on the CI for the HRs, using Equationequation (2)
(2)
(2) .
(1)
(1)
(2)
(2)
The proportion of patients with events in the VKA group was calculated based on the weighted average of incidence rates (IRVKA) across the studies included in the MA, using the number of patients as weightsCitation20,Citation21 (Equationequation 3(3)
(3) ).
(3)
(3)
The time horizon for the calculations (T) was set to 2 years. All calculations were performed using Microsoft Excel.
Results
A summary of the results of the MA and the NNT2Y calculation comparing NOACs vs VKAs is presented in . Detailed inputs can be found in the supplemental Appendix. Superiority of NOACs compared to VKAs was observed in 10 out of 15 comparisons ().
Figure 1. Number needed to treat at 2 years for non-vitamin K antagonist oral anticoagulants vs vitamin K antagonists. Dark bars represent significant results vs vitamin K antagonists. Light bars represent non-significant results. Abbreviations. API, apixaban; DAB, dabigatran; RIV, rivaroxaban.
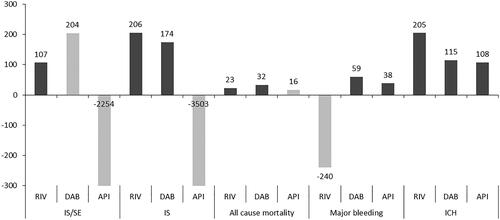
Table 1. Results of the meta-analysis comparing non-vitamin K antagonist oral anticoagulants vs vitamin K antagonists and number needed to treat at 2 years calculation.
NNT2y for efficacy outcomes: IS/SE, IS, and all-cause mortality
Treating patients for 2 years with rivaroxaban and dabigatran was associated with a reduced risk of IS, with one event prevented for every 206 and 174 patients, respectively. Rivaroxaban and dabigatran were also associated with a reduced risk of all-cause mortality compared to VKAs, with one death prevented for every 23 and 32 patients treated for 2 years, respectively. Only rivaroxaban was significantly associated with a reduced risk of IS/SE compared to VKA, with one death prevented for every 107 patients treated for 2 years. No significant differences were observed between apixaban and VKAs.
NNT2y for safety outcomes: major bleeding and ICH
Dabigatran and apixaban were associated with a reduced risk of major bleeding compared to VKA, with a NNT2y of 59 and 38 patients, respectively. No significant difference was observed between rivaroxaban and VKAs regarding major bleeding. A limitation of the analysis of major bleeding was the heterogeneity in the definition of major bleedings among the studies analysedCitation14.
Regarding ICH, rivaroxaban, dabigatran, and apixaban were significantly associated with a reduced risk, with one event prevented for every 205, 115, and 107 patients treated for 2 years, respectively.
Discussion
Clinical trialsCitation9–12 and RWECitation14 have shown that NOACs are at least as safe and effective as VKAs. Randomized controlled trials (RCTs) serve as a gold standard for the assessment of comparative efficacy and safety, since well-defined inclusion/exclusion criteria and clinical settings allow investigators to control for known and unknown confounding factors, thus minimizing the risk of bias. However, the experimental character of RCTs may limit the external validity of the findings, due to stringent inclusion/exclusion criteria which do not correspond to the characteristics of patients who qualify for the treatment in real practice. Indeed, real-world settings, the population of patients receiving treatment is usually much more diverse, treatment conditions are less tightly controlled, and the patients are less intensively encouraged to continue the treatment compared with RCTs. Due to the limited external validity of experimental trials, the confrontation of findings from RCTs with observations from RWE is recommended.
The estimates for between-treatment differences from different observational studies are usually more heterogeneous compared to the estimates from RCTs, which increases the uncertainty of the final estimate. However, this uncertainty may better reflect heterogeneous effectiveness in real clinical practiceCitation22. In this study, we present the NNT for NOACs vs VKAs for stroke prevention in patients with non-valvular AF, based on the experience from real clinical practice, in an effort to provide an easily interpretable measure of the absolute effectiveness of NOACs. We believe this will support not only clinicians in assessing the uncertainty associated with effects that are observed in real settings, but also decision makers in assessing the risk associated with drug reimbursement.
We calculated the NNT for a 2-year treatment period using incidence rates and hazard ratios (HRs) to account for both between- and within-study variation in follow-up times and premature drop-outs.
The choice of an adequate method for calculating NNT that fits the analyzed dataset is crucial to obtain a reliable estimate that reflects the effectiveness of the interventions assessedCitation23. Despite its popularity in the medical communityCitation19,Citation24 and being recommended by the Consolidated Standards of Reporting Trials (CONSORT) statementCitation25, the NNT is often misinterpreted, miscalculated, and misrepresentedCitation24,Citation26. This is partly due to an inadequate understanding of what the NNT is, but also due to use of the simple, classical method for calculating NNT in cases that require more complex methodsCitation17,Citation27.
The NNT is typically calculated using simple proportions, where the NNT is defined as the inverse of an absolute difference in proportions of patients with the event, and can be interpreted as the number of patients needed to be treated to prevent one adverse outcome. This basic method is adequate for studies with binary outcomes in which all patients are followed for a given period of time, but it is not appropriate for studies with a flexible duration of treatmentCitation17,Citation21. The reason is that simple proportions do not give a valid estimate of cumulative incidence when the follow-up time is not fixedCitation17. The exception seems to be short-term studies of acute conditionsCitation17,Citation28.
On the other hand, calculating the NNT using methods based on the survival function and incidence difference allows different follow-up periods to be taken into account, and is, therefore, more appropriate for time-to-event data. When the values of cumulative risks are available from the Kaplan–Meier curve for the treatment and control groups, the NNT can be calculated using a difference in survival estimates. The survival probabilities of both groups can be extracted from the Kaplan–Meier curve and used to calculate the risk reduction. Then the NNT is calculated as the inverse of that risk reductionCitation20. Alternatively, if the survival probability of the control group is known, the NNT can be calculated using the HRCitation20, which is the method we followed in this analysis. The NNT calculated with these two methods is interpreted as the number of patients needed to be treated for a specific amount of time to prevent one adverse outcome from happeningCitation20.
A third method to calculate the NNT uses an additive hazard modelCitation29. NNTs calculated with this method are interpreted as the number of time units needed to treat to avoid one event, on averageCitation29. It operates under the assumption of additivity of hazard. However, in order to use this method, the researcher needs to have available the hazard function for the two groups being comparedCitation29.
Calculating the NNT using the difference in the incidence rates, expressed as the number of events per unit of time, is also appropriate for time-to-event outcomes. However, NNTs calculated with this method represent the number of person-moments needed to be treated to prevent the occurrence of one event compared to the control groupCitation16,Citation17,Citation21,Citation28. Therefore, it cannot be interpreted in terms of patients needed to be treated. Some authors argue that this method can lead to false results. For example, Suissa et al.Citation17 commented on a trial evaluating the risk of hip fracture among elderly patients using a hip protector compared to control, with varying follow-up times. Using incidence rates, the authors calculated a NNT at 1 year of 41 personsCitation30. However, when using the Kaplan–Meier approach, the NNT was 35 patientsCitation17. The incidence difference method makes several important assumptions, including constant risk over timeCitation17, the existence of exponentially distributed survival timesCitation21, small baseline risks, and strong treatment effectsCitation21. Furthermore, it can only be used if the relative benefit of one drug over another does not increase over timeCitation17.
In this paper, we have calculated the NNT based on the results of a MA, which provides a more adequate estimate of the true effect compared to estimates from single studies. Mendes et al.Citation16 found that a considerable percentage of studies reporting NNTs did not follow recommended methods, particularly for MAs. NNTs can be calculated from a MA using pooled raw data, using the pooled absolute risk difference, using the pooled hazard ratio and risk difference, and using the pooled odds ratio (OR) or relative risk.
Using pooled raw data involves adding the number of patients with events in the control and treatment groups from all trials as if they were one single study. Although straightforward, this approach is not recommended because it can lead to an imbalance in the size of the trial arms and result in a reversed direction of the effect (Simpson’s paradox)Citation18,Citation31. Calculating the NNT based on pooled absolute risk differences is also not recommended because it can also lead to Simpson’s paradox. Absolute measures are very dependent on baseline risks, which often vary from trial to trial and on the duration of follow-upCitation31,Citation32.
The use of relative measures, particularly the pooled OR, is preferred over other methods of NNT calculationCitation18,Citation31 from a MA. When using pooled OR, compared to pooled relative risks, the weights and effect sizes are less dependent upon whether the data are entered as beneficial or adverse outcomesCitation18. The NNT calculated with pooled OR can also be converted into an NNT for individual types of patient by assessing their baseline riskCitation18,Citation33. In this paper, a two-step approach was adopted in order to provide a reliable estimate of NNT. First, the relative effects between respective NOACs and VKAs were calculated by pooling the HRs reported in respective studies with a MA using the inverse-variance weighting method. The probability of the event in the VKA group at 2 years was calculated using the pooled incidence rates from the identified studies, assuming exponential survival distribution. This method combined both relative effect measures with the estimate of the absolute risk in the reference group and allowed for straightforward interpretation. The use of pooled HRs and baseline risks for calculation of the NNT ensures the highest possible credibility of the estimatesCitation21,Citation28.
Our analysis confirms that, in real life, NOACs provide additional benefit compared to VKAs. In particular, only 23 and 32 patients need to be treated for 2 years with rivaroxaban and dabigatran, respectively, to avoid one outcome of all-cause mortality. The interpretation of these time-specific NNTs is straightforward and helps to better visualize the difference in effectiveness and safety between NOACs and VKAs for stroke prevention in AF. Some results were not statistically significant, but this does not necessarily indicate a lack of effect. The significance of the NNT depends on the precision of the HR estimates from the MA and may, therefore, be due to insufficient power of the MA. Worthy of note is that the NNTs calculated in this paper are for a 2-year follow-up period to provide an estimate of between-treatment differences in the long-treatment perspective. NNT estimates for different time periods would require recalculations.
All treatments were associated with a reduced risk of ICH and an at least similar efficacy profile compared with VKAs, which was consistent with the key clinical trials ROCKET AF, ARISTOTLE, and RE-LY, which assessed rivaroxaban, dabigatran, and apixaban, respectively. Interestingly real-world studies indicate that rivaroxaban and dabigatran may be associated with improved survival and a reduced risk of ischaemic strokes, something that was not demonstrated unambiguously in the key clinical trials. Our analysis is highly consistent with the experimental evidence regarding the risk of major bleeding showing that NOACs are not associated with an increased risk of major bleeding compared with VKA9–11.
Recently, Proietti et al.Citation34 conducted a SLR of RWE followed by a pairwise MA to assess real-world safety and effectiveness of apibaxan compared with warfarin and with other NOACs. Their work has, however, several methodological limitations. First, the authors included 16 RWE studies, of which six retrospectively analyse patient data from overlapping US insurance and medical databases (MarketScan, Medicare, Optum, Humana) recorded between 2010 and 2015, and four others that were based on information from Danish nationwide registries. These studies were pooled together in MAs, leading to an over-estimation of the total number of patients and the precision of the results. It cannot be excluded that the outcomes of the MAs and estimates for between-study heterogeneity were also affected. Second, the selection of OR as the measure of association is questionable, since the follow-up in respective groups of studies could not be considered equal.
The studies collected by Proietti et al.Citation34 indicate that NOACs used in the treatment of patients with AF are associated with a favourable efficacy/safety ratio compared to VKA, and may lead to better control of ischaemic events without increasing the risk of bleeding. The results were consistent with the outcomes of our MA: no significant differences regarding any thrombotic event (OR = 0.92 [0.72, 1.17]) or any stroke (OR = 0.81 [0.60, 1.10]) in their analysis, no significant differences regarding the composite endpoint of IS/SE (HR = 1.01 [0.86, 1.18]) or of IS alone (HR = 1.01 [0.87, 1.17]), and apixaban was found to be associated with a lower risk of major bleeding and ICH compared to VKA. Recently, Deitelzweig et al.Citation35 performed a network MA on 11 RWE studies in order to assess the differences between NOACs in the risk of major bleeding in patients with non-valvular AF. The authors concluded that NOACs were associated with a lower or similar risk of major bleeding compared with VKA.
Our study has some limitations. First, in the NNT calculation we used event rates calculated using the weighted average of the incidence rate, without considering between-trial heterogeneity. Therefore, our results should be interpreted with caution. Second, the efficacy and safety of the interventions were assessed using objective outcomes with high relevance in terms of a clinical decision. In particular, we did not attempt to assess the net clinical benefit for NOACs, as per the rare reporting of composite endpoints combining efficacy and safety parameters, as well as the between-study heterogeneity regarding the composition of such endpoints. We also did not attempt to combine safety and efficacy estimates assessed separately in respective studies, since this approach has many methodological limitations. Last, the NNTs were calculated based on the outcomes from a MA and, therefore, inherit the credibility from the evidence included in the MA.
Conclusions
In this study, we present NNTs for NOACs vs VKAs using data derived from a MA of RWE and calculated using a method appropriate for time-to-event outcomes. When calculated and interpreted properly, the NNT is a very practical measure of the effectiveness of a treatment. We show that NOACs are safe and effective alternatives to VKAs in real life. Rivaroxaban and dabigatran were associated with a significant reduction of all-cause mortality and IS compared to VKAs. All NOACs were associated with a significant reduction of ICH without an increase in major bleeding compared to VKAs.
Transparency
Declaration of funding
This study was funded by Bayer AG.
Declaration of financial/other relationships
At the time of the study, JB and KB were employees of Bayer AG, and VT, AM, and PW were employees of Creativ-Ceutical. MT is a consultant for Creativ-Ceutical. Peer reviewers on this manuscript have received an honorarium from JME for their review work. One of the peer reviewers discloses consulting for Boehringer-Ingelheim. The remaining peer reviewers have no other relevant financial relationships to disclose.
Supplemental Materials - Input Tables
Download MS Word (34 KB)Acknowledgements
None.
References
- Bunch TJ, Gersh BJ. Rhythm control strategies and the role of antiarrhythmic drugs in the management of atrial fibrillation: focus on clinical outcomes. J General Intern Med. 2011;26(5):531–537.
- Bajpai A, Savelieva I, Camm A. Epidemiology and economic burden of atrial fibrillation. US Cardiol. 2007;4(1):14–17.
- Kannel W, Benjamin E. Final draft status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92(1):17–ix.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(7):2N–9N.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary. Circulation. 2006;114(7):700–752.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962.
- Hart R, Pearce L, Aguilar M. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867.
- Warfarin 0.5 mg tablets. Electronic medicines compendium. 2017. https://www.medicines.org.uk/emc/medicine/27651. [cited 2018 Dec 10]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
- Granger CB, Alexander JH, McMurray JJ V, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104.
- Briere J, Bowrin K, Coleman C, et al. Real-world clinical evidence on rivaroxaban, dabigatran, and apixaban compared with vitamin K antagonists in patients with non-valvular atrial fibrillation: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2019;19(1):27–36.
- Coleman C, Briere J-B, Fauchier L, et al. Meta-analysis of real-world evidence comparing non-vitamin K antagonist oral anticoagulants with vitamin K antagonists for the treatment of patients with non-valvular atrial fibrillation. J Market Access HealthPolicy. 2019;7(1):1574541.
- Cowan C, Healicon R, Robson I, et al. The use of anticoagulants in the management of atrial fi brillation among general practices in England. Heart. 2013;99(16):1166–1172.
- Mendes D, Alves C, Batel-Marques F. Number needed to treat (NNT) in clinical literature: an appraisal. BMC Med. 2017;15(1):112.
- Suissa S. The number needed to treat: 25 years of trials and tribulations in clinical research. Rambam Maimonides Med J. 2015;6(3):e0033.
- Cates CJ. Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Med Res Methodol. 2002;2:1.
- Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. 1988;318(26):1728–1733.
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(December):1492–1495.
- Lubsen J, Hoes A, Grobbee D. Implications of trial results: the potentially misleading notions of number needed to treat and average duration of life gained. Lancet. 2000;356(9243):1757–1759.
- Schmitz S, Adams R, Walsh C. Incorporating data from various trial designs into a mixed treatment comparison model. Stat Med. 2013;32(17):2935–2949.
- Moore RA, Gavaghan DJ, Edwards JE, et al. Pooling data for number needed to treat: no problems for apples. BMC Med Res Methodol. 2002;2:2.
- McAlister FA. The “number needed to treat” turns 20—and continues to be used and misused. CMAJ. 2008;179(6):549–553.
- Altman D, Schulz K, Moher D, et al. The revised consort statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–694.
- Stang A, Poole C, Bender R. Common problems related to the use of number needed to treat. J Clin Epidemiol. 2010;63(8):820–825.
- Bender R. Using and interpreting adjusted NNT measures in biomedical research. Open Dent J. 2010;4:72–76.
- Mayne TJ, Whalen E, Vu A. Annualized was found better than absolute risk reduction in the calculation of number needed to treat in chronic conditions. J Clin Epidemiol. 2006;59(3):217–223.
- Ke C, Jiang Q. Benefit–risk assessment using number needed to treat and number needed to harm for time-to-event endpoints. Stat Biopharm Res. 2016;8(4):379–385.
- Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10(6):312–320.
- Altman DG, Deeks JJ. Meta-analysis, Simpson’s paradox, and the number needed to treat. BMC Med Res Methodol. 2002;2(1):3.
- Cates DH, Eggert C, Yang JL, et al. Comparison of effluents from TCF and ECF bleaching of kraft pulps. Tappi J. 1995;78(12):93–98.
- Furukawa T, Guyatt G, Griffith L. Can we individualize the “number needed to treat”? An empirical study of summary effect measures in meta-analyses. Int J Epidemiol. 2002;31(1):72–76.
- Proietti M, Romanazzi I, Romiti GF, et al. Real-world use of apixaban for stroke prevention in atrial fibrillation. Stroke. 2018;49(1):98–106.
- Deitelzweig S, Farmer C, Luo X, et al. Comparison of major bleeding risk in patients with non-valvular atrial fibrillation receiving direct oral anticoagulants in the real-world setting: a network meta-analysis. Curr Med Res Opin. 2018;34(3):487–498.
