Abstract
Aims: To evaluate the cost-effectiveness of adjuvant pembrolizumab relative to observation alone following complete resection of high-risk stage III melanoma with lymph node involvement, from a US health system perspective.
Materials and methods: A Markov cohort model with four health states (recurrence-free, locoregional recurrence, distant metastases, and death) was developed to estimate costs, life-years, and quality-adjusted life-years (QALYs) associated with pembrolizumab vs observation over a lifetime (46-year) horizon. Using a parametric multi-state modeling approach, transition probabilities starting from recurrence-free were estimated based on patient-level data from KEYNOTE-054 (NCT02362594), a direct head-to-head phase 3 trial. Post-recurrence transition probabilities were informed by real-world retrospective data and clinical trials in advanced melanoma. Health state utilities and adverse event-related disutility were derived from KEYNOTE-054 trial data and published literature. Costs of drug acquisition and administration, adverse events, disease management, and terminal care were estimated in 2018 US dollars. Deterministic and probabilistic sensitivity analyses were conducted to assess robustness.
Results: Over a lifetime horizon, adjuvant pembrolizumab and observation were associated with total QALYs of 9.24 and 5.95, total life-years of 10.54 and 7.15, and total costs of $489,820 and $440,431, respectively. The resulting incremental cost-effectiveness ratios (ICERs) for pembrolizumab vs observation were $15,009/QALY and $14,550/life-year. Across the range of input values and assumptions tested in deterministic sensitivity analyses, pembrolizumab ranged from being a dominant strategy to having an ICER of $57,449/QALY vs observation. The ICER was below a willingness-to-pay threshold of $100,000/QALY in 90.2% of probabilistic simulations.
Limitations: Long-term extrapolation of outcomes was based on interim results from KEYNOTE-054, with a median follow-up of 15 months.
Conclusions: Based on common willingness-to-pay benchmarks, pembrolizumab is highly cost-effective compared with observation alone for the adjuvant treatment of completely resected stage III melanoma in the US.
Introduction
Melanoma is a type of cancer that affects melanocytes, the melanin producing cells in the skin. Melanoma is the most serious form of skin cancer. In the US, the incidence of melanoma has risen by 1.5% annually over the last decade, with an estimated 91,270 newly-diagnosed cases and 9,320 deaths attributed to melanoma in 2018Citation1. The mortality risk associated with melanoma is directly proportional to the depth of the primary tumorCitation2. Five-year survival ranges from 92–97% for stage I disease (localized tumor, thin primary) to 53–81% for stage II, 40–78% for stage III (loco-regional disease), and 15–20% for stage IV (distant metastases)Citation3.
For localized melanoma, surgical excision is the standard of care and is often curativeCitation4; however, patients with lymph node-positive stage III melanoma have a high risk of recurrence or death after complete surgical resectionCitation5,Citation6. Recommended treatment for patients with node-positive, resectable stage III melanoma consists of surgery followed by either treatment with an approved adjuvant therapy or observation without therapyCitation7,Citation8.
Historically, adjuvant treatment options for melanoma in the US were limited to high-dose interferon-α2bCitation9 or peginterferon-α2bCitation10. Clinical trials have shown a sustained impact of adjuvant therapy with high-dose interferon or peginterferon-α2b on recurrence-free survival (RFS), but with considerable toxicity and no significant effect on overall survival (OS) relative to observationCitation11,Citation12. Immune checkpoint inhibitors and targeted therapies have dramatically improved the treatment landscape for advanced (i.e. unresectable stage III or IV) melanomaCitation13–15, and have more recently been evaluated as adjuvant therapies for patients with resected high-risk melanomaCitation16–20. In 2015, ipilimumab, a cytotoxic lymphocyte antigen-4 inhibitor, was approved by the US Food and Drug Administration (FDA) as an adjuvant treatment following complete resection of stage III melanoma based on interim results from the phase 3 European Organization for Research and Treatment of Cancer (EORTC) 18071 trial, which demonstrated significant improvements in RFS with ipilimumab vs placeboCitation17,Citation21–23; 5-year data from the same trial showed a similar benefit with respect to OS in 2016Citation18. Two additional adjuvant treatment options for melanoma were FDA-approved in 2017 based on RFS benefits in phase 3 trials, including the programmed death-1 (PD-1) checkpoint inhibitor nivolumab for resected stage III/IV melanomaCitation19, and the BRAF + MEK inhibitor combination therapy dabrafenib plus trametinib for resected stage III melanoma with BRAF V600E or V600K mutationsCitation20.
Pembrolizumab is a high-affinity monoclonal antibody that binds to and consequently blocks the activity of the PD-1 receptor. This reactivates the tumor-specific cytotoxic T-lymphocytes that destroy tumor cells, thereby re-establishing anti-tumor immunity in affected patients. Pembrolizumab has been approved by the FDA for the treatment of multiple oncology conditions, including unresectable or metastatic melanomaCitation24, and more recently approved by the European Medicines Agency for the treatment of high-risk stage III melanoma in the adjuvant settingCitation25,Citation26. KEYNOTE-054 (NCT02362594), a randomized phase 3 study conducted in collaboration with the EORTC, is underway to evaluate pembrolizumab vs placebo (i.e. observation) as adjuvant treatment for completely resected high risk stage III melanoma (stage IIIA [> 1 mm lymph node metastasis], IIIB and IIIC)Citation16. Patients were randomly assigned to receive either pembrolizumab 200 mg or placebo intravenously every 3 weeks for up to 1 year or to complete a maximum of 18 doses. Interim results based on a median follow-up of 15 months have demonstrated significant improvements in RFS with pembrolizumab vs placebo (HR = 0.57; 98.4% CI = 0.43–0.74; p < 0.0001)Citation16. The secondary endpoints of distant metastases-free survival (DMFS) and OS will be evaluated in the second interim and final data analyses of the KEYNOTE-054 trial.
With the advent of pembrolizumab as a new adjuvant treatment option, it will be important for payers and decision-makers to understand whether this immunotherapy represents good value for resources relative to standard of care in this indication. While studies have examined the cost-effectiveness of pembrolizumab as a treatment for advanced melanoma in the USCitation27 and European settingsCitation28,Citation29, there have been no published economic evaluations of pembrolizumab or any other novel immunotherapy as adjuvant treatment for melanoma. Prior economic evaluations in this setting have been limited to interferon-based regimensCitation30–34.
The objective of this study was to evaluate the cost-effectiveness of pembrolizumab relative to observation following complete resection of stage III melanoma with lymph node involvement, from a US health system perspective. Observation was considered the most relevant comparator because it was the only comparator with direct, head-to-head trial evidence vs pembrolizumab, and is a recommended management strategy for this indication in the USCitation8.
Methods
Model overview
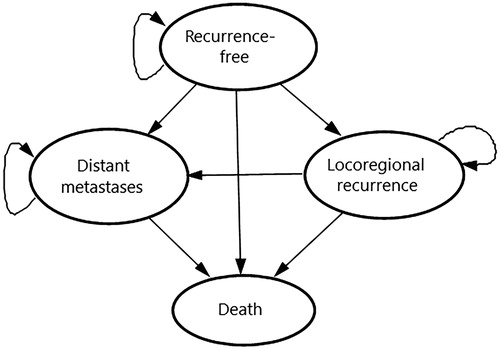
A Markov state transition model was developed in Microsoft Excel 2016 (Microsoft Corp., Redmond, WA) to evaluate the cost-effectiveness of adjuvant pembrolizumab vs observation following complete resection of high-risk stage III melanoma with lymph node involvement. The model consisted of four mutually exclusive health states (i.e. recurrence-free [RF], locoregional recurrence [LR], and distant metastases [DM], with death as the absorbing state) to track the disease course and survival of patients over time (). Patients' initial health state (RF), starting age (54 years), and gender distribution (38.4% female) at model entry reflected the baseline characteristics of patients in the KEYNOTE-054 trial (NCT02362594). From the RF state, patients’ risks of transitioning directly to LR, DM, or death varied depending on the adjuvant treatment strategy (i.e. pembrolizumab or observation). It was assumed that, upon transitioning to LR or DM, patients would receive no ongoing therapeutic benefit from adjuvant pembrolizumab relative to observation. Therefore, from the LR state, transition probabilities to DM and to death were assumed equivalent between the two model arms. After entering the DM state, patients were assumed to receive first- and second-line therapies for advanced melanoma. Transition probabilities from DM to death were determined by the efficacy of the various first-line therapies that patients may receive.
The cohort-level simulation was conducted in weekly cycles with half-cycle correction over 46 years (i.e. 100 years minus 54, the average starting age of patients in KEYNOTE-054), representing a lifetime horizon for this population. Based on the transition probabilities between different states, the distribution of patients across health states was traced over time in each adjuvant treatment arm. Quality-adjusted life years (QALYs) and costs could, therefore, be estimated based on the utility and costs assigned to each health state. In the base-case analysis, both costs and effectiveness were discounted at 3% annuallyCitation35, and all cost inputs were inflation-adjusted from their original reporting year to 2018 US dollars (USD)Citation36. The incremental cost-effectiveness ratio (ICER) of pembrolizumab vs observation was evaluated in terms of incremental cost per QALY gained and incremental cost per life year gained.
Model inputs
Transition probabilities
Transition probabilities were estimated using a parametric multi-state modeling approachCitation37,Citation38 in which different parametric functions were fitted to each individual transition. reports the base-case parameter estimates used to model each health state transition in the Markov model and data sources. Within each weekly cycle, all transition probabilities to death were constrained to be at least as high as background US mortality, as estimated from national life tables given the age and gender distribution of the cohort by that cycleCitation39.
Table 1. Parametric models of health state transitions in the model.
Transitions from the RF state
The clinical efficacy of each adjuvant treatment strategy was reflected in the transition probabilities starting from the RF state, which were derived using interim data from the KEYNOTE-054 trialCitation16. Parametric functions were fitted to the cause-specific hazards of each transition (RF → LR, RF → DM, and RF → death) using patient-level time-to-event data. For the base-case analysis, parametric models were separately fitted to the pembrolizumab and placebo arms of KEYNOTE-054, based on evidence from cumulative hazards plots that the proportional hazards assumption may not hold. To account for competing risks when analyzing time to each of the three types of RFS failure (i.e. LR, DM, or death), competing RFS failure types were treated as censoring eventsCitation37,Citation38,Citation41,Citation42. For example, to model the RF → DM transition, patients who experienced LR or death prior to DM were treated as being censored at the time of the earlier competing event. Analyses were conducted using the package flexsurvreg in R software (R Development Core Team, Vienna, Austria)Citation43.
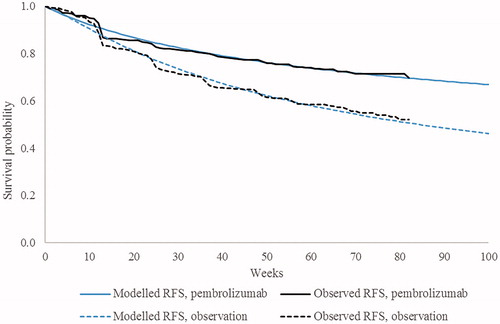
Six different parametric functions were considered to model transitions from RF → LR and RF → DM in each treatment arm, including exponential, Weibull, Gompertz, log-logistic, log-normal, and generalized gamma distributions. Exponential models were fitted to the RF → death transition due to the small number of direct transitions from RF to death observed in the KEYNOTE-054 trial (i.e. two in the pembrolizumab arm, one in the placebo arm)Citation16. In each treatment arm, the transition probability from the RF state to any other state, as well as the probability of staying in RF state, depended upon all three cause-specific hazard functions. Therefore, in order to select base-case parametric functions, all 36 possible combinations of parametric functions for RF → LR and RF → DM were considered. Base-case parametric functions were selected with consideration of: goodness-of-fit (assessed by mean squared error and visual assessment) between predicted vs observed RFS in each treatment arm during the trial period; and plausibility of long-term extrapolations based on external dataCitation18 and clinical expert opinion. Consistent with recommended practiceCitation44, the same functional forms were used in both model arms.
Based on these criteria, the Gompertz function for RF → LR and generalized gamma function for RF → DM were used in the base-case analysis (). Among all 36 possible combinations of parametric functions, this combination was ranked first in the observation arm and fifth in the pembrolizumab arm in terms of mean squared error. plots the resulting predictions of RFS during the trial period against the observed Kaplan-Meier curve in each treatment arm. In scenario analyses, transitions from RF were instead modeled using alternative parametric distributions, or incorporating a function of hazard ratio (HR), time-constant or time-varying, for pembrolizumab vs observation.
Transitions from the LR state
Due to limited post-recurrence follow-up in KEYNOTE-054 as of the interim data cutoff, the cause-specific hazard of LR → DM was estimated using real-world US electronic health record data from the Flatiron Health database (January 1, 2011 to February 28, 2018) for melanomaCitation40. The analytical sample included 147 patients who had undergone complete resection of stage III melanoma and experienced LR. An exponential parametric function was fitted using observed data on time from the date of LR to DM or until censoring at the earlier of end of data availability or January 28, 2018. The exponential distribution is commonly assumed when estimating transition probabilities starting from intermediate health states in a Markov model, as the hazard rate does not depend on time since entry into the health stateCitation45. Because no direct transitions from LR → death occurred in the Flatiron Health melanoma sample, the cause-specific hazard for this transition was approximated based on an exponential model of RF → death derived from KEYNOTE-054.
Transitions from the DM state
Once the disease progresses to distant metastasis, patients will be eligible for several options for advanced melanoma; transition probabilities from DM → death were, therefore, assumed to depend on the market shares of first-line treatments for advanced melanoma received in each adjuvant treatment arm. The base-case model also considered the cost of second-line therapies received in the DM state; however, survival within the DM state was assumed to depend on the choice of first-line therapy.
To reflect clinical practice in the US, in the observation arm, market shares were obtained from US-specific unpublished market research (pembrolizumab: 32.5%; nivolumab: 32.5%; nivolumab + ipilimumab: 15.0%; vemurafenib + cobimetinib: 2.8%; dabrafenib + trametinib: 17.1%). Patients who have received pembrolizumab adjuvant treatment may not have the same treatment options as patients with no adjuvant treatment, depending on when the DM occurs. If DM occurs 24 months after adjuvant treatment initiation, 50% of patients were assumed to receive rechallenge with an immunotherapy (25% with pembrolizumab, 25% with nivolumab), while the remaining 50% were assumed to receive other therapies (ipilimumab: 13.5%; nivolumab + ipilimumab: 21.5%; vemurafenib + cobimetinib: 5.0%; dabrafenib + trametinib: 10.0%). If DM occurs within 24 months of adjuvant treatment initiation, patients were assumed to be ineligible for retreatment with pembrolizumab following adjuvant pembrolizumab, and market shares of other advanced melanoma treatments were 20% for ipilimumab, 10% for nivolumab, 40% for nivolumab + ipilimumab, 5% for vemurafenib + cobimetinib, and 25% for dabrafenib + trametinib.
For each first-line treatment option, exponential models of OS and progression-free survival (PFS) were estimated using data from clinical trials conducted in the advanced melanoma settingCitation13,Citation15,Citation46–51. For pembrolizumab, exponential models of OS and PFS were fitted to patient-level data from the pembrolizumab 10 mg/kg Q3W arm (first-line sub-group only) of the KEYNOTE-006 trial, a multi-center, randomized, open-label phase III trial among ipilimumab-naïve unresectable or advanced melanoma patientsCitation13. For other first-line subsequent treatments, HRs for OS and PFS failure vs pembrolizumab were each obtained from a network meta-analysis (NMA) of trials that evaluated first-line treatments for advanced melanoma. Expected OS within the DM state was calculated in each adjuvant treatment arm as a market share-based weighted average of expected OS associated with different first-line treatments for advanced melanoma. Expected OS was then translated into a weekly hazard of DM → death. Expected PFS was estimated for each adjuvant treatment arm using a similar approach.
Adverse events
The model considered the disutility and cost impact of drug-related grade 3–5 AEs. Specifically, AEs of grade 3–5 were included if they occurred with a frequency of ≥ 5% at any grade in either the pembrolizumab or placebo arm of the KEYNOTE-054 trial. Diarrhea of grade 2 was also considered based on the high expected cost of managing this AE. Risks of the included AEs in each arm were obtained from KEYNOTE-054. Mean durations for the AEs were collected from KEYNOTE-054.
Utilities
Different utility values were assigned to each health state (). Health state utilities for the RF and LR states were derived through primary analyses of EuroQol-five dimension-three level questionnaire (EQ-5D-3L) data collected during the KEYNOTE-054 trial. Linear mixed-effects regression analyses with patient-level random effects were performed using repeated measures data from patient visits in which both health state (defined according to RECIST V1.1 criteria) and EQ-5D-3L were assessed. EQ-5D-3L index scores were calculated using a published, validated US-specific algorithmCitation52.
Table 2. Utility and cost inputs.
Utility in the DM state was computed as a weighted average of the utilities associated with pre- and post-progression DM, based on the expected proportion of time spent in each sub-state within the DM state (i.e. the ratio of expected PFS to expected OS after entering the DM state). This ratio was estimated based on the market shares of different first-line treatments received for advanced melanoma. The base-case utility for pre-progression DM was estimated based on EQ-5D-3L data from KEYNOTE-054. The base-case utility for post-progression DM was instead obtained from an external literature sourceCitation53, based on the expectation that the available follow-up in KEYNOTE-054 would be too limited to capture average utility over the entire post-progression disease course until death. The cross-sectional study by Beusterien et al.Citation53 used the standard gamble method to elicit utilities for advanced melanoma health states from members of the general public. The utility estimated for progressive disease was extracted for use in the present model as the utility associated with post-progression DM. In sensitivity analyses, alternative utility scenarios were tested in which: utilities for all health states (including post-progression DM) were informed by KEYNOTE-054 data; or utilities for all health states were obtained from a different literature sourceCitation54.
The health-related quality-of-life impact of AEs was also considered. For all AE types considered in the model, disutility was based on the estimated difference in utility associated with RF (without toxicity) vs RF (during any grade 3+ AE) in KEYNOTE-054. In each adjuvant treatment arm, the one-time QALY decrement associated with AEs (applied in the first model cycle) was calculated as a function of: treatment-specific AE risks, mean durations of these AEs, and the AE-related disutility value.
Resource utilization and costs
Cost of adjuvant therapy
Drug acquisition costs per infusion of adjuvant pembrolizumab was calculated in the model as a function of the list price ($4,719.40 per 100 mg vial; Analysource.com) and defined dosing schedule (200 mg every 3 weeks, as in KEYNOTE-054)Citation16. In the base case, the relative dose intensity observed in the pembrolizumab arm of KEYNOTE-054 (99.7%) was applied to the drug acquisition cost per infusion of adjuvant pembrolizumab to account for any delays or interruptions in administration (e.g. due to AEs). Drug administration cost per 30-minute infusion of pembrolizumab was based on the Centers for Medicare & Medicaid Services (CMS) Physician Fee ScheduleCitation55.
The proportion of patients remaining on adjuvant pembrolizumab at each scheduled infusion was based on the observed Kaplan-Meier curve for time to treatment discontinuation in KEYNOTE-054. In the trial, patients randomized to adjuvant pembrolizumab received treatment for up to 1 year or until completion of 18 doses (i.e. the number of scheduled doses over 1 year). Based on this maximum duration, there was sufficient follow-up data from the trial to directly measure time on adjuvant treatment without extrapolation (mean number of doses: 14).
Cost of subsequent therapy
Drug acquisition and administration costs associated with first- and second-line therapies for advanced melanoma were applied as a one-time cost upon entry into the DM state. Drug acquisition and administration costs of these subsequent therapies were based on the same sources as those used for adjuvant therapyCitation55. Dosing schedules were based on the respective FDA labelsCitation24,Citation56–61. For drugs with weight-based dosing, the average number of whole vials required per infusion was calculated under a base-case assumption of no vial-sharing, using the weight distribution of US patients in the KEYNOTE-006 trialCitation13. For each treatment regimen, the exponential rate of PFS failure was used to approximate the treatment discontinuation rate, up to the label-recommended maximum duration where applicable. For all second-line regimens, mean time on treatment was assumed to be 21 weeks, consistent with prior health technology appraisals in the advanced melanoma settingsCitation62,Citation63; as an exception, ipilimumab as monotherapy or in combination was subject to the maximum duration of 12 weeks.
Based on the estimated mean duration of each drug component in a regimen, the model estimated the mean total cost of each first- and second-line regimen for advanced melanoma. The mean cost of subsequent treatment was then calculated for each adjuvant treatment arm as a weighted average based on market shares of subsequent treatments. First-line market shares are described above. Second-line market shares in the observation arm were 31.7% for pembrolizumab, 5.3% for ipilimumab, 31.7% for nivolumab, 21.1% for nivolumab + ipilimumab, 1.0% for vemurafenib + cobimetinib, 4.0% for dabrafenib + trametinib, and 5.3% for best supportive care (dacarbazine). The same second-line market shares were assumed for patients in the pembrolizumab arm who were eligible for rechallenge with immunotherapies; for those ineligible for rechallenge, second-line market shares were set to 0% for pembrolizumab, nivolumab, and nivolumab + ipilimumab, and increased to 89.7% for ipilimumab.
Cost of adverse events
The cost of AE management was applied as a one-time cost in the first model cycle based on treatment-specific AE risks and the unit costs per episode for included AEs. Unit costs of AEs were obtained from the CMS Acute Inpatient Prospective Payment System (PPS)Citation64 and other literature sourcesCitation65–67.
Disease management cost by health state
The weekly cost of medical resource use in the RF state included outpatient services (i.e. physician office visits, radiologic assessments) as reported in a study by Zhang et al.Citation68. This weekly cost was reduced by 50% in years 2–5 and by an additional 50% (relative to years 2–5) in years 5–10; this assumption was supported by the National Comprehensive Care Network guidelines, that recommend reductions in the frequency of screening among patients who have remained recurrence-free for longer periods of timeCitation8. Patients remaining in the RF state beyond 10 years were assumed to incur no further disease management costs in this state.
For patients who transitioned into the LR state, a one-time cost of salvage surgery was applied based on the cost of surgery described in Zhang et al.Citation68, and the percentages of patients in the KEYNOTE-054 trial who underwent lymphadenectomy, skin lesion resection, in-transit metastases resection, or other surgery after experiencing LRCitation69. Subsequent medical cost per week in the LR state was assumed to be the same as during years 0–2 in the RF state.
Upon entry into the DM state, a one-time cost of medical resource use was applied based on the cost associated with first-line treatment initiation in Seidler et al.Citation70. In subsequent cycles, the recurring medical costs associated with pre- and post-progression DM were applied based on reported costs during PFS (on treatment) and after progression in Tarhini et al.Citation71, a retrospective chart review of US patients with unresectable stage III/IV melanoma who received first-line ipilimumab therapy. Overall, weekly medical cost in the DM state was computed as a weighted average of the weekly cost associated with pre- and post-progression DM, similar to the approach used to compute utility in the DM state.
Terminal care costs
Patients who transitioned to death from the DM state were assumed to incur a one-time cost associated with palliative/terminal care based on Chastek et al.Citation72.
Sensitivity analyses
To assess the robustness of the model results, one-way deterministic sensitivity analyses (DSAs) were conducted by varying one model input at a time. Utilities, costs of drug administration, AEs, disease management, and terminal care, patient weight, and exponential rates of transitions from the LR and DM states were individually varied above and below their base-case values by 25%. Annual discount rates for costs and effectiveness were each varied from 0–6%. Additionally, numerous scenario analyses were undertaken to assess the impact of using shorter time horizons (10 years, 20 years), alternative methodologies for modeling transitions starting from the RF states, alternative sources for health state utilities, and different assumptions regarding the mix of subsequent treatments received in each adjuvant treatment arm.
A probabilistic sensitivity analysis (PSA) was conducted to estimate the probability of pembrolizumab being cost-effective relative to observation under different willingness-to-pay thresholds. A Monte-Carlo simulation with 1,000 iterations was conducted. In each iteration, model inputs were randomly drawn from their specified distributions. To represent uncertainty in the transition probabilities starting from RF or LR, the parameter estimates underlying each parametric function were varied according to multivariate normal distributions (or univariate normal for exponential rate parameters). For exponential rates of OS and PFS failure with pembrolizumab in the advanced melanoma setting, normal distributions were used. Log-normal distributions were assumed for HRs of OS and PFS failure for other subsequent treatments vs pembrolizumab in the advanced melanoma setting. Beta distributions were assumed for health state utilities to reflect their allowable range between zero and one. For the disutility associated with AEs, a normal distribution was used. Gamma distributions were assumed for medical management, drug administration, and AE cost parameters that can range between zero and infinity. In the absence of data on the variability around health state costs, the standard error for each cost parameter was set equal to 20% of the base-case input value. For all other inputs, the standard error or variance-covariance matrix of the selected distribution was obtained directly from the same data source that informed the base-case value.
Results
Base-case results
Adjuvant treatment with pembrolizumab was predicted to extend RFS, DMFS, and OS relative to the strategy of observation in patients with complete resection of stage III melanoma. presents the modeled RFS, DMFS, and OS curves in each treatment arm.
Figure 3. Modeled RFS, DMFS, and OS in the long-term. Abbreviations. DMFS, distant metastases-free survival; OS, overall survival; RFS, recurrence-free survival.
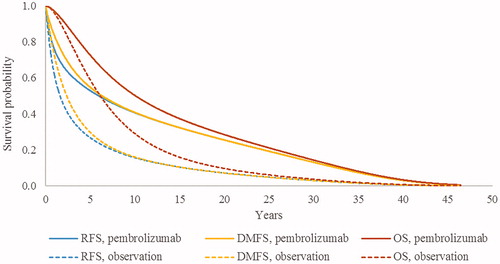
Base-case results are presented in . Over a 46-year time horizon, total QALYs were estimated to be 9.24 for pembrolizumab vs 5.95 for observation. Total life years were estimated to be 10.54 and 7.15, respectively, in these adjuvant treatment arms; thus, adjuvant pembrolizumab was estimated to prolong life expectancy by 3.39 years. The proportion of total life years spent in the recurrence-free state was 80.3% (8.46 years) in the pembrolizumab arm, compared with 57.3% (4.10 years) in the observation arm.
Table 3. Base-case cost-effectiveness results.
Total costs over the 46-year time horizon were $489,820 for pembrolizumab and $440,431 for observation. Differences in total costs across the treatment arms were largely attributable to adjuvant treatment costs (which were zero dollars under the observation strategy vs $133,895 for pembrolizumab) and subsequent treatment costs in the advanced melanoma setting (which were $368,798 for observation vs $305,438 for pembrolizumab). Disease management costs were also lower in the pembrolizumab arm ($40,975) relative to observation ($58,879), reflecting the lower incidence of disease recurrence achieved with pembrolizumab. Terminal care costs were similarly lower for pembrolizumab compared to observation ($9,213 vs $12,678).
The resulting ICERs for pembrolizumab vs observation were $15,009 per QALY gained and $14,550 per life year gained.
One-way DSA and scenario analysis results
DSA and scenario analysis results are shown graphically in a tornado diagram (). Sensitivity analyses are sorted from the widest to narrowest range of ICER values to highlight parameters with the strongest influence on the cost-effectiveness results.
Figure 4. Tornado diagram based on DSA results for pembrolizumab vs observation. Abbreviations. DM, distant metastases; LR, locoregional recurrence; OS, overall survival; PFS, progression-free survival; RF, recurrence-free.
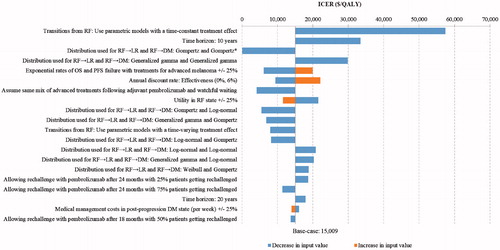
Across the sensitivity analyses, pembrolizumab ranged from being a dominant strategy to having an incremental cost of $57,449 per QALY vs observation. The ICER was most sensitive to parameters determining transition probabilities from the RF state to other states, as the model structure linked RFS to long-term projections of survival in each treatment arm. The highest ICERs were measured in the scenario analyses that used generalized gamma distributions to model the cause-specific hazards of RF → LR and RF → DM (ICER = $29,837 per QALY), or that used parametric models with a time-constant treatment effect to model the cause-specific hazards of each transition from the RF state (ICER = $57,449 per QALY). These parametric modeling approaches were considered as sensitivity analyses only, as they resulted in comparatively worse fit with the observed trial data than the base-case approach of using parametric models (Gompertz for RF → LR, generalized gamma for RF → DM) individually fitted to the pembrolizumab and placebo arms of the KEYNOTE-054.
Compared to the base-case ICERs (reflecting a 46-year time horizon), the ICER of pembrolizumab vs observation ($15,009/QALY) was similar when using a 20-year horizon ($17,963 per QALY), and was moderately higher when using a 10-year horizon ($33,431 per QALY). The ICERs for pembrolizumab vs observation decreased to $4,081 when the market shares of subsequent treatments in the pembrolizumab arm were set equal to those in the observation arm. The ICERs of pembrolizumab vs observation showed moderate-to-small variation when changing the discount rate for effectiveness, the utility in the RF state, the proportion of patients being rechallenged, or when shortening the time to allow rechallenge in the pembrolizumab arm (from 24 to 18 months after treatment initiation; that is, from 12 to 6 months after treatment completion). The cost-effectiveness results were not sensitive to: hazard rates from LR to DM and to death; drug administration costs; patient weight; use of 100% relative dose intensity for adjuvant pembrolizumab; state-specific medical management costs; terminal care costs; AE costs or disutilities; or utility values associated with the LR or DM states.
PSA results
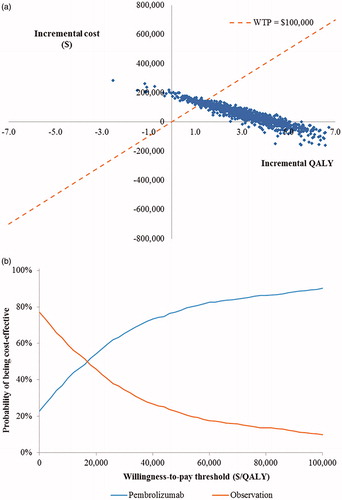
Across the 1,000 iterations of the PSA, the average incremental cost was $49,895, and the average incremental QALY gain was 3.16 for pembrolizumab vs observation. The resulting probabilistic ICER for pembrolizumab vs observation was $15,776 per QALY gained.
presents scatterplots of simulated incremental cost and QALY pairs for pembrolizumab vs observation. The cost-effectiveness acceptability curves in show the probability of each adjuvant treatment arm being cost-effective vs the other arm over a range of different willingness-to-pay thresholds. Based on a willingness-to-pay threshold of $100,000 per QALY gained, pembrolizumab had a 90.2% probability of being cost-effective vs observation.
Discussion
In the US, observation continues to be one of the recommended management strategies for completely resected stage III melanoma, even though a large proportion of patients with high-risk stage III disease develop disease recurrence when managed with surgery alone. In the KEYNOTE-054 trial, for example, 39% of patients randomized to observation experienced RFS failure (i.e. locoregional recurrence, distant metastases, or death) by 1 year, and 64% of those with RFS failure in this arm experienced distant metastases as their first recurrence eventCitation16. The present study sought to evaluate cost-effectiveness of pembrolizumab compared with observation for the adjuvant management of patients who have undergone complete resection of stage III melanoma with lymph node involvement.
Results from this economic evaluation suggest that adjuvant therapy with pembrolizumab is a highly cost-effective strategy for improving the health outcomes of patients following complete resection of stage III melanoma. Under base-case assumptions, the ICER of pembrolizumab vs observation from a health system perspective was $15,009 per QALY gained, well below the common willingness-to-pay benchmark of $100,000 per QALY in the USCitation73,Citation74. In the DSA and scenario analyses, the ICER value varied depending on several different factors, including the parametric assumptions determining RFS in each arm, the choice of time horizon, and assumptions regarding the distribution of subsequent treatments received in each adjuvant treatment arm after distant metastases. Nevertheless, the cost-effectiveness of pembrolizumab was robust across the range of plausible input values and alternative assumptions tested, with the most conservative scenario resulting in an ICER of $57,449 per QALY. In the PSA, the average ICERs across all 1,000 iterations were consistent with base-case ICERs. At a willingness-to-pay threshold of $100,000 per QALY gained, pembrolizumab had a 90.2% probability of being cost-effective vs observation.
For patients receiving observation alone, the model estimated total QALYs of 5.95 and total life years of 7.15 over a lifetime horizon. By prolonging RFS, pembrolizumab was projected to yield an improvement of 3.29 QALYs and 3.39 life years relative to observation over the lifetime model horizon. Transition probabilities determining RFS were modeled parametrically in each arm using patient-level data from KEYNOTE-054, the phase 3 trial that directly compared pembrolizumab and placebo; however, in the absence of mature DMFS and OS data from the trial, post-recurrence transition probabilities were estimated using real-world data and clinical trials in the advanced melanoma setting. The use of RFS to extrapolate long-term survival within the model aligns with recent evidence on the natural history of resected high-risk melanoma. In a meta-analysis of randomized controlled trials of adjuvant interferon, Suciu et al.Citation75 found a high degree of correspondence between the HR for recurrence or death and the HR for death, and concluded that RFS appears to be a valid surrogate endpoint for OS. Five-year data from the EORTC 18071 trial similarly showed that relative reductions in recurrence or death with ipilimumab vs placebo (HR = 0.76) closely aligned with relative reductions in death (HR = 0.72)Citation18. At 5 years, reported OS in the placebo arm of the EORTC 18071 trial (54.4%) was comparable to predicted OS in the observation arm of the model (56.8%), which supports the plausibility of the base-case survival extrapolations.
Until recently, limited options were available for the adjuvant treatment of resected high-risk melanoma with lymph node involvement. A number of earlier studies examined the cost-effectiveness of high-dose interferon as an adjuvant treatment of resected high-risk melanomaCitation30–34, including two US-based Markov models developed by Hillner et al.Citation30 and Cormier et al.Citation31. Based on 7-year data from the Eastern Cooperative Oncology Group 1684 trial, Hillner et al.Citation30 estimated an ICER of $26,953/QALY (adjusted to 2018 USD) for high-dose interferon vs observation over a 35-year timeframe, with the ICER increasing ∼ 2.8-fold when focusing on the 7-year trial timeframe. Cormier et al.Citation31 instead used pooled effectiveness data from a meta-analysis of randomized controlled trials, and estimated a higher ICER of $118,629/QALY (adjusted to 2018 USD) among all patients with resected stage III melanoma, ranging from $234,486/QALY for stage IIIA to $105,203/QALY for stage IIIC disease.
Given the recent introduction of several new adjuvant treatments for resected stage III melanoma in the US, rigorous economic evaluations of these treatments are needed to inform reimbursement decision-making by payers. To the best of our knowledge, the present study is the first cost-effectiveness analysis of any adjuvant treatment for melanoma to be published in over a decade. Strengths of this study include the use of direct comparative evidence from the KEYNOTE-054 trial to model the efficacy of each treatment arm with respect to RFS. Selection of the underlying parametric functions in each arm considered both goodness of fit with the observed data and clinical plausibility of long-term projections, consistent with recommended practiceCitation44. Because pembrolizumab is subject to a 1-year/18-dose maximum treatment duration in the adjuvant setting, time on treatment in the adjuvant pembrolizumab arm was precisely estimated based on observed Kaplan-Meier data from KEYNOTE-054, without the need for extrapolation.
Nevertheless, several limitations should be considered when interpreting the results of this study. First, DMFS and OS data were not included in the pre-specified first interim analyses of the KEYNOTE-054 trial, and therefore were not used to inform transition probabilities starting from the LR and DM states. In the absence of such data, the model conservatively assumed that adjuvant pembrolizumab would have no ongoing therapeutic benefit once patients experienced a recurrence event. DMFS and OS predictions from the model are, thus, subject to greater uncertainty and should be validated against long-term efficacy data from the KEYNOTE-054 trial as this data becomes available. Second, there is some uncertainty regarding whether patients treated with adjuvant pembrolizumab will be eligible for subsequent immunotherapies after recurrence. To address this limitation, survival and subsequent treatment costs within the distant metastases state were explicitly modeled based on the market shares and efficacy of subsequent treatments received in each adjuvant treatment arm. Using this approach, it was possible to conduct meaningful sensitivity analyses that varied assumptions about the mix of subsequent treatments received following adjuvant pembrolizumab. Third, the model does not account for the potential introduction of future treatments in the advanced melanoma setting, such as the triple combination of BRAF + MEK inhibitors and immunotherapy or the combination of different checkpoint inhibitors. Therapeutic advancements in the metastatic melanoma setting could impact survival within the DM state. Fourth, because costs were estimated from a health system perspective, indirect costs related to work productivity loss or caregiver burden were not considered in this economic evaluation. Inclusion of indirect costs under a societal perspective would be expected to further offset the cost of treatment with pembrolizumab, resulting in a lower ICER. Fifth, the target population of the model was restricted to patients with resected high risk stage III melanoma with lymph node involvement, in accordance with the eligibility criteria of the KEYNOTE-054 trial; thus, the cost-effectiveness analysis does not consider off-label usages of adjuvant pembrolizumab (e.g. by patients with satellite or in-transit metastases). Lastly, this study focused on the comparison of costs and effectiveness between pembrolizumab and its within-trial comparator, a strategy of observation. While beyond the scope of this initial economic evaluation, further research is warranted to evaluate the cost-effectiveness of pembrolizumab relative to other adjuvant treatments recently approved in the US, including ipilimumab, nivolumab, and dabrafenib in combination with trametinib, based on indirect comparative evidence across trials.
Conclusion
The results of this economic evaluation suggest that, from a US health system perspective, adjuvant pembrolizumab is highly cost-effective compared with the observation strategy following complete resection of stage III melanoma with lymph node involvement. The cost-effectiveness of pembrolizumab was robust in probabilistic simulations, and across a range of alternative scenarios and parameter values. Future studies are needed to compare the clinical and economic value of pembrolizumab relative to other novel adjuvant treatment options for resected high-risk melanoma.
Transparency
Declaration of funding
This study was supported by funding from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other interests
AGB, ZYZ, MJ, YS, WG, and JS are employees of Analysis Group, Inc., which received consultancy fees from Merck and Co. in connection with this study. FXL, JW, and RAI are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ. Peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, two reviewers disclose the following: receipt of honorariums for lectures and advisory board service from BMS, MSD, Novartis, Roche, Amgen, Pierre Fabre, and role as a sub-investigator in the KEYNOTE 054 clinical trial, as well as serving as an advisor for Merck Sharp & Dohme. The reviewers have no other relevant financial relationships or otherwise to disclose.
Previous presentations
This research has not been previously presented elsewhere.
Acknowledgements
The authors would like to thank the following individuals for their contributions to the analyses of clinical and quality-of-life data from KEYNOTE-054 to populate the model: Ruifeng Xu, Principal Scientist Outcomes Research; Qian Wang, Principal Scientist Statistical Programming; Thao Nguyen, Associate Director, Quantitative Sciences; and Harris Kampouris, Associate Director, Statistical Programming. The authors would also like to thank the following individuals for their contributions to the analyses of Flatiron registry data, which was also used to populate the model: Qian Xia, Associate Director, Outcomes Research; and Wanmei Ou, Director, Outcomes Research.
References
- National Cancer Institute Surveillance Epidemiology and End Results Program. Cancer stat facts: melanoma of the skin; [cited 2018 Oct 30]. Available from: https://seer.cancer.gov/statfacts/html/melan.html
- Rastrelli M, Tropea S, Rossi CR, et al. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005–1011.
- American Cancer Society. Survival Rates for Melanoma; 2016 [cited 2018 October 31]. Available from: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-staging/survival-rates-for-melanoma-skin-cancer-by-stage.html
- Stone M. Surgical management of metastatic melanoma: UpToDate; 2016 [cited 2018 October 30]. Available from: https://www.uptodate.com/contents/surgical-management-of-metastatic-melanoma
- Mayo Clinic. Melanoma diagnosis and treatment 2016 [cited 2018 October 31]. Available from: https://www.mayoclinic.org/diseases-conditions/melanoma/diagnosis-treatment/drc-20374888
- Kudchadkar RR, Michielin O, van Akkooi A. Practice-changing developments in stage III melanoma: surgery, adjuvant targeted therapy, and immunotherapy. Am Soc Clin Oncol Educ Book. 2018;23:759–762.
- Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline - Update 2016. Eur J Cancer. 2016;63:201–217.
- National Comprehensive Cancer Network. Melanoma. NCCN clinical practice guidelines in oncology. Version 1.2019. [cited 2019 Feb 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- Sondak VK, Wolfe JA. Adjuvant therapy for melanoma. Curr Opin Oncol. 1997;9:189–204.
- Herndon TM, Demko SG, Jiang X, et al. US Food and Drug Administration Approval: peginterferon-alfa-2b for the adjuvant treatment of patients with melanoma. Oncologist. 2012;17:1323–1328.
- Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: first analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol.. 2000;18:2444–2458.
- Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6:S11–S14.
- Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853–1862.
- Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39.
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801.
- Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530.
- Eggermont AMM, Chiarion-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med.. 2016;375:1845–1855.
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med.. 2017;377:1813–1823.
- Food and Drug Administration. Highlights of Prescribing Information; 2011 [cited 2018 October 30]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125377s087lbl.pdf
- Abdul-Karim RM, Cowey CL. Challenging the standard of care in advanced melanoma: focus on pembrolizumab. Cancer Manag Res. 2017;9:433–442.
- European Medicines Agency. Yervoy: EPAR – Product Information. 2011 [cited 2018 July 26]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/yervoy.
- Merck & Co. Keytruda (pembrolizumab): prescribing information; 2018. [cited 2018 July 26]. Available from: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
- Committee for Medicinal Products for Human Use. Summary of opinion (post authorisation): Keytruda; 2018 [cited 2018 July 26]. Available from: https://www.ema.europa.eu/documents/smop/chmp-post-authorisation-summary-positive-opinion-keytruda-ii-42ii-43_en.pdf
- European Society for Medical Oncology. EMA recommends pembrolizumab for the adjuvant treatment of melanoma; 2018 [cited 2018 October 25]. Available from: https://www.esmo.org/Oncology-News/EMA-Recommends-Pembrolizumab-for-the-Adjuvant-Treatment-of-Melanoma
- Wang J, Chmielowski B, Pellissier J, et al. Cost-effectiveness of pembrolizumab versus ipilimumab in ipilimumab-naive patients with advanced melanoma in the United States. JMCP. 2017;23:184–194.
- Miguel LS, Lopes FV, Pinheiro B, et al. Cost effectiveness of pembrolizumab for advanced melanoma treatment in Portugal. Value Health. 2017;20:1065–1073.
- Marriott E, Praet C, Aguiar-Ibanez R, et al. Cost-effectiveness of pembrolizumab for unresectable metastatic melanoma after progression with ipilimumab in England. Value Health. 2015;18:A453.
- Hillner BE, Kirkwood JM, Atkins MB, et al. Economic analysis of adjuvant interferon alfa-2b in high-risk melanoma based on projections from Eastern Cooperative Oncology Group 1684. J Clin Oncol. 1997;15:2351–2358.
- Cormier JN, Xing Y, Ding M, et al. Cost effectiveness of adjuvant interferon in node-positive melanoma. J Clin Oncol. 2007;25:2442–2448.
- Crott R, Ali F, Burdette‐Radoux S. Cost-utility of adjuvant high-dose interferon alpha therapy in stage III cutaneous melanoma in Quebec . Value Health. 2004;7:423–432.
- Gonzalez-Larriba JL, Serrano S, Alvarez-Mon M, et al. Cost-effectiveness analysis of interferon as adjuvant therapy in high-risk melanoma patients in Spain. Eur J Cancer. 2000;36.18:2344–2352.
- Messori A, Becagli P, Trippoli S, et al. A retrospective cost-effectiveness analysis of interferon as adjuvant therapy in high-risk resected cutaneous melanoma. Eur J Cancer. 1997;33:1373–1379.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103.
- Bureau of Labor Statistics. Consumer price index: Medical care commodities in U.S. city average, all urban consumers, not seasonally adjusted. 2019. [cited 2019 Jan 5]. Available from: https://beta.bls.gov/dataViewer/view/timeseries/CUUR0000SAM1
- Williams C, Lewsey JD, Briggs AH, et al. Cost-effectiveness analysis in R using a multi-state modeling survival analysis framework: a tutorial. Med Decis Making. 2017;37:340–352.
- Williams C, Lewsey JD, Mackay DF, et al. Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov Decision-Analytic modeling. Med Decis Making. 2017;37:427–439.
- Arias E, Heron M, Xu J. United States Life Tables, 2014. Natl Vital Stat Rep. 2017;66:1–64.
- Flatiron Health. Flatiron database. 2018. [cited 2018 Dec 15]. Available from: http://www.flatiron.com/real-world-evidence
- National Institute for Health and Care Excellence. DSU Technical Support Document 19: Partitioned survival analysis for decision modelling in health care: a critical review. 2017. [cited 2018 March 19]. Available from: http://nicedsu.org.uk/technical-support-documents/partitioned-survival-analysis-tsd/
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430.
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [cited 2019 Jan 5]. Available from: http://www.R-project.org/
- National Institute for Health and Care Excellence. DSU Technical Support Document 14: Survival analysis for economic evaluations alongside clinical trials – extrapolation with patient-level data; 2013. [cited 2018 March 9]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York (NY): Oxford University Press; 2011.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330.
- Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271.
- Hodi FS, Chesney J, Pavlick AC, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568.
- Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAFV600E and BRAFV600K mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323–332.
- Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451.
- Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220.
- Beusterien KSS, Kotapati S, et al. Societal preference values for advanced melanoma health states in the United Kingdom and Australia. Br J Cancer. 2009;101:385–387.
- Middleton MR, Atkins MB, Amos K, et al. Societal preferences for adjuvant melanoma health states: UK and Australia. BMC Cancer. 2017;17:689.
- Centers for Medicare & Medicaid Services. Physician Fee Schedule Search;2018. [cited 2019 Jan 5]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- Bristol-Myers Squibb Company. Opdivo (nivolumab): prescribing information; 2018. [cited 2018 Dec 17]. Available from: https://packageinserts.bms.com/pi/pi_opdivo.pdf
- Bristol-Myers Squibb Company. Yervoy (ipilimumab): prescribing information; 2018. [cited 2018 Dec 17]. Available from: https://packageinserts.bms.com/pi/pi_yervoy.pdf
- Genentech. Cotellic (cobimetinib): prescribing information; 2015. [cited 2018 Dec 17]. Available from: https://www.gene.com/download/pdf/cotellic_prescribing.pdf
- Genentech. Zelboraf (vemurafenib): prescribing information; 2017. [cited 2018 Dec 17]. Available from: https://www.gene.com/download/pdf/zelboraf_prescribing.pdf
- Novartis Pharmaceuticals Corporation. Tafinlar (dabrafenib): prescribing information; 2018. [cited 2018 Dec 17]. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tafinlar.pdf
- Novartis Pharmaceuticals Corporation. Mekinist (trametinib): prescribing information; 2018. [cited 2018 Dec 17]. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/mekinist.pdf
- National Institute for Health and Care Excellence. Ipilimumab for previously untreated advanced (unresectable or metastatic) melanoma [TA319]; 2014. [cited 2018 March 9]. Available from: https://www.nice.org.uk/guidance/ta319
- National Institute for Health and Care Excellence. Pembrolizumab for treating advanced melanoma after disease progression with ipilimumab [TA357]; 2015. [cited 2018 March 9]. Available from: https://www.nice.org.uk/guidance/ta357
- Centers for Medicare & Medicaid Services. Acute inpatient prospective payment system. 2018. [Cited 2019 Jan 9]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2018-IPPS-Final-Rule-Home-Page.html
- Rashid N, Koh HA, Baca HC, et al. Economic burden related to chemotherapy-related adverse events in patients with metastatic breast cancer in an integrated health care system. BCTT. 2016;8:173–181.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13:e0196007.
- Barzey V, Atkins MB, Garrison LP, et al. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost-effectiveness analysis. J Med Econ. 2013;16:202–212.
- Zhang Y, Le TK, Shaw JW, et al. Retrospective analysis of drug utilization, health care resource use, and costs associated with IFN therapy for adjuvant treatment of malignant melanoma. CEOR. 2015;7:397–407.
- Merck S, Dohme C. Adjuvant immunotherapy with anti-PD-1 monoclonal antibody Pembrolizumab (MK-3475) versus placebo after complete resection of high-risk Stage III melanoma: a randomized, double-blind Phase 3 trial of the EORTC Melanoma Group: Clinical study report. 2018. Data cutoff date: 2 Oct 2017. Data on file.
- Seidler AM, Pennie ML, Veledar E, et al. Economic burden of melanoma in the elderly population: population-based analysis of the surveillance, epidemiology, and end results (SEER)-Medicare data. Arch Dermatol. 2010;146:249–256.
- Tarhini A, Corman SL, Rao S, et al. Healthcare resource utilization and associated costs in patients with advanced melanoma receiving first-line ipilimumab. JCT. 2015;06:833–840.
- Chastek B, Harley C, Kallich J, et al. Health care costs for patients with cancer at the end of life. J Oncol Pract. 2012;8:75s–80s.
- Institute for Clinical and Economic Review. Overview of the ICER value assessment framework and update for 2017–2019.
- Institute for Clinical and Economic Review. Overview of the ICER value assessment framework and update for 2017-2019. 2018. [cited 2019 Feb 1]. Available from: https://icer-review.org/wp-content/uploads/2017/06/ICER-value-assessment-framework-Updated-050818.pdf
- Suciu S, Eggermont AMM, Lorigan P, et al. Relapse-free survival as a surrogate for overall survival in the evaluation of stage II–III melanoma adjuvant therapy. J Natl Cancer Inst. 2018;110:87–96.