Abstract
Aims: To evaluate the cost-effectiveness of percutaneous patent foramen ovale (PFO) closure, from a US payer perspective. Lower rates of recurrent ischemic stroke have been documented following percutaneous PFO closure in properly selected patients. Stroke in patients aged <60 years is particularly interesting because this population is typically at peak economic productivity and vulnerable to prolonged disability.
Materials and methods: A Markov model comprising six health states (Stable after index stroke, Transient ischemic attack, Post-Transient Ischemic Attack, Clinical ischemic stroke, Post-clinical ischemic stroke, and Death) was constructed to evaluate the cost-effectiveness of PFO closure in combination with medical management versus medical management alone. The base-case model employed a 5-year time-horizon, with transition probabilities, clinical inputs, costs, and utility values ascertained from published and national costing sources. Incremental cost-effectiveness ratio (ICER) was evaluated per US guidelines, utilizing a discount rate of 3.0%.
Results: At 5 years, overall costs and quality-adjusted life-years (QALYs) obtained from PFO closure compared with medical management were $16,323 vs $7,670 and 4.18 vs 3.77, respectively. At 5 years, PFO closure achieved an ICER of $21,049, beneficially lower than the conventional threshold of $50,000. PFO closure reached cost-effectiveness at 2.3 years (ICER = $47,145). Applying discount rates of 0% and 6% had a negligible impact on base-case model findings. Furthermore, PFO closure was 95.4% likely to be cost-effective, with a willingness-to-pay (WTP) threshold of $50,000 and a 5-year time horizon.
Limitations: From a cost perspective, our economic model employed a US patient sub-population, so cost data may not extrapolate to other non-US stroke populations.
Conclusion: Percutaneous PFO closure plus medical management represents a cost-effective approach for lowering the risk of recurrent stroke compared with medical management alone.
Introduction
Epidemiologic data support a relationship between the presence of a patent foramen ovale (PFO), defined as an embryonic defect in the interatrial septum, and cryptogenic strokeCitation1,Citation2. In a series of studies of cryptogenic stroke patients, echocardiographic imaging revealed a PFO in 47–56% of the subjects, compared with only 4–18% in non-cryptogenic stroke counterpartsCitation3.
The mechanism of cryptogenic stroke in PFO is presumed to be paradoxical embolization of a venous clot into the arterial circulation. Many studies have explored the hypothesis that closure of the septal defect is an effective therapy for secondary prevention of recurrent ischemic strokeCitation4. In these studies, PFO closure has been an adjunctive therapy to medical management with antiplatelets or anticoagulantsCitation5,Citation6. Early series included surgical repairCitation6–8, but developments in endovascular technologies have led to the emergence of transcatheter devices specifically designed for percutaneous closure of a PFO. Four recently completed randomized controlled trials of such devices found that the stroke incidence was significantly lower in patients who received PFO closure as compared with those assigned medical therapy aloneCitation9–12.
These results establish the clinical utility of transcatheter devices for percutaneous closure of a PFO in properly selected patients; however, we need more studies that investigate the cost-effectiveness of such devicesCitation13–15. Cryptogenic stroke as a diagnosis, although generally applicable to a wide spectrum of stroke patients, is specifically applicable to younger-aged patients; the mean age at enrollment in prior studies was 45 yearsCitation9,Citation10. Considering that the average age of first stroke is 70 years, the economic impact of a stroke in such a young patient population is 2-fold: it prevents the patient from being a productive member of the economy at the peak of their employable years, and it imparts a potential long-term disability cost of many decades on the healthcare system.
In light of the high costs and societal burden imposed by stroke in general and this population in particular, we developed an economic appraisal of a device-based therapy. Considering the variability in studies and costs, the specific aim of this analysis is to perform a detailed cost-analysis of percutaneous closure of a PFO using the GORE CARDIOFORM Septal Occluder (W.L. Gore & Associates, Flagstaff, AZ) in combination with standard medical management compared with standard medical management alone for prevention of recurrent stroke in cryptogenic stroke patients with PFO. This analysis assesses whether medical management or a device-based therapy provides the greatest reduction in neurologic events at the most affordable price. We submit that this analysis may assist in guiding clinical practice and physician-based decisions in optimizing the use of health services.
Methods
Model structure and overview
A Markov cohort model was developed () to assess the cost-effectiveness of PFO closure by use of the GORE CARDIOFORM Septal Occluder (in combination with standard medical management), compared with standard medical management alone (reference therapy) in a population of cryptogenic stroke patients with PFO. In line with the REDUCE trialCitation9,Citation16, standard medical management consisted of aspirin alone, a combination of aspirin and dipyridamole, or clopidogrel. Within the Markov model, health states were based on the presentation of a stable state, severity of neurologic episodes (i.e. ischemic stroke), or death. In brief, the model comprised six health states including: stable after index stroke, transient ischemic attack (TIA), post-transient ischemic attack (a near-stable state that many patients return to after a TIA), clinical ischemic stroke, post-clinical ischemic stroke, and death. The duration of the model cycle was set at 3 months, with this length deemed appropriate for providing sufficient granularity to capture the outcome of interest (a composite of recurrent non-fatal ischemic stroke, fatal ischemic stroke, or early death). Model assumptions, resource utilization, and unit cost data were acquired from numerous sources as described in . The latest healthcare expenses accounting for treatment and physician costs, long-term care, as well as first-year medical device costs were primarily derived from RED BOOK and the Centers for Medicare & Medicaid Services, as well as several other relevant published data sources ()Citation17,Citation18. For the purpose of this model, outcomes were expressed as costs using 2018 USD ($) per quality-adjusted life-year (QALY).
Figure 1. Markov cohort model structure for cost-effectiveness of percutaneous PFO closure. Abbreviation. PFO, patent foramen ovale.
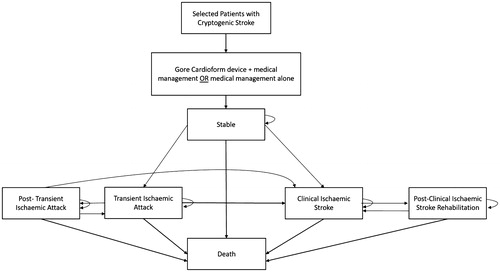
Table 1. Key resource utilization, treatment costs, and clinical utility inputs for the model.
Transition probabilities and utilities
Transition probabilities were estimated between each health state in the Markov model in an effort to evaluate the likelihood of transitioning to a different health state at the end of each model cycle for each treatment arm. Transition probabilities and clinical inputs based on ischemic stroke were ascertained from a number of recently published reportsCitation9,Citation10,Citation19. Transition probabilities for ischemic stroke were modeled at the end of the first 3-month cycle. The proportion of patients belonging to each health state beyond model cycle 1 (i.e. the first 3 months) served as a basis for the transition probabilities applied in subsequent model cycles. Transition probabilities for the remainder of the timeframe were acquired directly from patient-level sources for each respective time pointCitation9,Citation10,Citation19.
Utility values were calculated using the Modified Rankin Scale, EQ-5D (EuroQol-5 Dimensions) health-related quality-of-life (HRQoL) classification system, along with a set of unique HRQoL weights for ischemic stroke, all of which were extrapolated from several pertinent studies, reviews, and meta-analyses aimed at investigating HRQoL estimates for ischemic strokeCitation14,Citation20–22. For the present model, each health state was assigned a value reflecting both the utility and costs. The overall costs and utilities of receiving each treatment were estimated by multiplying the number of patients in each health state at the beginning of each model cycle by their corresponding utility and cost value, and then summing across health states and model cycles. HRQoL was further assessed across each 3-month model cycle over the 5-year follow-up study duration, with assumptions translated into utilities that ranged from 0.00 (death) to 0.88 (stable state in device arm). Five-year QALYs were then calculated using a combination of the utility and survival data.
Base-case model
We compared the overall costs and effects of the PFO closure device in combination with standard medical management vs standard medical management alone. The base-case analysis utilizes a 3% discount rate that was applied to both costs and benefits (i.e. QALYs)Citation23. The model analyzed data over a 5-year time horizon with an in-use age of ≥45 years applied, to reflect the maximum follow-up period and mean age of patients with a PFO who had had a cryptogenic stroke in the REDUCE clinical trialCitation9. The model assumes a 5-year time horizon for the base-case analysis, because this allows a sufficient length of time to reflect the chronic nature of clinical ischemic stroke, and captures key costs and effects related to all treatments that were assessed.
Serious adverse events
The model accounts for a number of serious adverse events (SAEs), along with the incurred costs. Data referring to device-related SAEs were acquired from several pertinent data sourcesCitation24,Citation25 and included: costs associated with thrombosis on a device, thrombus on the left disk, atrial fibrillation, and tachycardia. The frequency of patients who experienced any of these SAEs was low (1.4%), which reflected an overall cost of $35.15 per-patient SAE. Data based on procedural-related SAEs and their costs incurred was includedCitation24,Citation25. SAEs that occurred among patients included: anxiety, generalized right-side weakness, non-cardiac chest pain, intraprocedural device embolization, arteriovenous fistula, tamponade in pericardium, chest discomfort due to pericardial effusion, bleeding from femoral vein access site, groin hematoma, and post-procedural hypotension. The frequency of experiencing any of these SAEs was low (2.5%), with associated costs incurred totaling $121.47 per patient. These adverse event costs in the model included a low proportion of patients with “a serious bleeding event” (1.8% in the PFO closure + medical management arm, 2.7% in the medical management only arm) based on the REDUCE trialCitation9. Sondergaard et al.Citation9 reported that the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism did not differ significantly between the REDUCE study groups. Provision for additional costs associated with major bleeding would make no difference to the cost-effectiveness model as both arms are equally affected.
Scenario and sensitivity analyses
The model estimates incremental cost-effectiveness ratios (ICERs) for a range of scenario analyses to assess key assumptions that include: repeating the model according to 3-, 10-, and 20-year time horizons, as well as applying cost and benefit discount rates of 0% and 6%, in an effort to independently establish whether the chronic nature of stroke and its associated treatments became more cost-effective over time through implementation of PFO closure among patients who had had a cryptogenic stroke. The cost-effectiveness models assume a willingness-to-pay (WTP) threshold of $50,000 USDCitation26. Parameter uncertainty was explored by use of deterministic 1-way sensitivity analyses (OWSAs) and probabilistic sensitivity analyses (PSAs). Fundamental model parameters were individually varied over a suitable range determined by the standard error of each variable. In the likelihood, a measure of uncertainty was absent for parameters in OWSAs, the range estimate was calculated as ±10% (user-modifiable) of the point estimate for costs data, and defined as ±5% of the point estimate for utilities. For PSAs, model parameters were allocated a distribution according to the underlying data. PSAs were conducted using 2,000 iterations based on a conventional bootstrapping approach.
Results
Base-case analysis
At 5 years, the overall costs and QALYs for the PFO closure device compared with standard medical management are $16,323 vs $7,670, and 4.18 vs 3.77 (). The incremental costs and QALYs for the PFO closure device are $8,653 and 0.41, which is cost-effective compared with standard medical management, with a cost/QALY of $21,049 (). The PFO closure device reaches cost-effectiveness compared with standard medical management at 2.3 years. Compared with standard medical therapy at this time point, the incremental cost of the PFO closure device was $9,406, with patients achieving an incremental QALY of 0.20, for a cost/QALY of $47,145.
Table 2. Economic model results for the cost-effectiveness of percutaneous PFO closure.
Scenario and sensitivity analyses
Using a WTP threshold of $50,000/QALY at a 5-year time horizon, percutaneous PFO closure by use of the PFO closure device displayed a 95.4% likelihood of being cost-effective (). The incremental cost and QALYs for the PFO closure device at a 3-year time horizon are $9,236 and 0.26, with a cost/QALY of $35,755. The incremental cost and QALYs at 10 years are $6,655 and 0.76, for a cost/QALY of $8,753; the incremental cost and QALYs at 20 years are $2,412 and 1.27, with a cost/QALY of $1,894 (). Applying discount rates of 0% and 6% had a negligible impact on the base-case model findings (). OWSAs across a 5-year time horizon revealed the results of both treatments were predominantly influenced by stable state utilities ( and ). The costs per QALY ranged from $14,013 to $42,367 as utilities of the stable state varied from 0.924 to 0.836 within the PFO closure device arm, and from $14,628 to $37,593 as the utility values assigned to the stable state varied from 0.840 to 0.760 within the standard medical management arm (). When assessing joint parameter uncertainty through PSAs, a cost-effectiveness acceptability curve demonstrated that PFO closure using the PFO closure device was cost-effective over varying WTP thresholds ().
Figure 2. Acceptability curve for the probability of percutaneous PFO closure to be cost-effective at the willingness-to-pay threshold on the x-axis. Acceptability curve assessed at a 5-year time horizon. Abbreviation. PFO, patent foramen ovale.
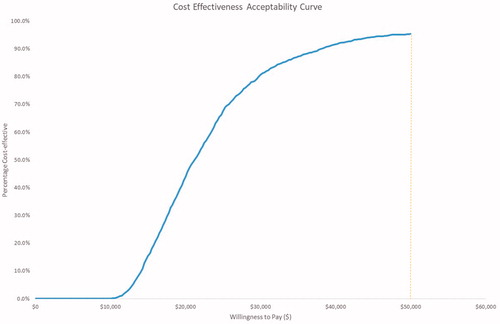
Figure 3. One-way sensitivity analysis for the cost-effectiveness of percutaneous PFO closure at a 5-year time horizon. Abbreviations. QALY, quality-adjusted life-years; TIA, transient ischemic attack.
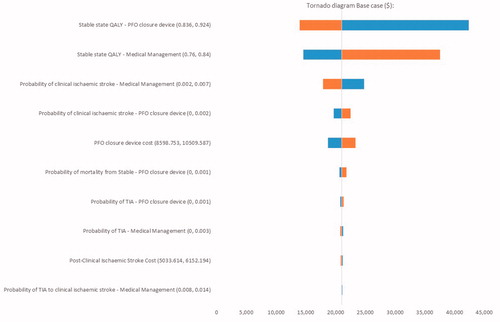
Figure 4. Cost-effectiveness acceptability curve for evaluating the cost-effectiveness of percutaneous closure of a patent foramen ovale by use of the GORE CARDIOFORM septal occluder. The orange data point indicates the mean incremental cost ($8,643) and quality-adjusted life-years (QALY; 0.41).
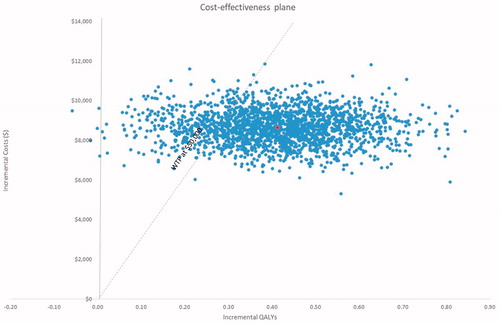
Table 3. One-way sensitivity analysis for the cost-effectiveness of percutaneous closure of a patent foramen ovale at a 5-year time-horizon.
Discussion
In a properly selected population of US patients with cryptogenic stroke and PFO, device closure utilizing the GORE CARDIOFORM Septal Occluder in combination with standard medical management is cost-effective compared with standard medical management alone at 2.3 years. The cost-effectiveness of PFO closure increases over time, as shown at 3-, 5-, 10-, and 20-year time horizons, because there are incrementally more TIAs, clinical ischemic strokes, and ultimately deaths in the “medical management only” arm compared with PFO closure + medical management.
The aim of this study was to model the GORE CARDIOFORM Septal Occluder based on the REDUCE study population and outcomes. As device utilities and prices vary, a comparison with other devices was not part of the study scope. Nonetheless, the present study is consistent with the literature on other economic evaluations related to PFO closure, which have also demonstrated cost-effectiveness. In one such analysis, a 15-year time horizon was used in combination with a meta-analysis of five randomized controlled trials from the perspective of a US payerCitation15. That study determined that PFO closure for cryptogenic stroke is cost-effective, with a benefit in QALYs gained alongside improved cost savingsCitation15. Similar to the present study, another cost-effectiveness model found PFO closure was cost-effective at 2.6 yearsCitation13.
As with any procedure-based intervention, the key economic question is whether the high upfront cost of the device prevents future costs. Both our model and others predict that, over time, patients who receive medical therapy alone tend to encounter more adverse events leading to higher medication expenses, which at 2–3 years exceed the cost for PFO closure.
Other non-US healthcare system models have also been established. From a UK perspective, the cost-effectiveness of PFO closure using the AMPLATZER PFO Occluder (St Jude Medical, St Paul, MN) plus medical therapy vs medical therapy alone achieved cost-effectiveness at 4.2 yearsCitation14. Similar to our model, the benefit of PFO closure derives from reducing recurrent neurologic episodes and improving HRQoLCitation9,Citation10,Citation14. The difference in time to cost-effectiveness between these UK and US models may be determined by various factors, including differences in health system costs, procedure costs, and potentially a difference in device performance. Analysis of these variables is outside the scope of the current study. However, to summarize the base case parameters of the current model, the cost of the entire PFO closure + medical management protocol, including the device, is $9,554. This includes the cost of the device plus pre-operative, peri-operative, and post-operative costs. Post-operative costs include two office visits made during the first year post-discharge with a cardiologist. After the first year, patients are discharged to their primary care physician, with no ongoing cardiology follow-up. Ongoing prescription therapy includes indefinite antiplatelet therapy. Medical management costs alone are lower, but the effectiveness of this strategy gives way to increased risk of clinical ischemic stroke and TIA, which costs substantially more than PFO device closure.
Stroke remains the leading cause of long-term disability and imposes a substantial economic burden on patients and the healthcare systemCitation27–29. In the US, the overall direct and indirect costs of stroke are anticipated to reach ∼ $185 billion by 2030Citation29, and $2.2 trillion by 2050Citation30. Most of these expenditures are direct costs related to hospitalization, rehabilitation, medication use, and physician servicesCitation29. Nonetheless, one study found that the median proportion of indirect costs attributable to stroke (e.g. loss in work productivity) was considerable, reflecting 32% of the overall cost of strokeCitation27. As a result, the rise in indirect costs due to stroke will persist, owing to the relatively young age of cryptogenic stroke patients. While stroke patients in this younger age strata are more likely to survive their subsequent stroke, it is this survival with long-term disability that leads to considerable indirect costs from home care and lost work productivityCitation14,Citation30. It is estimated that 1,100 individuals in their prime working years in the US are disabled by cryptogenic PFO-related stroke each yearCitation13.
Efforts to prevent stroke and thereby alleviate the direct and indirect costs attributable to this chronic condition are beneficial to patients and their healthcare system. Despite the initial costs, PFO closure using the PFO closure device not only lowers the rate of recurrent ischemic strokeCitation9, but offsets the overall costs attributable to stroke in the long-term when compared with medical management alone. On this basis, the clinical and economic utility of the GORE CARDIOFORM Septal Occluder as an interventional tool for stroke prevention is particularly beneficial among stroke patients who recover to independence after their index stroke, as it can prevent decades of costs and lost work associated with a subsequent stroke.
Limitations
From a cost perspective, our economic model employed a US patient sub-population, so cost data may not extrapolate to other non-US stroke populations. Certain device-related complications (e.g. atrial fibrillation) occurred within the PFO closure groupCitation9; because these events were rare in the pivotal study, they require further surveillance and investigation to understand the risks and costs associated with them. In this model, difficulties were encountered when attempting to classify the various types of clinical ischemic stroke. The REDUCE trial was unable to define stroke severity or rehabilitation directly, and these outcomes had to be imputed from the extant literature, which may have influenced the probability of reaching a certain health state within the Markov model and could have affected subsequent cost implications. Information regarding the indirect costs that might have been incurred by stroke were not accounted for in this economic analysis. In general, studies comprising data that provide a comprehensive description of the indirect costs that are accrued due to stroke are scarce. Thus, efforts to quantify the indirect economic burden associated with stroke are urgently needed to facilitate prospective cost analyses that aim to establish a robust estimate of the overall costs incurred as a consequence of stroke.
Conclusions
From a US healthcare perspective, percutaneous closure of a PFO with the GORE CARDIOFORM Septal Occluder plus standard medical management represents a cost-effective approach for lowering the risk of recurrent PFO-related cryptogenic stroke compared with standard medical management alone.
Transparency
Declaration of funding
The study was supported by W.L. Gore & Associates, Inc.
Declaration of financial/other interests
J.V.V. is a consultant and speaker for W.L. Gore & Associates, and a speaker for Amgen and Janssen. M.N. is an employee of Envision Pharma Group Ltd and was commissioned by the sponsor to provide economic modeling and scientific support for the manuscript. J.R.R. is an employee of W.L. Gore & Associates and may hold corporate stock. J.F.R. is a REDUCE study clinical investigator and has received financial support from W.L. Gore & Associates. L.S. is a national principal investigator for the REDUCE study and is compensated for his time by W.L. Gore & Associates. S.E.K. is a national principal investigator for the REDUCE study and is supported by a research grant from W.L. Gore & Associates. Peer reviewers on this manuscript have received an honorarium from J.M.E. for their review work. In addition, one reviewer on this manuscript discloses their role as a member of the RESPECT Trial Steering Committee (Abbott), while another reviewer discloses receiving speaker fees from Abbott. The reviewers have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
Daniel Ribes and Bríain Ó Hartaigh, PhD, of Envision Pharma Group, Inc were commissioned by the sponsor to provide economic modeling and scientific support throughout the manuscript process.
References
- Handke M, Harloff A, Bode C, et al. Patent foramen ovale and cryptogenic stroke: a matter of age? Semin Thromb Hemost. 2009;35:505–514.
- Handke M, Harloff A, Olschewski M, et al. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med. 2007;357:2262–2268.
- Bedeir K, Volpi J, Ramlawi B. Cryptogenic stroke with a patent foramen ovale: Medical therapy, percutaneous intervention, or surgery. J Card Surg. 2016;31:156–160.
- Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr. 2010;23:144–155. quiz 220.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236.
- Li J, Liu J, Liu M, et al. Closure versus medical therapy for preventing recurrent stroke in patients with patent foramen ovale and a history of cryptogenic stroke or transient ischemic attack. Cochrane Database Syst Rev. 2015;9:CD009938.
- Bogousslavsky J, Garazi S, Jeanrenaud X, et al. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology. 1996;46:1301–1305.
- Homma S, Di Tullio MR, Sacco RL, et al. Surgical closure of patent foramen ovale in cryptogenic stroke patients. Stroke. 1997;28:2376–2381.
- Sondergaard L, Kasner SE, Rhodes JF, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. 2017;377:1033–1042.
- Saver JL, Carroll JD, Thaler DE, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032.
- Mas JL, Derumeaux G, Guillon B, et al. Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. 2017;377:1011–1021.
- Lee PH, Song JK, Kim JS, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol. 2018;71:2335–2342.
- Pickett CA, Villines TC, Ferguson MA, et al. Cost effectiveness of percutaneous closure versus medical therapy for cryptogenic stroke in patients with a patent foramen ovale. Am J Cardiol. 2014;114:1584–1589.
- Tirschwell DL, Turner M, Thaler D, et al. Cost-effectiveness of percutaneous patent foramen ovale closure as secondary stroke prevention. J Med Econ. 2018;21:1–10.
- Leppert MH, Poisson SN, Carroll JD, et al. Cost-effectiveness of patent foramen ovale closure versus medical therapy for secondary stroke prevention. Stroke. 2018;49:1443–1450.
- Kasner SE, Thomassen L, Sondergaard L, et al. Patent foramen ovale closure with GORE HELEX or CARDIOFORM Septal Occluder vs. antiplatelet therapy for reduction of recurrent stroke or new brain infarct in patients with prior cryptogenic stroke: Design of the randomized Gore REDUCE Clinical Study. Int J Stroke. 2017;12:998–1004.
- W. L. Gore and Associates. Health data on file. Newark, DE: W.L. Gore and Associates Company; 2017.
- Johnson BH, Bonafede MM, Watson C. Short- and longer-term health-care resource utilization and costs associated with acute ischemic stroke. Clinicoecon Outcomes Res. 2016;8:53–61.
- Amarenco P, Lavallee PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374:1533–1542.
- Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: ten-year results of the Oxford Vascular Study. Neurology. 2013;81:1588–1595.
- Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke. 2001;32:1425–1429.
- Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21:191–200.
- Health Economic Resource Center (HERC). What is cost-effectiveness analysis? [cited 2018 Aug 30]. Available from: https://www.herc.research.va.gov/include/page.asp?id=cost-effectiveness-analysis.
- Centers for Medicare & Medicaid Services. Medicaid costs. [cited 2018 Aug 30]. Available from: https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-as-of-2018-02-14/ke5k-twun.
- cms.gov. Centers for Medicare and Medicaid Services [website]. [cited 2018 Aug 30]. Available from: https://www.cms.gov/.
- Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19:929–935.
- Joo H, George MG, Fang J, et al. A literature review of indirect costs associated with stroke. J Stroke Cerebrovasc Dis. 2014;23:1753–1763.
- Demaerschalk BM, Hwang HM, Leung G. US cost burden of ischemic stroke: a systematic literature review. Am J Manag Care. 2010;16:525–533.
- Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603.
- Brown DL, Boden-Albala B, Langa KM, et al. Projected costs of ischemic stroke in the United States. Neurology. 2006;67:1390–1395.
