Abstract
Purpose: The EF-14 trial demonstrated that adding tumor treating fields (TTFields) to maintenance temozolomide (TMZ) significantly extends progression-free survival (PFS) and overall survival (OS) for newly-diagnosed glioblastoma (GBM) patients. This study assessed the cost-effectiveness of TTFields and TMZ for newly-diagnosed GBM from the US healthcare system perspective.
Methods and materials: Outcomes for newly-diagnosed GBM patients were estimated over a lifetime horizon using an area under the curve model with three states: stable disease, progressive disease, or death. The survival model integrated the 5-year EF-14 trial results with long-term GBM epidemiology data and US background mortality rates. Adverse event rates were derived from the EF-14 trial data. Utility values to determine quality-adjusted life-years, adverse event costs, and supportive care costs were obtained from published literature. A 3% discount rate was applied to future costs and outcomes. One-way and probabilistic sensitivity analyses were performed to assess result uncertainty due to parameter variability.
Results: Treatment with TTFields and TMZ was estimated to result in a mean increase in survival of 1.25 life years (95% credible range [CR] = 0.89–1.67) and 0.96 quality-adjusted life years (QALYs) (95% CR = 0.67–1.30) compared to treatment with TMZ alone. The incremental total cost was $188,637 (95% CR = $145,324–$225,330). The incremental cost-effectiveness ratio (ICER) was $150,452 per life year gained and $197,336 per QALY gained. The model was most sensitive to changes in the cost of TTFields treatment.
Conclusions: Adding TTFields to maintenance TMZ resulted in a substantial increase in the estimated mean lifetime survival and quality-adjusted survival for newly-diagnosed GBM patients. Treatment with TTFields can be considered cost-effective within the reported range of willingness-to-pay thresholds in the US.
Introduction
Glioblastoma (GBM) is the most aggressive and common primary brain malignancy, with an estimated incidence of 12,760 new cases each year in the USCitation1. The disease rapidly progresses, and median survival is ∼15 monthsCitation2–4. However, clinical and epidemiological literature indicates that ∼3–5% of patients survive beyond 5 yearsCitation5–9.
Tumor treating fields (TTFields), a novel therapy that can be added to radiochemotherapy for the treatment of GBM, rely on a physics-based mechanism of action that is unlike previous applications of electricity or ionizing radiation in medicineCitation10. TTFields are low-intensity alternating electric fields delivered at intermediate frequencies that have the ability to arrest mitosis, induce apotosis, and kill tumor cells. TTFields have been studied since 2000 in pre-clinical models and in clinical trials for GBM and other solid tumorsCitation11,Citation12.
The EF-14 trial, a pivotal randomized controlled trial for newly-diagnosed GBM patients, demonstrated that adding TTFields to maintenance temozolomide (TMZ) chemotherapy significantly prolonged progression free and overall survival compared to maintenance TMZ alone without increasing systemic toxicity or decreasing quality-of-lifeCitation13,Citation14. Importantly, the combination of TTFields and TMZ has resulted in the first report of 5-year GBM survival greater than 10% from a large clinical trialCitation13.
The National Comprehensive Cancer Network (NCCN) has assigned a category 1 recommendation for TTFields in newly-diagnosed GBM based on the EF-14 trial resultsCitation15. The manufacturer of the delivery system has reported that 30% of eligible GBM patients are now being treated with the systemCitation16. Given the clinical adoption of TTFields as a GBM treatment, economic assessment of the therapy is warranted. Our objective was to estimate the cost-effectiveness of adding TTFields to maintenance TMZ chemotherapy as the standard-of-care in newly-diagnosed GBM patients.
Methods
Approach
We developed a three-state area under the curve (AUC) framework, also known as a partitioned survival model. Modeled health states were stable disease (SD), progressive disease (PD), and death. The AUC framework estimated time spent in each state for a hypothetical cohort of patients, based on the progression-free survival (PFS) and overall survival (OS) data from the EF-14 trial. Patients without progression were assigned to the SD state, and patients alive after progression were assigned to the PD state. Patients in either state could be on or off treatment, as patients may finish therapy but remain alive.
We conducted the analysis from a US payer perspective and focused on direct medical care costs only. We used weekly cycles and modeled patients starting TTFields with maintenance TMZ compared to patients receiving TMZ alone. Patients started treatment at age 56 in the model, consistent with the EF-14 trial population. The model estimated mean survival outcomes over a lifetime horizon. We applied a 3% discount rate for all future outcomes and costs based on current guidance for US health economic modelsCitation15.
Clinical trial results
The EF-14 trial enrolled 695 patients with histologically confirmed supratentorial GBM who had stable disease after having undergone surgery and standard concomitant radiochemotherapy with TMZ. Patients were randomized 2:1 to receive TTFields with maintenance TMZ or maintenance TMZ aloneCitation17. The patient characteristics were balanced for both patient and molecular characteristicsCitation13.
The primary endpoint was PFS, and the powered secondary endpoint was OS, with both endpoints measured in the intent-to-treat populationCitation13,Citation18. Median PFS for TTFields plus TMZ was 6.7 months vs 4.0 months in the TMZ monotherapy group (hazard ratio [HR] = 0.63; 95% confidence interval [CI] = 0.52–0.76]; p < 0.001). Median OS for TTFields plus TMZ was 20.9 months vs 16.0 months in the TMZ monotherapy group (HR = 0.63; 95% CI = 0.53–0.76, p < 0.001).
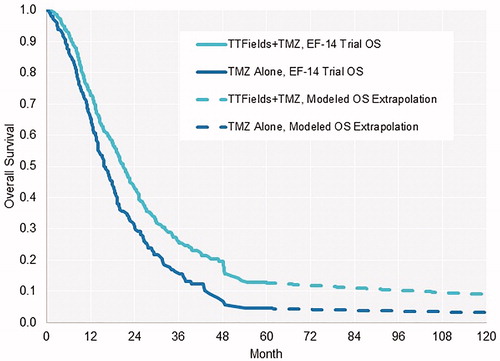
The EF-14 trial demonstrated that the OS benefit of adding TTFields was maintained through 5 years after starting treatment. The reported survival at 5-years was 13% for patients treated with TTFields and maintenance TMZ and 5% for patients treated with maintenance TMZ alone (p = 0.004). The landmark annual survival rates by year through year-5 are reported for each arm in Citation13,Citation18.
Table 1. EF-14 overall survival rates—estimates according to the Kaplan-Meier method.
Epidemiology data
Our literature review of long-term GBM OS has been described previouslyCitation19. Given the material number of 5-year survivors in the EF-14 trial, we reviewed epidemiological literature reporting long-term GBM survival to determine the best modeling approach to extrapolate the EF-14 survival curves. We searched the MEDLINE database using a Boolean word search of the keyword combination “glioblastoma” AND “long-term survival” OR “conditional survival”. Of the 541 publications screened, 23 publications were reviewed in full text based on their relevance, as indicated by title and abstract, and seven publications were selected for a detailed review based on the time horizon and suitability of reported results. All seven publications indicated that the probability of surviving GBM increased the longer patients survived since diagnosis, and that the first 2 years after diagnosis were the period of the highest mortality hazard ratesCitation6,Citation7,Citation20–24. We excluded four publications based on single institution reports in favor of larger epidemiological populationsCitation6,Citation22–24.
Upon reviewCitation19, we concluded that the introduction of TMZ in 2005 was potentially a confounding factor for estimating survival beyond 5 years in the remaining three studies, as they included pre- and post-2005 populationsCitation7,Citation20,Citation21. Therefore, we selected the study by Porter et al.Citation7, which was based on 5,991 GBM patients in the National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER) registries, to model long-term overall survival due to its homogeneous population of pre-TMZ patients, long-term data horizon, and data maturity. Conditional survival probabilities were available up to 15 years post-GBM diagnosis. GBM patients alive 5 years after diagnosis had a 70.4% (95% CI = 55.6–81.2%) probability of surviving to 10 years after diagnosis. GBM patients alive 10 years after diagnosis had an 84.0% (95% CI = 38.9–96.8) probability of surviving to 15 years after diagnosisCitation7.
Survival curves
We fit several parametric functions to the EF-14 trial PFS and OS Kaplan-Meier data; our approach to modeling long-term OS for EF-14 trial patients has been described previouslyCitation19. The fitted distributions included exponential, Weibull, log-logistic, and log-normal. The parametric functions were assessed for goodness of fit to the data using the Akaike Information Criterion (AIC) as well as assessment for face validity. We rejected the resultant OS curves based on face validity considerations due to observed under-estimation of 5-year OS compared to both the EF-14 trial data and epidemiological data.
We then evaluated modeling the TTFields curves using the trial-reported hazard ratios. We tested the proportional hazards assumption by plotting the log-cumulative hazards for PFS and OS curves, estimated as log(time) vs log(–log(S(time)). The log-cumulative hazards plots of EF-14 survival data converged over time, thus the proportional hazards assumption was inappropriate for the base case analysis.
Given the inadequacy of the above approaches to replicate both EF-14 trial and long-term overall survival and the availability of epidemiological data, we instead used a 3-phase approach to model mean lifetime survival that integrated: (1) the EF-14 data to year-5, (2) long-term conditional survival probabilities from the epidemiological literature up to year 15, and (3) US background mortality for patients who survived beyond year 15Citation25. This multi-segment approach has been utilized in previous modeling studies when both clinical trial data and long-term epidemiology data are availableCitation26,Citation27. The EF-14 trial Kaplan-Meier curves for OS were directly incorporated in the first phase without curve fitting (). We converted the Porter et al.Citation7 long-term conditional survival probabilities to weekly mortality probabilities in the second phase.
We directly incorporated PFS data from the EF-14 trial in the model and then used a parametric function to extrapolate survival thereafter. Parametric extrapolation was necessary due to the lack of epidemiology reports of PFS outcomes. We used Weibull distribution based on its best fit according to AIC testing.
For sensitivity analysis we constructed parametric functions for both OS and PFS curves. We fit separate parametric functions to distinct phases of the Kaplan-Meier PFS curves to minimize the effect of the reducing hazard rates with time from diagnosisCitation28,Citation29. The PFS curves were log-normal distribution in year-1 followed by Weibull distribution in subsequent years. The OS curve was log-normal for TTFields and TMZ, and log-logistic through year-4 and Weibull thereafter for TMZ alone.
Treatment parameters in the model
The treatment duration time for TTFields and TMZ was taken from the EF-14 Trial (). We used the drug wholesale acquisition cost for TMZ. The TMZ cost per patient per administration was calculated using the average patient baseline characteristics and the recommended dose for the first maintenance cycle of 150 mg/m2 per day for 5 days, every 28 days. The model used the current supplier list price for TTFields therapy in the US without consideration of discounts negotiated by payers.
Table 2. Model parameters.
We did not consider surgical, radiological, or pre-maintenance chemotherapy costs. These would be similar for both arms and, thus, have no impact on the model. Supportive care costs in both the SD and PD health states reflected utilization rate-weighted estimates of physician visits, laboratory tests, MRI/CT scans, and hospitalizationCitation30. We assumed that long-term survivors required the same intensive care as recently progressed patients, given a lack of data on GBM patient healthcare utilization rates over extended periods of time.
Adverse event parameters in model
We included all EF-14-reported grade 3–4 adverse events that occurred in at least 5% of patients or greaterCitation17. The frequency of each event was modeled as the total number of occurrences in the trial. This frequency was then multiplied by the average cost per event to derive a total adverse event cost for each regimen. Costs per adverse event were based on data from the Centers for Medicare and Medicaid Services (CMS) list of Medicare Severity-Diagnosis Related Groups (MS-DRGs) for the fiscal year 2017Citation30. All adverse events were costed as acute events, and did not account for chronic costs or outcomes related to the event, which were assumed to be reflected in supportive care costs.
Quality-of-life parameters
We used the utility values for GBM developed by Garside et al.Citation31 for their cost-utility analysis of carmustine implants and TMZ in the UK GBM population. We did not include adverse-event related disutilities, due to the low frequency of adverse events in both treatment armsCitation13. We also noted that the EF-14 trial indicated that combining TTFields with TMZ did not reduce the quality-of-life compared to treatment with TMZ aloneCitation14.
Analysis
We calculated lifetime direct medical costs, life years, and quality-adjusted life years (QALYs) for TTFields with maintenance TMZ and for TMZ alone. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in costs divided by the difference in life years and QALYs. We performed one-way and probabilistic sensitivity analyses to assess the impacts of uncertainty in model parameter estimates on the results. In one-way sensitivity analysis, one parameter at a time is varied to its low and high value, while keeping all other parameters constant. In probabilistic sensitivity analysis, all model parameters were simultaneously and randomly varied according to an assigned probability distribution over 5,000 simulations, and 95% credible ranges (CR) were calculated for each model result.
Results
The results represent mean costs and outcomes for one patient with glioblastoma over their lifetime. Base case results are presented in and are reported after applying the 3% discount rate to future costs and outcomes. The addition of TTFields to maintenance TMZ increased survival and QALYs with additional incremental costs.
Table 3. Model results.
Mean lifetime survival of patients treated with TTFields and TMZ was 3.34 years, an incremental 1.25 life years gained compared to treatment with TMZ alone. The average patient treated with TTFields and TMZ achieved 2.57 QALYs, an increase of 0.96 QALYs compared to patients treated with TMZ alone. The incremental life years gained with TTFields treatment reflected both the survival benefit observed through year-5 and the presence of more 5-year survivors on the TTFields and TMZ arm vs the TMZ alone arm. The increase in QALYs was driven by the increase in life years gained and the extended period of SD for TTFields treated patients.
The average costs per patient are presented in . Incremental costs were $188,637 (95% CR = $145,324–$225,330) for patients treated with TTFields. The primary drivers of incremental costs were the acquisition cost of TTFields and the cost of managing progressive disease.
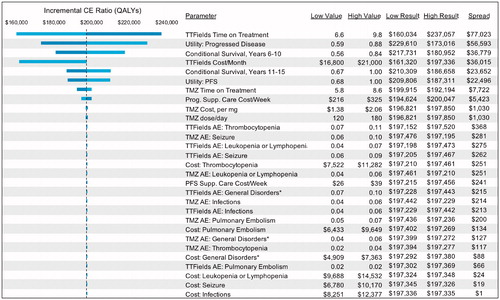
The incremental cost-effectiveness ratio (ICER) was $150,452 (95% CR = $105,135–$214,318) per life year gained and $197,336 (95% CR = $134,839–$287,249) per QALY. The ICER estimate was generally robust to changes in model parameters (). One-way sensitivity analysis showed that our results were most sensitive to the treatment duration of TTFields followed by the cost of TTFields treatment per month, health state utility values, and conditional survival probabilities for patients who survived beyond 5 years.
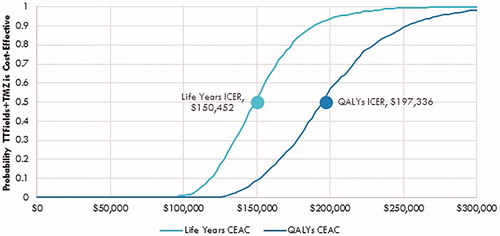
Probabilistic sensitivity analyses results are presented as a cost-effectiveness acceptability curve, showing the probability that TTFields are cost-effective at increasing willingness-to-pay per QALY thresholds (). The addition of TTFields to maintenance TMZ was 57% and 98% likely to be cost-effective at willingness-to-pay thresholds per QALY of $200,000 and $300,000, respectively.
Discussion
The EF-14 trial demonstrated that adding TTFields to maintenance TMZ resulted in a significant increase in overall survival for newly-diagnosed glioblastoma patients, the first pivotal trial report of increased GBM survival in more than a decade. We present here the first analysis of TTFields treatment for newly-diagnosed GBM from a US healthcare payer perspective.
Our model estimated that the mean number of life years and QALYs gained by treating a newly-diagnosed GBM patient with TTFields would be 1.25 and 0.96, respectively. The greater incremental benefit in estimated mean survival compared to the reported median survival in the EF-14 trial (1.25 years mean vs 4.9 months median) reflects the observed long-term survival benefit for TTFields patients in the EF-14 trialCitation18. For patients treated with TTFields and TMZ in the EF-14 trial, 12% were progression-free as of 30 months, and 13% were alive as of 5-years after randomization. For patients treated with TMZ alone, only 5% were progression-free as of 30 months, and only 5% were alive as of 5-years after randomizationCitation18.
Adding TTFields to TMZ resulted in a higher mean incremental cost per patient compared to treatment with TMZ alone, as would be expected when adding a new oncology treatment to an existing standard of care. The incremental costs were primarily due to the acquisition costs of TTFields and the costs of supportive medical care in the PD state. The model used the supplier list price for TTFields without considering any discounts negotiated between payers and the manufacturer. The model also assumed that supportive care costs were constant in the PD state, regardless of time from diagnosis or progression. Both assumptions could be considered conservative. Specifically, the use of the supplier list price for TTFields can be viewed as the maximum acquisition cost.
The resulting ICERs for TTFields and TMZ in the base case were $150,600 per life year gained and $198,000 per QALY gained. To put these figures in perspective, we reviewed health economic literature related to willingness-to-pay thresholds in the US. Willingness-to-pay thresholds are theoretical benchmarks indicating how much a payer should spend to gain an additional year of survival for a patient. Economic studies of implied willingness-to-pay levels in the US suggest thresholds in the range of $100,000–$300,000 per QALY gained, with thresholds increasing for treatments that extend survivalCitation32–35. The range of proposed thresholds reflects the different academic approaches to determining this benchmark. Specifically, the lower end of the range typically reflects simplified estimates based on multiples of per capital annual incomeCitation36.
More advanced economic studies focused on the value of extending life, resource allocation trade-offs, and revealed preference/job risk scoring have estimated willingness-to-pay thresholds at ∼$200,000 or above, with the revealed preference/job risk method reaching as high as $400,000Citation33–35,Citation37. Thus, the estimated ICERs for TTFields in newly-diagnosed GBM, even at the maximum acquisition cost, may be considered cost-effective by current standards of willingness-to-pay for cancer in the US. Over time, the potential for lower costs as technologies become generic and/or TTFields competitors enter the market may further reduce the current ICER.
Our survival model integrated the EF-14 trial data through year-5 with real-world outcomes for GBM patients alive 5–15 years after entering the model. This integrated modeling approach uses epidemiological reported real-world outcomes to estimate future survival and did not rely on statistical extrapolation of the EF-14 trial results for the base case scenario. The limitations of extrapolating trial data for oncology treatments using regression-based models have been reported in health economic literatureCitation28,Citation29,Citation38. The principal limitation of regression-based extrapolation is that it is unable to account for changing (i.e. reducing) hazard rates as patients live longer.
The limitations of regression-based survival forecasting can be observed in a previous cost-effectiveness analysis based on the EF-14 trial data. Bernard-Arnoux et al.Citation39 estimated GBM survival using exponential extrapolation of median EF-14 survival rates. We previously observed this approach to be a poor visual fit to the actual EF-14 K-M survival curves, and that the exponential extrapolation had the worst fit of four estimated parametric approaches according to Akaike’s information criterion testingCitation19; the Bernard-Arnoux et al.Citation39 study consequently estimated that the TTFields and TMZ arm and TMZ alone arm converged by year-5, which was not observed in the trial.
The benefit of integrating epidemiological data with clinical trial data in oncology survival models has been previously reportedCitation26. Of note, the National Institute for Health and Care Excellence (NICE) in the UK considered such a survival model structure in its decision to license ipilimumabCitation27,Citation40. Recent academic research has also relied on this approach to assess ipilimumab and pembrolizumabCitation41,Citation42. We also note that the survival model presented here was the basis of successful health technology assessment filings for TTFields in both Japan and SwedenCitation43,Citation44. However, this is the first publication of the cost-effectiveness model.
Our model relied on a single pivotal trial for TTFields in newly-diagnosed GBM. Nonetheless, the EF-14 trial was sufficiently powered to detect the proposed study outcomes, and the survival results for the control arm were consistent with a previous study of TMZ for GBMCitation45, as well as that reported in the epidemiological literatureCitation7,Citation20–23. We also assumed the age of the patient entering the model was consistent with the EF-14 trial, which was younger than the reported age of GBM patients in real-world populationsCitation2. Using the reported age and outcomes from EF-14 allows for a consistent application of trial data in the model. The minor difference in age for patients entering the model compared to real-world reports (56 vs 64 years of age) has an immaterial impact on mean lifetime survival gains, estimated at less than 8% of QALYs gained. Also, we noted that a post-hoc analysis of the EF-14 trial patients over age 65 achieved the greatest survival benefit (HR = 0.51; 95% CI = 0.22–0.77) and the cost-effectiveness for the therapy in this sub-group may be greater than for the total trial population. Future research could segment the EF-14 trial population by age and apply the age-specific EF-14 trial outcomes data to each group.
The analysis of longitudinally collected quality-of-life data in the EF-14 trial showed that role and social functioning were not statistically different to the control armCitation14. While adherence to treatment may be different between trial and real-world settings, the majority of TTFields patients display a high or very high daily usage of the device in both settings and only a few patients are unable to use the device as recommendedCitation46.
The EF14 trial protocol stipulated a TMZ dose of 150–200 mg/m2 for 5 days every 28 daysCitation13. The average dose was not reported, and our model assumes a TMZ dose of 150 mg/m2 for 5 days every 28 days for the duration of maintenance therapy in both arms. Real world dosing may be increased to 200 mg/m2, particularly after the first treatment cycle; however, the results of our one-way sensitivity analysis indicate that TMZ dosage has little impact on model results given that dosage is equal between treatment arms. Also, while skin irritation is a common side-effect of TTFields treatment, the trial reported incidence of grade 3 skin irritation events were 2%. Lower grade skin irritation would not usually require specific treatment and, therefore, would not notably impact the model results.
The model assumes a constant utility score for the SD and PD states, regardless of time from diagnosis or progression. This modeling approach is consistent with standard methods to model oncology outcomes. The limitation of this approach is that it assumes that no improvement in quality-of-life is possible as time passes from diagnosis or progression. A patient alive and progression free at year-5 is modeled with the same utility value as a patient starting TTFields therapy shortly after diagnosis. Additional health outcomes research would be needed to understand quality-of-life and utility values for long-term glioblastoma survivors. Accordingly, our model relies on the standard approach, which may be conservative for long-term survivors.
Conclusion
Adding TTFields to maintenance TMZ resulted in a substantial increase in the estimated mean lifetime survival and quality-adjusted survival for newly-diagnosed GBM patients. Treatment with TTFields can be considered cost-effective within the reported range of willingness-to-pay thresholds in the US.
Transparency
Declaration of funding
This study was funded by Novocure, Inc.
Declaration of financial/other interests
G.G. and B.W. are paid consultants to Novocure, Inc. V.S. is a speaker for the Novocure Speaker Bureau and a member of Novocure Advisory Boards. E.P. and L.G. have no financial or other relationships to disclose. Peer reviewers on this manuscript have received honorariums from JME for their review work. In addition, one of the reviewers discloses being employed by Novocure, Inc. The remaining reviewers have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:iv1–iv63.
- Ostrom QT, Gittleman H, Xu J, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18:v1–v75.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996.
- Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722.
- Gittleman H, Boscia A, Ostrom QT, et al. Survivorship in adults with malignant brain and other central nervous system tumor from 2000–2014. Neuro Oncol. 2018;20(suppl_7):vii6–vii16.
- Davis FG, McCarthy BJ, Freels S, et al. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85:485–491.
- Porter KR, McCarthy BJ, Berbaum ML, et al. Conditional survival of all primary brain tumor patients by age, behavior, and histology. Neuroepidemiology. 2011;36:230–239.
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466.
- Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci. 2018;54:7–13.
- Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104:10152–10157.
- Hottinger AF, Pacheco P, Stupp R. Tumor treating fields: a novel treatment modality and its use in brain tumors. NEUONC. 2016;18:1338–1349.
- Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295.
- Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–2316.
- Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495–504.
- National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Central Nervous System Cancers. 2018; v 1.2018 [cited 2018 Mar 20]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- Novocure I. SEC form 10-K for the year-ended December 31, 2017. 2018.
- Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus Temozolomide vs Temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314:2535–2543.
- Stupp R, Hegi ME, Idbaih A, et al. Abstract CT007: tumor treating fields added to standard chemotherapy in newly diagnosed glioblastoma (GBM): final results of a randomized, multi-center, phase III trial. Cancer Res. 2017;77(13 Suppl):CT007.
- Guzauskas GF, Salzberg M, Wang BC. Estimated lifetime survival benefit of tumor treating fields and temozolomide for newly diagnosed glioblastoma patients. CNS Oncology. 2018;7:Cns23.
- Farah P, Blanda R, Kromer C, et al. Conditional survival after diagnosis with malignant brain and central nervous system tumor in the United States, 1995–2012. J Neurooncol. 2016;128:419–429.
- Johnson DR, Ma DJ, Buckner JC, et al. Conditional probability of long-term survival in glioblastoma: a population-based analysis. Cancer. 2012;118:5608–5613.
- McNamara MG, Lwin Z, Jiang H, et al. Conditional probability of survival and post-progression survival in patients with glioblastoma in the temozolomide treatment era. J Neurooncol. 2014;117:153–160.
- Polley MY, Lamborn KR, Chang SM, et al. Conditional probability of survival in patients with newly diagnosed glioblastoma. JCO. 2011;29:4175–4180.
- Roh TH, Park HH, Kang SG, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: \ single-center analysis. Medicine. 2017;96:e7422.
- Arias E, Heron M, Xu J. United States life tables, 2013. Natl Vital Stat Rep. 2017;66(3):1–64.
- Holland RR, Ellis CA, Geller BM, et al. Life expectancy estimation with breast cancer: bias of the declining exponential function and an alternative to its use. Med Decis Making. 1999;19:385–393.
- Larkin J, Hatswell AJ, Nathan P, et al. The predicted impact of ipilimumab usage on survival in previously treated advanced or metastatic melanoma in the UK. PLoS One. 2015;10:e0145524.
- Gibson E, Koblbauer I, Begum N, et al. Modelling the survival outcomes of immuno-oncology drugs in economic evaluations: a systematic approach to data analysis and extrapolation. Pharmacoeconomics. 2017;35:1257–1270.
- Huang M, Latimer N, Zhang Y, et al. Estimating the Long-term outcomes associated with Immuno- oncology therapies: challenges and approaches for overall survival extrapolations. ISPOR Value Outcomes Spotlight 2018;28–30 [cited 2019 Feb]. Available from: https://www.ispor.org/valueoutcomesspotlight_long-term-immuno-oncology-therapies_January-February_2018.pdf.
- Centers for Medicare and Medicaid Services. Medicare Fee-for-Service Part B Drugs. 2017 [cited 2017 Nov 1]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2017ASPFiles.html.
- Garside R, Pitt M, Anderson R, et al. The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation. Health Technol Assess. 2007;11:iii–iiv. ix–221.
- Becker G, Murphy K, Philipson T. The value of life near its end and terminal care. 2007 [cited 2018 May 21]. Available from: http://www.nber.org/papers/w13333.
- Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356.
- Hirth RA, Chernew ME, Miller E, et al. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–342.
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797.
- Seabury SA, Goldman DP, Maclean JR, et al. Patients value metastatic cancer therapy more highly than is typically shown through traditional estimates. Health Aff. 2012;31:691–699.
- Annemans L, Asukai Y, Barzey V, et al. Outcomes assessment: extrapolation in oncology modelling: novel methods for novel compounds. ISPOR Connections. 2012;18.
- Bernard-Arnoux F, Lamure M, Ducray F, et al. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18:1129–1136.
- National Institute for Clinical Excellence. Ipilimumab for previously treated advanced (unresectable or metastatic) melanoma | Guidance and guidelines | NICE. 2012 (TA268) [cited 2018 May 21]. Available from: https://www.nice.org.uk/guidance/ta268.
- Barzey V, Asukai Y, Gueron B, et al. Cost-effectiveness of ipilimumab in previously untreated patients for advanced melanoma in Sweden. Value Health. 2014;17:A642–A643.
- Huang M, Lou Y, Pellissier J, et al. Cost effectiveness of pembrolizumab vs. standard-of-care chemotherapy as first-line treatment for metastatic NSCLC that expresses high levels of PD-L1 in the United States. Pharmacoeconomics. 2017;35(8):831–844.
- Japan Ministry of Health Labour and Welfare. MHLW Official Gazette. 2017;154.
- Swedish Association of Local Authorities and Regions. 2017 [cited 2018 May 21]. Available from: http://www.janusinfo.se/Documents/Nationellt_inforande_av_nya_lakemedel/Motesprotokoll/Protokoll-NT-radet-180207.pdf.
- Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. JCO. 2013;31:4085–4091.
- Onken J, Staub-Bartelt F, Vajkoczy P, et al. Acceptance and compliance of TTFields treatment among high grade glioma patients. J Neurooncol. 2018;139:177–184.