Abstract
Aims: To assess the real-world clinical burden and healthcare resource utilization (HRU) among patients with chronic hypoparathyroidism, overall and by adequately controlled (AC) vs not adequately controlled (NAC) disease, informed by guideline-recommended clinical management targets, including biochemistry and symptoms.
Materials and methods: In this retrospective online chart review, endocrinologists in the US, Canada, the UK, France, Germany, Italy, and Spain were randomly selected to review the medical charts of adult patients with chronic hypoparathyroidism receiving calcium and activated vitamin D. Patients’ demographics, disease characteristics, symptoms, comorbidities, and hypoparathyroidism-related HRU during the 1 year before the review date were assessed. Clinical burden and HRU were compared between patients with NAC and AC hypoparathyroidism.
Results: Of 614 patients with hypoparathyroidism (AC, N = 442; NAC, N = 172), the mean age was 43.6 years, and the majority were female (61.6%), Caucasian (78.8%), and had post-surgical hypoparathyroidism (74.4%). Mean duration of hypoparathyroidism was 46.0 months. Hypoparathyroidism-related symptoms and comorbidities were reported in 59.4% and 46.7% of patients, respectively; 90.7% of patients had ≥1 hypoparathyroidism-related HRU event. More patients with NAC (57.6%) vs AC (42.5%) hypoparathyroidism experienced ≥1 comorbidity including calcium/phosphate imbalances, and brain, cardiovascular, metabolic, and renal disorders (all p < 0.01). More patients with NAC vs AC hypoparathyroidism incurred ≥1 hypoparathyroidism-related hospitalization (27.9% vs 16.3%) and emergency room visits (47.7% vs 38.5%), and patients with NAC vs AC hypoparathyroidism had a higher number of outpatient visits (3.6 vs 2.6; all p < 0.05), in the 1-year observation period.
Limitations and conclusions: Limitations of this online chart review include possible under-estimation of disease burden, limited sample size, and the inability to rule out selection bias. Findings indicate that patients with chronic hypoparathyroidism experience substantial symptomatic and comorbid burdens resulting in frequent HRU, suggesting an unmet need, particularly in NAC disease.
Introduction
Parathyroid hormone (PTH), produced by the parathyroid gland, is a primary regulator of serum calciumCitation1. Chronic hypoparathyroidism is a rare endocrine disorder presenting with low serum calcium (hypocalcemia) and inappropriately low PTH levelsCitation2. The most common cause of chronic hypoparathyroidism is damage to or removal of the parathyroid glands during neck surgeryCitation3–5. Rarely, hypoparathyroidism may be idiopathic or result from congenital conditions (e.g. DiGeorge syndrome or Kearns-Sayre syndrome), autoimmune disease (e.g. polyglandular autoimmune syndrome type 1), or cancerCitation3.
Classic hypoparathyroidism-related symptoms are multi-faceted and related to neuromuscular irritability as a result of hypocalcemia (e.g. paresthesia and muscle contractions)Citation6. Manifestations of hypoparathyroidism that may be less specifically associated with hypocalcemia include symptoms such as difficulty in concentrating (brain fog), effects on mood and ideation, insomnia, and fatigueCitation7–9. The goals in the treatment of chronic hypoparathyroidism are to maintain serum calcium in the lower part of the normal range, maintain serum phosphate within the normal range, reduce comorbidities and symptoms, and improve quality of lifeCitation10,Citation11. Hypoparathyroidism is often treated with conventional therapy, which includes oral calcium and calcitriol (activated vitamin D [1,25-dihydroxyvitamin D (1,25[OH]2D)]), in addition to thiazide diuretics and magnesium supplementation as needed. However, in some patients high doses of calcium (up to 9 g/day) and activated vitamin D (up to 1,250 mcg/day) are required to achieve serum calcium within the target rangeCitation2. Chronic hypoparathyroidism and conventional therapy are often associated with calcifications in the brain and other organs.
In addition, as PTH stimulates renal tubular calcium reabsorption, patients deficient in PTH receiving calcium supplementation can have elevations of serum phosphate and urinary calcium excretion, increasing the risk of nephrocalcinosis, nephrolithiasis (renal stones), and renal complications, including renal insufficiencyCitation12,Citation13. In an attempt to prevent these complications, urinary and serum calcium and serum phosphate need to be frequently monitored in clinical practiceCitation14.
A proportion of patients with chronic hypoparathyroidism do not achieve recommended biochemical targets with conventional therapy and may experience a spectrum of physical, cognitive, and emotional symptoms, including muscle and joint pain, paresthesia, tetany, cognitive impairment (brain fog, difficulty focusing), anxiety, and depressionCitation10. In addition, long-term treatment with conventional therapy may be associated with soft tissue calcification and organ damageCitation10. In a 2014 survey (PARADOX; N = 374) of patients with hypoparathyroidism who were receiving conventional treatment (e.g. calcium, activated vitamin D, etc.) in the US, 69% of patients self-reported that they had experienced hypoparathyroidism-related comorbidities, with cardiac arrhythmia and renal stones being the most frequentCitation15. In that survey, 72% of patients had experienced >10 hypoparathyroidism-related symptoms, 45% reported that their symptoms significantly interfered in their daily life, and 79% reported hypoparathyroidism-related hospitalizations or emergency room (ER) visits in the 12-month period prior to the surveyCitation15. In another patient survey (in 2015) of German patients with chronic hypoparathyroidism, 96% of patients reported potential hypoparathyroidism-related symptoms during the prior year, with paresthesia (86%), carpopedal spasm (59%), bone pain (56%), and muscle pain (56%) being the most commonly reportedCitation16. These symptoms led to outpatient (OP) treatment and hospitalization in the prior year for 31% and 5% of patients, respectively, and 40% of patients had visited the ER because of severe hypocalcemic symptoms since primary hypoparathyroidism diagnosisCitation16. In addition, a population-based study in Minnesota found that the medical care costs of patients with hypoparathyroidism was three times higher than those of demographic-matched controlsCitation17.
These studies suggest that the burden of hypoparathyroidism is high from the perspectives of patients and healthcare systems. However, few studies in the real world have quantified the clinical and healthcare resource utilization (HRU) burdens experienced by patients with chronic hypoparathyroidism, particularly among patients with not adequately controlled disease despite calcium and activated vitamin D supplementation. To address this question, this retrospective chart review study was conducted to investigate the clinical burden in terms of hypoparathyroidism-related symptoms, comorbidities, treatments, and HRU among adult patients with chronic hypoparathyroidism in the US, UK, France, Germany, Italy, Spain, and Canada. It also compared patient demographic and disease characteristics, hypoparathyroidism-related comorbidities, treatments, and HRU between patients with adequately controlled (AC) vs not adequately controlled (NAC) hypoparathyroidism, classified according to guideline-recommended clinical management targetsCitation10,Citation11.
Methods
Study overview
This study was a retrospective, cross-sectional physician-administered chart review of adult patients with chronic hypoparathyroidism receiving calcium and activated vitamin D supplementation in an endocrinology practice setting. Chart collection was conducted between May 27, 2016, and August 2, 2016. Endocrinologists in the US, UK, France, Germany, Italy, Spain, and Canada contributed eligible patient charts. This study received an exemption from institutional review from the New England Institutional Review Board on April 26, 2016. The identities of physicians and patients were anonymized, and all data were compliant with the US Health Insurance Portability and Accountability Act of 1996 and the Declaration of Helsinki. Patient consent was not required for this retrospective chart review of anonymized data.
Physician selection
Endocrinologists were recruited from the Medefield physician panel (http://www.medefield.com/), a large geographically representative online physician panel that included ∼4,000 endocrinologists from North America and 4,500 endocrinologists from Europe. Endocrinologists were screened and selected to review and abstract the medical charts if they had treated at least 10 patients with chronic hypoparathyroidism at any time, and had at least one patient who met the patient selection criteria for this study.
Patient selection
Endocrinologists used the following patient selection criteria: (1) diagnosed with chronic hypoparathyroidism for at least 1 year; (2) ≥18 years of age at the time of hypoparathyroidism diagnosis; (3) required calcium and activated vitamin D treatment; and (4) had available and complete medical records relevant to hypoparathyroidism and its comorbidities, as well as information on hypoparathyroidism-related HRU (i.e. inpatient, outpatient, and ER visits, if any), for at least 12 months before the chart abstraction date. Patients diagnosed with pseudo-hypoparathyroidism or hypoparathyroidism caused by calcium-sensing receptor mutations were excluded.
Hypoparathyroidism-related HRU refers to resource utilization deemed to be associated with hypoparathyroidism or its complications by participating physicians. The requirement for complete records on hypoparathyroidism-related HRU in the study period was to ensure that the data were complete for this key outcome of interest. If patients did not have any hypoparathyroidism-related HRU in the study period, but physicians had the full record of their HRU to confirm this, these patients would still be eligible for the study.
Patient cohorts
Using the collected data from patients’ medical records, patients were classified as NAC retrospectively based on the treatment targets suggested in two international clinical guidelines for hypoparathyroidism (the European Society of Endocrinology’s Clinical Guideline and First International Conference on the Management of Hypoparathyroidism)Citation10,Citation11. The criteria adapted from these guidelines were: (1) most recent serum calcium levels <2.0 mmol/L (<8.0 mg/dL) despite using activated vitamin D (≥3 µg/day of alfacalcidol or ≥1.5 µg/day of calcitriol); or (2) most recent serum phosphate levels >1.45 mmol/L (>4.5 mg/dL); or (3) most recent 24-hour urinary calcium excretion >7.5 mmol (300 mg)/24 h in males or >6.25 mmol (250 mg)/24 h in females; or (4) at least one of the following symptoms during the study period: blepharospasm, facial spasm, hypoesthesia, irritability, muscle fatigue, muscle spasms, muscle tightness, muscle twitching, musculoskeletal pain, musculoskeletal stiffness, myalgia, nervousness, esophageal spasm, paresthesia, throat tightness, or tremor. Patients who were not classified as NAC hypoparathyroidism were considered as AC with conventional therapy (calcium and activated vitamin D supplements). In addition, the endocrinologists who reviewed the medical charts were requested to provide their classification of whether the patients were AC or NAC, based on all the information available in the charts.
Study measures
The study period was the 12-month period prior to the chart abstraction date. The following data were collected from the medical charts: (1) patient demographic and disease characteristics at the time of hypoparathyroidism diagnosis (age, sex, race, hypoparathyroidism type, physician-assessed hypoparathyroidism status [AC vs NAC] and hypoparathyroidism duration); (2) most recent treatment dosing (including calcium and activated vitamin D supplements) and laboratory test values prior to or on the date of chart abstraction (i.e. current treatments and current laboratory values); (3) symptoms and comorbidities related to hypoparathyroidism during the study period; and (4) hypoparathyroidism-related HRU during the study period, if assessment possible by participating endocrinologists (). Laboratory tests related to hypoparathyroidism included serum calcium, 24 h urine calcium, serum phosphate, serum magnesium, PTH, serum creatinine, glomerular filtration rate (GFR), and bone density. For patients with missing GFR and available serum creatinine information, the estimated GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equationCitation18.
Figure 1. Cross-sectional burden of illness design. Demographic characteristics were collected as of the day of initial hypoparathyroidism diagnosis. Hypoparathyroidism-related HRU, symptoms, and comorbidities were collected during the 1-year period prior to the date of chart abstraction. Current treatments and laboratory values were collected on the chart abstraction date and reflect the last recorded treatments and tests. Abbreviation. HRU, health resource utilization.
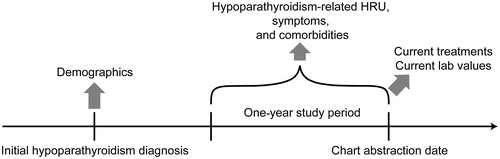
Symptoms included physical, cognitive, and emotional symptoms; comorbidities included those related to brain/central nervous system (CNS), blood, cardiovascular and metabolic disorders, connective tissue disease, edema, eyes, gastrointestinal system, hemiplegia, imbalance of calcium/phosphate, infectious disease, lung/respiratory, musculoskeletal, neuropsychiatric, renal solid tumor with and without metastasis and others. To efficiently collect hypoparathyroidism-related symptoms and comorbidities, the case report form included drop-down lists of common symptoms and comorbidities. The lists of these symptoms and comorbidities were identified based on a literature review and clinical experts’ input during the study design. Physicians could also choose the ‘other’ category in the drop-down lists to enter symptoms or comorbidities not in the pre-specified lists.
HRU included hospitalizations, OP visits, and ER visits related to chronic hypoparathyroidism or associated complications.
Statistical analyses
Patient demographic and disease characteristics, current treatments, current laboratory values, hypoparathyroidism-related symptoms and comorbidities, and hypoparathyroidism-related HRU were summarized for the overall population, pooled across countries. Means and standard deviations (SD) were reported for continuous variables, and counts and proportions were reported for categorical variables. Sub-group analyses were conducted in the US, the country with the largest sample size, as well as in the sub-groups defined by hypoparathyroidism etiology (i.e. autoimmune, congenital, idiopathic, and post-surgical).
The overall study population was stratified into NAC and AC cohorts. Patient demographics, current treatments (oral calcium, activated vitamin D, thiazide diuretics, and parathyroid hormone), hypoparathyroidism-related comorbidities, and hypoparathyroidism-related HRU were then compared between patients with NAC vs AC hypoparathyroidism using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables. Current laboratory values and hypoparathyroidism-related symptoms were not compared between the two cohorts because they were used to define NAC vs AC.
Patients with NAC vs AC hypoparathyroidism were compared in the overall study population only. The comparisons were not performed in the sub-groups by country or etiology due to the limited sample size in these sub-groups, other than post-surgical type.
Analyses were conducted using SAS v. 9.4 (SAS Institute, Cary, NC) and R v.3.2.4 (R Foundation for Statistical Computing, Auckland, New Zealand), and statistical significance was assessed at the 0.05 level.
Results
Clinical burden and HRU of hypoparathyroidism
Patient characteristics, current treatments, and current laboratory values
A total of 161 endocrinologists contributed medical charts for 614 adult patients with hypoparathyroidism who met the inclusion criteria. The distribution of endocrinologists included 44 from the US (n = 173 patients), 27 from Italy (n = 98 patients), 25 from the UK (n = 106 patients), 20 each from France and Spain (n = 72 and 75 patients, respectively), 15 from Germany (n = 51 patients), and 10 from Canada (n = 39 patients). The mean age of the overall sample was 43.6 (SD = 14.8) years, and the majority were female (61.6%), Caucasian (78.8%), and had acquired hypoparathyroidism post-surgically (74.4%; ). The mean duration of hypoparathyroidism was 46.0 (SD = 56.8) months ().
Table 1. Demographic and disease characteristics, current treatments,Table Footnotea and current laboratory valuesTable Footnotea of all patients with hypoparathyroidism.
In the overall sample, 91.9% and 86.3% were currently taking oral calcium or activated vitamin D supplements, respectively; 79.2% of patients were currently taking both (). The most common form of calcium supplementation was oral calcium carbonate (82.7% of patients), and the mean dose was 1.8 (SD = 1.0) g/day. The most common form of activated vitamin D supplementation was calcitriol (58.3% of patients), with a mean dose of 0.9 (SD = 0.9) mcg/day and a median dose of 0.8 mcg/day. Among patients taking thiazide diuretics, hydrochlorothiazide was more common than chlorthalidone (13.5% vs 0.8% of patients, respectively) (). PTH treatments were prescribed to 7.2% of patients in the study cohort, with 4.1% prescribed with recombinant human PTH 1-84 (rhPTH 1-84), 3.3% prescribed with rhPTH (1-34), and 0.2% having used both rhPTH (1-84) and rhPTH (1-34). The mean doses were 49.2 mcg/day and 25.8 mcg/day for rhPTH 1-84 and rhPTH (1-34), respectively. rhPTH 1-84 was used primarily in North America (96.0%) and rhPTH (1-34) was used primarily in Europe (75.0%).
Almost all patients (98.2%) had received a serum calcium test, and the mean serum calcium level was 2.4 (SD = 2.2) mmol/L (). Over half of the patients had laboratory values for serum creatinine (63.2%; mean [SD] = 82.7 [28.5] µmol/L), serum phosphate (62.4%; mean [SD] = 1.2 [0.7] mmol/L), and intact PTH levels (54.1%; mean [SD] = 9.5 [14.3] ng/L). About one-third of patients had information on GFR (38.6%; mean [SD] = 82.4 [22.3] mL/min/1.73 m2), serum magnesium (30.5%; mean [SD] = 0.6 [0.3] mmml/L), and urine calcium levels (30.3%; mean [SD] = 5.5 [30.3%] mmol/24 h).
Hypoparathyroidism-related symptoms and comorbidities
Based on information from the collected medical records, more than half (59.4%) of patients experienced at least one symptom of chronic hypoparathyroidism over the 12-month study period (). The most frequently reported physical symptoms were fatigue (33.6%) and a group of symptoms consisting of muscle cramping, pain, fatigue, and myalgia (23.1%). Compared to physical symptoms, cognitive or emotional symptoms of chronic hypoparathyroidism were less frequent. The most commonly reported cognitive symptoms were the inability to focus or concentrate (14.7%), brain fog/mental lethargy (10.7%), and memory loss/forgetfulness (10.4%), while the most commonly reported emotional symptoms were anxiety, fear, and inner unrest (13.2%), irritability (11%), and emotional sensitiveness (9.8%).
Table 2. Hypoparathyroidism-related symptoms during the 1-year study period among all patients with hypoparathyroidism.
Approximately half (46.7%) of patients had at least one selected comorbidity over the 12-month study period (). Imbalance of calcium/phosphate, which included hypercalcemia, hypercalciuria, hyperphosphatemia, and hypocalcemia, was reported among 21.2% of patients, with hypocalcemia (16.4%) being the most prevalent form. In addition, 20.2% of patients had cardiovascular and metabolic disorders, such as hypertension (12.4%). Neuropsychiatric, gastrointestinal, brain/CNS, musculoskeletal, and renal comorbidities were reported in 16.8%, 11.2%, 10.6%, 6.8%, and 5.7% of patients, respectively.
Table 3. Hypoparathyroidism-related comorbidities during the 1-year study period among all patients with hypoparathyroidism.
Hypoparathyroidism-related HRU
Most of the patients in the overall sample (90.7%) had at least one hypoparathyroidism-related HRU event (as assessed by the endocrinologists) during the 12-month study period; 87.8% had ≥1 OP visit, 41.0% had ≥1 ER visit, and 19.5% had ≥1 hospitalization (). Of the patients who had OP visits, the mean number of visits was 3.3 (SD = 2.2) per year, and most visits were for management of hypoparathyroidism (mean visits = 2.8 [SD = 1.6]/year). Of the patients who had a specialist OP visit, the mean number of visits was 3.1 (SD = 3.0)/year. Other than endocrinologists, nephrologists were the most commonly consulted specialist (17.9%), followed by ophthalmologists (15.5%), psychiatrists (12.7%), cardiologists (12.4%), and neurologists (11.9%). Of the patients who visited the ER, the mean number of visits was 2.4 (SD = 1.8) per year, and more visits were related to the management of hypoparathyroidism (mean visits = 2.0 [SD = 1.2]/year) than the management of hypoparathyroidism-related comorbidities (1.5 [SD = 1.1]/year) or treatment-related adverse events (1.4 [SD = 0.7]/year) (). Of the patients who had a hospitalization, the mean number of admissions was 1.5 (SD = 1.4) per year, and most events were related to hypoparathyroidism-related comorbidities (mean admissions = 1.5 [SD: 1.6]/year).
Table 4. Hypoparathyroidism-related health resource utilization during the 1-year study period among all patients with hypoparathyroidism.
Sub-group analyses
The results among the 173 patients in the US were generally consistent with the overall population. The two notable differences were that the proportion of patients with any hypoparathyroidism-related HRU was 83.8% in the US vs 90.7% in the overall population, and that the rate of NAC hypoparathyroidism was slightly higher in the US (31.2%) vs the overall population (28.0%).
Similarly, results in the sub-groups defined by etiology were generally consistent with those of the overall study population, although there were several differences. Specifically, patients with congenital (n = 16) and idiopathic (n = 57) hypoparathyroidism were more likely to have a complication related to the brain/central nervous system (31.2% and 31.6%, respectively) than the other patient etiology groups (10.6% in the overall population), and patients with congenital hypoparathyroidism were more likely to have cardiovascular and metabolic disorders (37.5% vs 20.2% in the overall population). In addition, patients with congenital hypoparathyroidism tended to have more hypoparathyroidism-related hospitalizations (mean [SD] = 3.8 [4.7] vs 1.5 [1.4] in the overall population), ER (mean [SD] = 3.7 [4.6] vs 2.4 [1.8] in the overall population), and outpatient visits (mean [SD] = 4.3 [4.1] vs 3.3 [2.2] in the overall population) related to the management of hypoparathyroidism-related complications, and treatment of adverse events in the past 12 months compared to patients with other etiologies (results not presented in Tables due to the small sample sizes).
Clinical burden and HRU of NAC vs AC hypoparathyroidism
Patient characteristics and current treatments of NAC vs AC
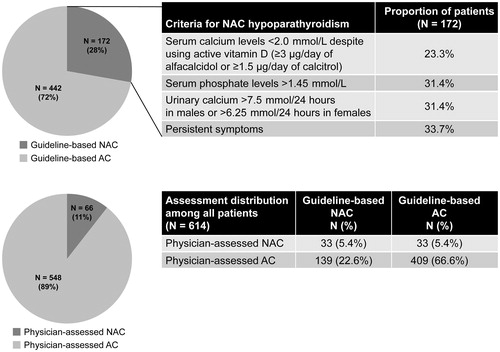
A total of 172 (28.0%) patients were identified as NAC hypoparathyroidism adapted from hypoparathyroidism clinical guidelines ()Citation10,Citation11. Of the 172 patients with NAC hypoparathyroidism, 23.3% had serum calcium levels <2.0 mmol/L, despite using activated vitamin D (≥3 μg/day of alfacalcidol or ≥1.5 μg/day of calcitriol), 31.4% had serum phosphate levels >1.45 mmol/L, 31.4% had urinary calcium >7.5 mmol/24 h in males or >6.25 mmol/24 h in females, and 33.7% had persistent symptoms (). Based on the physicians' assessments, 66 (10.7%) patients had NAC hypoparathyroidism. Most (97%) physicians used serum calcium level to categorize NAC hypoparathyroidism, and 62% used symptoms to do so. Among patients assessed by endocrinologists who did not use symptoms as a criterion (38%), only 6.3% were considered as NAC, whereas 31.3% of the same patients would be identified as such using the clinical guideline ().
Figure 2. Proportions of patients with AC and NAC hypoparathyroidism. The definitions of guideline-based NAC are described in the ‘Patient cohorts’ section of the Methods. Abbreviations. AC, adequately controlled; N, number; NAC, not adequately controlled.
The demographics of patients with NAC and AC hypoparathyroidism were balanced (). When comparing patients with AC vs NAC hypoparathyroidism, there were no significant differences in terms of the use of oral calcium or activated vitamin D supplements (). The distribution of etiology was consistent among patients with NAC or AC hypoparathyroidism. However, patients with NAC hypoparathyroidism on average were treated with a significantly higher dose of calcitriol compared to those with AC hypoparathyroidism (mean: 1.1 vs 0.9 mcg/day, respectively; p < 0.05; median: 0.8 vs 0.6 mcg/day). In addition, significantly more patients with NAC vs AC hypoparathyroidism used hydrochlorothiazide (19.2% vs 11.3%, respectively; p < 0.05) and PTH treatments (rhPTH: 7.6% vs 2.7%, respectively; rhPTH (1-34): 5.8% vs 2.3%, respectively; both p < 0.05). Of the patients using rhPTH (1–34), the mean dose was significantly higher for patients with AC hypoparathyroidism (31.5 mcg/day) compared to patients with NAC hypoparathyroidism (20.0 mcg/day; p < 0.01).
Table 5. Comparisons of demographic and disease characteristics and current treatmentsTable Footnotea between patients with NAC and AC disease.
Hypoparathyroidism-related comorbidities of NAC vs AC
Compared with patients with AC hypoparathyroidism, those with NAC hypoparathyroidism had significantly higher rates of any comorbidities (57.6% [NAC] vs 42.5% [AC]; p < 0.01) over the study period, including those related to brain/CNS (16.9% vs 8.1%; p < 0.01), basal ganglia calcification (6.4% vs 2.3%; p < 0.05), cardiovascular and metabolic disorders (26.2% vs 17.9%; p < 0.05), calcification of cardiac arteries (2.9% vs 0.5%; p < 0.05), QT prolongation (4.1% vs 0.9%; p < 0.05), imbalance of calcium/phosphate (29.7% vs 17.9%; p < 0.01), hyperphosphatemia (8.1% vs 3.2%; p < 0.05), hypocalcemia (22.1% vs 14.3%), depression (11.6% vs 5.0%; p < 0.01), renal disorders (10.5% vs 3.8%; p < 0.01), and nephrocalcinosis (2.9% vs 0.7%; p < 0.05) (). Other comorbidities were relatively balanced between patients with NAC and patients with AC hypoparathyroidism.
Table 6. Comparisons of hypoparathyroidism-related comorbidities during the 1-year study period between patients with NAC and AC disease.
Hypoparathyroidism-related HRU of NAC vs AC
Compared with patients with AC hypoparathyroidism, patients with NAC hypoparathyroidism had significantly higher rates of hypoparathyroidism-related hospitalizations (27.9% [NAC] vs 16.3% [AC]; p < 0.01) and ER visits (47.7% vs 38.5%; p < 0.05), but similar rates of HRU-related OP visits (89.5% vs 87.1%; p = 0.49) (). In addition, patients with NAC hypoparathyroidism had a higher number of hospitalizations (mean visits/year: 0.5 [NAC] vs 0.2 [AC]), ER visits (1.2 vs. 0.9), and OP visits (3.6 vs 2.6; all p < 0.05). Patients with NAC hypoparathyroidism visited the ER more frequently for the management of hypoparathyroidism-related complications than those with AC disease (0.2 vs 0.1, respectively; p < 0.01). In addition, patients with NAC hypoparathyroidism had more frequent OP visits for management of hypoparathyroidism (2.9 vs 2.3, respectively) and hypoparathyroidism-related complications (0.5 vs 0.2; both p < 0.01) compared with patients with AC disease.
Table 7. Comparisons of hypoparathyroidism-related health resource utilization between patients with NAC and AC disease during the 1-year study period.
Discussion
Despite the use of calcium and activated vitamin D supplements, many patients with chronic hypoparathyroidism continue to experience physical, cognitive, and emotional symptoms and comorbidities with current treatments (e.g. persisting hypocalcemia)Citation10,Citation19–21. It is important to understand the real-world clinical burden and HRU experienced by patients with chronic hypoparathyroidism, especially those with NAC disease. To our knowledge, the present medical chart review provides the first real-world evidence of the clinical burden of chronic hypoparathyroidism in endocrinology practice settings in North America and Europe, assessed by symptoms, comorbidities, treatments, and HRU. The majority of patients were receiving oral calcium (91.9%), activated vitamin D (86.3%), or both (79.2%). Despite treatment, 59.4% of all patients experienced at least one symptom and 46.7% experienced at least one comorbidity related to hypoparathyroidism in the 12-month study period. This poor control of symptoms and comorbidities may also contribute to the high rate of HRU among patients in the same period; ∼20% had a hospitalization (with a median stay of 4.5 days), and 88% and 41% had an OP or ER visit, respectively.
The current findings on the clinical burden of hypoparathyroidism are consistent with prior patient surveys, all suggesting a high burden associated with chronic hypoparathyroidism. The PARADOX study found that 69% of patients with chronic hypoparathyroidism reported comorbidities (46.7% in the current study), and 79% reported hypoparathyroidism-related hospitalization or ER visits in the past yearCitation15. That study also identified that 78% of chronic hypoparathyroidism happened post-surgically, similar to the current study (74.4%). However, fewer patients in PARADOX were receiving calcium + activated vitamin D (66.6%) than in the current study (79.2%)Citation15. In addition, the patients surveyed in PARADOX had a shorter hypoparathyroidism disease duration than the population evaluated in the present study (mean = 46.0 vs 151.2 months, respectively)Citation15. A German patient survey also supported significant disease burden associated with hypoparathyroidism, reporting that 96% of patients had symptoms that resulted in OP visits in 31% and IP admissions in 5% of casesCitation16. The higher frequency of symptoms and comorbidities and lower HRU reported in the two studies compared to the current study may be due to the differences in study methodologies and disease duration. It is likely that patients reported more comorbidities but fewer treatments compared to the records in medical charts.
This study also assessed patients with NAC chronic hypoparathyroidism based on the clinical treatment target guidelinesCitation10,Citation11. Given the heterogeneity of the disease, it is important to understand this patient population. The burden of illness related to hypoparathyroidism was compared between patients with NAC vs AC chronic hypoparathyroidism. In the current study, approximately one-third of the patients with chronic hypoparathyroidism were identified as having NAC disease based on the clinical guidelines, while only 10.7% were assessed as NAC hypoparathyroidism by the contributing endocrinologists. The hypoparathyroidism clinical guidelines recommend symptom reduction and the achievement of target laboratory values as goals of therapy for this disease. In the current study, 97% of physicians indicated that they used serum calcium level to categorize NAC hypoparathyroidism, while only 62% used symptoms to do so. Among patients assessed by endocrinologists who did not use symptoms as a criterion (38%), only 6.3% were considered as NAC, whereas 31.3% of the same patients would be identified as such using the clinical guideline-based definitions. These results suggest that a certain number of patients are not identified by endocrinologists as having NAC, as would have been if using treatment target guidelines, potentially due to not including symptoms as criteria for defining NAC.
Patients with NAC hypoparathyroidism (assessed by the guidelines) had significantly higher rates of comorbidities compared with patients with AC hypoparathyroidism, including potentially severe comorbidities such as those related to the brain/CNS, the cardiovascular system, and the renal system. In addition, significantly more patients with NAC hypoparathyroidism had a hospitalization or ER visit in the study period compared with patients with AC hypoparathyroidism. The rates of hypoparathyroidism-related hospitalizations and ER visits were 27.9% and 47.7%, respectively, in NAC. The findings suggest that this sub-population with NAC chronic hypoparathyroidism has a particularly high unmet need.
This study required participating physicians’ assessments of whether certain key outcomes, such as symptoms, comorbidities, and HRU, were related to hypoparathyroidism. This was a main advantage of using a treating physicians-administered chart review as the physicians understood the disease and treatment histories of their patients, and their assessments were based on patients’ clinical information. The prevalence and severity of symptoms and comorbidities, and high utilization of health services, identified among patients with chronic hypoparathyroidism in this study highlight the unmet need in chronic hypoparathyroidism, particularly among patients with NAC hypoparathyroidism. This view is shared by the majority of physicians treating patients with hypoparathyroidism, and 69% of treating physicians indicated high unmet treatment needs in hypoparathyroidism in a recent surveyCitation5. rhPTH treatment (i.e. Natpara in the US and Natpar in EU) has been approved for chronic hypoparathyroidism, although this treatment was not widely used at the time of the study period. Studies of new therapies to control the symptoms and comorbidities associated with hypoparathyroidism are still needed.
The results of this study should be considered in light of its limitations, some of which are inherent to retrospective studies. First, the overall burden of illness is likely to be under-estimated in this study because only the hypoparathyroidism-related HRU in the medical charts accessible to endocrinologists could be collected. Other comorbidity-related HRU that was not recorded in the endocrinologists’ medical charts was not included (e.g. renal or cardiovascular conditions managed by specialists). Second, because the sample size for each country was small, country-specific results are not presented. However, the results from the US (the country with the largest sample size) were generally consistent with the pooled results. Third, as this was an online chart review, the data were collected through voluntary participation of physicians who selected eligible charts; therefore, selection bias may exist and the sample may not be representative of the overall hypoparathyroidism population. Fourth, the list of events defining NAC was based on the medical records. Performance of certain laboratory tests and assessment of symptoms varied across practices. Therefore, the procedures by which NAC classification data were collected could not be standardized. Some patients may not be classified as NAC due to a lack of laboratory test or symptom recording. Thus, if there is any classification error, it would under-estimate the frequency of NAC. Misclassification also leads to under-estimation of the differences in outcomes between the NAC and AC groups. The actual differences between the two groups may be larger than the ones observed in the current analysis.
Conclusion
In this retrospective medical chart review of patients with chronic hypoparathyroidism in North America and Europe, the overall burden of the disease, in terms of symptoms, comorbidities, and HRU, remains high, despite the current conventional therapy. Patients with NAC hypoparathyroidism were potentially under-diagnosed by physicians according to guidelines and were observed to have a higher burden of comorbidities and HRU compared with those with AC hypoparathyroidism.
Transparency
Declaration of funding
Funding for this research was provided by Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies. The study sponsor was involved in all stages of the study research and manuscript preparation.
Declaration of financial/other relationships
KC is an employee of Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies, and owns stock/stock options in Takeda. AK was an employee at Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies, at the time of the study’s conduct. CQX, TT, and JX are employees of Analysis Group Inc., which has received consultancy fees from Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies. NL was an employee of Analysis Group, Inc. during the conduct of the study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Medical writing assistance was provided by Shelley Batts, PhD, an employee of Analysis Group, Inc., and was funded by Shire Human Genetic Therapies, Inc., a member of the Takeda group of companies. The authors thank Ariadne Souroutzidis, a former employee of Analysis Group, Inc., and Catherine Fernan, an employee of Analysis Group, Inc., for assistance with the analysis. A synopsis of the current research was presented in poster format at the 19th European Congress of Endocrinology during May 20–23, 2017 in Lisbon, Portugal, and at the 99th Annual Meeting of the Endocrine Society (ENDO 2017) during April 1–4, 2017 in Orlando, FL.
References
- Brown EM. Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev. 1991;71:371–411.
- Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337.
- Marx SJ. Hyperparathyroid and hypoparathyroid disorders. N Engl J Med. 2000;343:1863–1875.
- Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on cancer patient care evaluation study. Cancer. 2000;89:202–217.
- Powers J, Joy K, Ruscio A, et al. Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res. 2013;28:2570–2576.
- Behaghel A, Donal E. Hypocalcaemia-induced transient dilated cardiomyopathy in elderly: a case report. Eur J Echocardiogr. 2011;12:E38.
- Rubin MR, Manavalan JS, Dempster DW, et al. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab. 2011;96:176–186.
- Arlt W, Fremerey C, Callies F, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146:215–222.
- Velasco PJ, Manshadi M, Breen K, et al. Psychiatric aspects of parathyroid disease. Psychosomatics. 1999;40:486–490.
- Bollerslev J, Rejnmark L, Marcocci C, et al. European Society of Endocrinology clinical guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173:G1–G20.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101:2273–2283.
- Gesek FA, Friedman PA. On the mechanism of parathyroid hormone stimulation of calcium uptake by mouse distal convoluted tubule cells. J Clin Invest. 1992;90:749–758.
- Kurokawa K. Calcium-regulating hormones and the kidney. Kidney Int. 1987;32:760–771.
- Mortensen L, Hyldstrup L, Charles P. Effect of vitamin D treatment in hypoparathyroid patients: a study on calcium, phosphate and magnesium homeostasis. Eur J Endocrinol. 1997;136:52–60.
- Hadker N, Egan J, Sanders J, et al. Understanding the burden of illness associated with hypoparathyroidism reported among patients in the PARADOX study. Endocr Pract. 2014;20:671–679.
- Dropmann S, Horn S, Hahner S. Disease burden and hypocalcemic emergencies in chronic hypoparathyroidism – a patient survey. Exp Clin Endocrinol Diabetes. 2015;123:P12–14.
- Leibson C, Clarke B, Ransom J, et al. Medical care costs for persons with and without prevalent hypoparathyroidism: A population-based study. Annual Meeting of the American Society for Bone and Mineral Research San Diego, CA; September 16–20, 2011.
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
- Bell NH, Stern PH. Hypercalcemia and increases in serum hormone value during prolonged administration of 1alpha,25-dihydroxyvitamin D. N Engl J Med. 1978;298:1241–1243.
- Kanis JA, Russell RG. Rate of reversal of hypercalcaemia and hypercalciuria induced by vitamin D and its 1alpha-hydroxylated derivatives. Br Med J. 1977;1:78–81.
- Underbjerg L, Sikjaer T, Mosekilde L, et al. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow‐up study. J Bone Miner Res. 2013;28:2277–2285.
