Abstract
Background: Phenylketonuria is a well-known disease, yet the characteristics of the affected population and their use of healthcare resources have not been comprehensively evaluated. Patient characteristics and use of resources are subjects of interest for most governments, especially for a disease included in newborn screening programs.
Objective: The aim of this study was to determine characteristics and use of healthcare resources of patients with phenylketonuria in the region of Catalonia.
Methods: Records of 289 patients admitted with phenylketonuria between 2007 and 2017 were extracted from the PADRIS database that includes admission data from primary care centers, hospitals (inpatient and outpatient care), extended care facilities, and mental health centers.
Results: The patient population was composed of 140 male patients and 149 female patients, and 102 patients were registered via newborn screening during the study period. Patients were admitted on average 2.19 times per year, mostly into primary care centers which concentrated the largest portion of direct medical expenses. Similar percentages of urgent and scheduled admissions were registered both in primary care and hospitals. Annual direct medical cost of treating patients with phenylketonuria was €667 per patient. Finally, 66.80% of the patients suffered from chronic conditions affecting two or more systems, likely to correspond to a wide variety of conditions.
Conclusions: Altogether, phenylketonuria patient demographics and direct medical costs in Catalonia have been revised. Patients diagnosed with phenylketonuria appeared 1.3-times more likely to suffer from chronic conditions in distinct organ systems, which is expected to have an effect on their use of healthcare resources. These results support the need to adapt and improve the healthcare system, taking multimorbidity into consideration in an effort to control the medical expenses derived.
Introduction
Phenylketonuria (PKU) is a genetic condition in which the enzyme that processes phenylalanine, phenylalanine hydroxylase (PAH), is deficient or absent. As a consequence, phenylalanine accumulates in the blood, causing a series of symptoms that vary in accordance with age of diagnosis and underlying genetic heterogeneity. Once hyperphenylalaninemia (HPA) is detected, the patient is tested for PAH deficiency and to exclude a non-phenylketonuric HPA caused by the deficiency of its cofactor, tetrahydrobiopterin (BH4)Citation1.
When PKU is properly diagnosed the first days of life, patients benefit from nutritional, clinical and biochemical follow-up, and few symptoms are observed; contrarily, when undiagnosed, symptoms include eczema, seizures, autism, behavioral issues, and, eventually, severe intellectual disabilityCitation2. Disease prevalence varies among geographical regions. In Europe, it affects one per 10,000 live birthsCitation3, although in Turkey it increases to one in 4,000Citation4. On the contrary, in Finland, prevalence is as low as one per 100,000 live birthsCitation5. In Spain, the geographical distribution of the underlying mutations causing PKU has been analyzed, revealing differences between Mediterranean descendants and originals from the north-west of the countryCitation6.
PKU is currently diagnosed the first days of life due to the well-established newborn blood screening. Such screenings were established in Spain in 1978 (Real Decreto 2176/1978, August 25) and were soon implemented in the whole countryCitation7. Their benefits have been evident, with practically all symptoms of the disease being reduced only by dietary restrictionCitation8. Phenylalanine-restricted diets are still the primary treatment for PKU, often in combination with sapropterin, which reduces blood phenylalanine, or enzyme substitution therapyCitation9. In general terms, PKU is a well-known disease, yet the characteristics of the affected population and possible regional discrepancies have not been comprehensively evaluated. Similarly, patients’ use of healthcare resources in a certain region is a subject of interest for most governments, especially for the diseases part of newborn screening programs. In such cases, neonatal screenings are justified based on the reduction of costs associated with treating patients later onCitation10. A previous study analyzed the costs associated to PKU in Spain, together with its epidemiologyCitation11; however, in Spanish regions such as Catalonia (7.5 million inhabitants) the regional government is in charge of the healthcare system, hence the interest of regional evaluations. In order to facilitate the management and examination of patient data, the local government has developed a specialized program that recruits patient information: the data analysis for research and innovation in health program (PADRIS)Citation12.
With this in mind this study was designed to evaluate the current status of PKU in the region of Catalonia, focusing on analyzing patient characteristics, revising disease management, and calculating the direct medical costs of the disease.
Materials and methods
Records of all registered patients with a diagnosis of PKU in the region of Catalonia (7.5 million inhabitants) between 2007 and 2017 were analyzed. Data was obtained from project PADRIS via the approval from the University of Barcelona’s Bioethics Commission. Parameters such as health centers and medical history identifiers were re-coded prior to extraction to maintain records anonymized in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
The database includes detailed information on patients’ use of healthcare resources, comprising primary care centers, hospitals (inpatient and outpatient care), emergency rooms (ER), extended care facilities and mental health centers. Records in the database are validated automatically via the evaluation of data consistency. Patient diagnoses and procedures were determined by means of the 9th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-9). When necessary, the extraction of single-patient information was carried out by eliminating repeated records corresponding to separated admissions, relying on the first admission registered during the study period as the index event.
The classification assigns patients an adjusted morbidity group (GMA), according to the number of systems they have affected by chronic diseases, and into five levels of complexity or risk. As it increases, GMA level correlates with a higher number of healthcare admissions of these patients and major pharmaceutical costsCitation13. Direct medical costs associated with the use of healthcare resources were calculated based on the mean costs of medical procedures determined by the Catalan government for the year 2013Citation14,Citation15.
Data presentation is mainly descriptive. Statistical analyses were performed using Microsoft Excel Professional Plus 2010 (Microsoft Corporation, Redmond, WA).
Results
The study evaluated records of 289 patients diagnosed with PKU in the region of Catalonia between 2007 and 2017 (). Not significant differences were observed in the percentage of male and female patients, 48.44% vs 51.56%. Surprisingly, patients’ mean age at first admission was 14.90 years (SD = 18.07), although age median was 8 years. In total, 102 patients included in the study were registered after diagnosis via newborn screening.
Table 1. Description of the studied population based on index event (first admission).
A total number of 15,018 healthcare admissions were analyzed, corresponding to primary health centers, hospitals, ER, extended care facilities, and mental health consultations. Data was gathered between the years 2007 and 2017, averaging 2.19 annual admissions per patient. Significantly, 8.78 admissions were registered annually in primary care centers and only 0.23 annual admissions per patient were into hospitals.
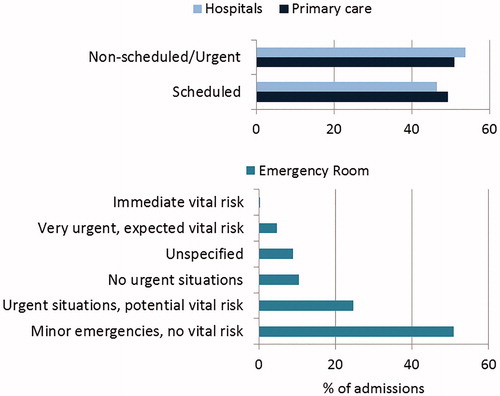
All available information was used to determine the origin of admissions in primary care and hospitals, and the nature of emergency admissions (). A similar proportion of scheduled and urgent visits were observed both in hospitals and primary care, while admissions into ER were mainly due to minor conditions with no vital risk associated.
Mean hospitalization time was 4.57 days, while the mean time patients spent in extended care facilities was 9.8 days. Generally, admissions into ER required stays of less than a day. After hospital discharge, 95.03% of the patients returned to their residence, a percentage slightly lower, 89.73%, in ER admissions ().
Table 2. Patients’ destination after discharge from hospitals and ER.
Patient admission files include a principal diagnosis (hospitalization motive), which was PKU in all cases, and a series of secondary diagnoses registered during the hospitalization. Secondary conditions diagnosed in more than 1.5% of admissions were acute nasopharyngitis (4.40%), unspecified essential hypertension (4.06%), asthma (3.41%), diabetes mellitus type II (2.59%), diabetes mellitus type I (1.94%), and acute tonsillitis (1.58%) ().
Table 3. Secondary conditions diagnosed in more than 1.5% of admissions of patients with phenylketonuria.
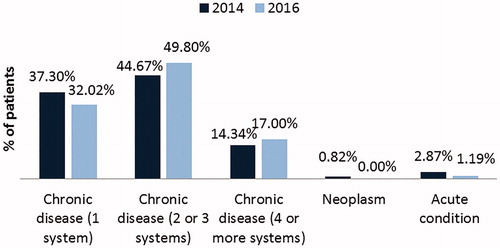
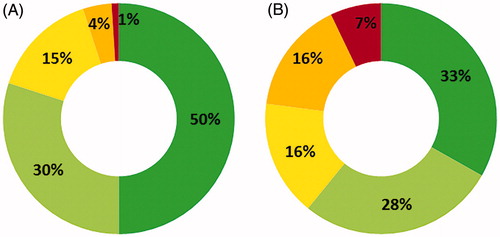
The Catalan Institute of Health (ICS) assigns patients a value according to the number of systems they have affected by chronic conditions. Inr 2016 (last available data), 49.80% of patients with PKU had two or three systems affected by a chronic condition, and 17.00% of patients had four or more systems affected (). A 5.13% and 2.66% increase was observed in each of these two categories since 2014. Two distinct approaches have been used to approximate the costs that PKU patients represent for the healthcare system. In terms of disease complexity or risk associated with patients’ condition, in 2016 there were seven times more patients in this group at very high risk, and 67% of patients displayed a certain level of risk vs the 50% in the general population (). This data suggests that increased medical costs will derive from the treatment of patients with PKU in comparison with the general population.
Figure 3. Disease complexity levels associated to risk (1 = green, low risk to 5 = red, very high risk) in (A) general populationCitation13 and (B) patients with phenylketonuria in 2016.
On the other hand, an approximation to the direct medical costs of treating patients with PKU was made based on the mean costs of medical procedures established by the regional government (). Resources destined to primary healthcare were estimated based on the mean cost per admission according to its urgency. Thus, primary attention costs were the most relevant, summing €641,134. ER costs were calculated equally. Hospitalization costs were estimated per admission in outpatient consultations, and based on the average days of stay (1 day or more) and the cost per day. Finally, resources associated to stays in extended care facilities were determined by the cost per admission. Altogether, the annual direct medical cost per patient equaled €667, a total amount that reflects the cost to treat a patient with PKU.
Table 4. Direct medical costs (2007–2017) based on mean costs of medical procedures established by the regional government.
Finally, patients’ contribution to pharmaceutical expenses was considered of significance to infer medical costs. Patients are assigned a co-payment percentage according to income, age, and disease. The majority of patients, 54.21%, contributed to 40% of pharmaceutical expenses (). 8.30% of patients were exempted from contribution due to age or long-term unemployment.
Table 5. Patients’ contribution to pharmaceutical costs.
Discussion
The present study revised all admission records of patients registered with PKU between 2007 and 2017 in the region of Catalonia. Altogether, results showed a predominance of admissions into primary care centers vs secondary care, with an annual cost per patient of €667. A significant frequency of multimorbidities was also measured among these patients.
The analysis revealed few differences to previous evaluations in the whole country. Patients’ age at first admission, 14.90 years, was relatively elevated in comparison with the 4.5 years determined in SpainCitation11. However, patients’ diagnoses are registered in the database solely after 2007, and diagnoses made before that year are not included. Thus, patients born and diagnosed before the study period elevate this parameter preventing a correlation with PKU’s first diagnosis age.
Patients were admitted on average 2.19-times per year into a healthcare center, mostly into primary care centers. The Spanish healthcare system, as many others, promotes the use of primary care as a method to increase prevention and maximize healthcare resourcesCitation16. In addition, the characteristics of PKU and its relatively simple control through diet determine a need for general attention received in primary care facilities, and a minority need for urgent attentionCitation2, consistent with the results of this study.
In terms of secondary diagnoses and comorbidities, no clear tendencies have been identified. A number of patients suffer from chronic conditions such as diabetes type I and II, hypertension, or asthma, but GMA indicators reflect much higher percentages; 66.8% of the patients had chronic conditions affecting two or more systems in 2016, while in the general population this percentage was 51.7% in the same yearCitation17. The low percentages obtained in the analysis of secondary diagnoses in admission data suggest that patients’ comorbid conditions correspond to a great variety of diseases. This data supports the findings of previous studies that described an elevated presence of multimorbidities in PKU patients affecting various organ systems, including conditions such as allergic and chronic rhinitis, esophageal disorders, overweight, anemia, gastroesophageal reflux disease, dermatitis and eczema, all significantly higher than matched non-PKU controlsCitation18. Therein, it is speculated that HPA could have consequences beyond neurologic, neurocognitive and neuropsychiatric conditions, but further research is required to evaluate that option. Equally, the restrictive diet necessary to control PKU could have consequences in other organ systems as differences in parameters such as fat oxidation have been confirmedCitation19.
On the other hand, the typical neurologic manifestations of an untreated PKUCitation20 were not detected in this study; the low prevalence of these conditions indicated that the elevated age of first admission does not reflect age of diagnosis in the region.
In general terms, multimorbidity groups have been associated with an increased complexity of disease management and, subsequently, increased medical costsCitation21. Additionally, in Catalonia, the ICS has determined a correlation between the presence of multimorbidities and an increased risk and subsequent use of healthcare resourcesCitation13. Health systems are rarely prepared to handle multimorbidityCitation22, hence the interest of governments in evaluating disease costs and their relation with multimorbid patients.
The direct medical costs of PKU patients summed €1,348,364 between 2007 and 2017, with a mean annual cost per patient of €667. This cost is presumed higher than in the general population, as a consequence of the major use of healthcare resources that derives from the higher portion of patients with chronic comorbid conditions. In Spain cost estimations situated annual costs of PKU at around €4,239 in 2015, however, therein, calculation methods were based on patients’ diagnoses and are not entirely comparableCitation11. Currently, the majority of costs associated with the disease are indirect costs, covered by families and patients, due to the extended use of a dietary treatmentCitation23,Citation24, although prospective treatments facing all disease outcomes might increase medical costsCitation25, dramatically rising as well by the presence of comorbid conditions.
Finally, a number of limitations could have influenced the results of this study. Data recording in the distinct healthcare sources did not begin uniformly in 2007, and no previous information is available for patients born before that year. Moreover, patients with PKU may not need medical attention in relation with the illness for several years, and a number of patients can remain unregistered in the database.
Conclusions
The characteristics and use of healthcare resources of patients with PKU in the region of Catalonia have been revised. The increased presence of multimorbid conditions affecting distinct organ systems supports the need to evaluate the weight of secondary symptoms derived from this illness. Further research will be required to determine the importance of multimorbid conditions in healthcare use patterns, which will be essential in order to adapt and improve healthcare systems.
Transparency
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
The author declares no competing interests. One reviewer discloses serving as an advisory board member for Biomarin, Applied Pharma Research SA, and Nutricia and receiving speaker fees from Merck Serono, Biomarin, Nutricia, Vitaflo, and Cambrooke. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Ethics approval and consent to participate
Project approved by the University of Barcelona’s Bioethics Commission. Patient consent was not required for this study.
Acknowledgements
Not applicable.
Data availability statement
Deidentified individual participant data will not be made available due to legal stipulations from the Catalan Health Department.
References
- Van Wegberg AMJ, MacDonald A, Ahring K, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12:162.
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427.
- Loeber JG. Neonatal screening in Europe; the situation in 2004. J Inherit Metab Dis. 2007;30:430–438.
- El-Metwally A, Yousef Al-Ahaidib L, Ayman Sunqurah A, et al. The prevalence of phenylketonuria in Arab Countries, Turkey, and Iran: a systematic review. Biomed Res Int. 2018;2018:1.
- Kääriäinen H, Muilu J, Perola M, et al. Genetics in an isolated population like Finland: a different basis for genomic medicine? J Community Genet. 2017;8:319–326.
- Desviat LR, Pérez B, Gámez A, et al. Genetic and phenotypic aspects of phenylalanine hydroxylase deficiency in Spain: molecular survey by regions. Eur J Hum Genet. 1999;7:386–392.
- Vicente E, Casas L, Ardanaz E. Origin of newborn screening programs and their beginnings in Spain [Origen de los programas de cribado neonatal y sus inicios en España]. An Sist Sanit Navar. 2017;40:131–140.
- van Spronsen FJ. Phenylketonuria: a 21st century perspective. Nat Rev Endocrinol. 2010;6:509–514.
- Lichter-Konecki U, Vockley J. Phenylketonuria: current treatments and future developments. Drugs. 2019;79:495–500.
- Brosco JP, Sanders LM, Seider MI, et al. Adverse medical outcomes of early newborn screening programs for phenylketonuria. Pediatrics. 2008;122:192–197.
- Darbà J, Ascanio M. Admissions and cost of hospitalisation of phenylketonuria: Spanish claims database analysis. Clin Drug Investig. 2019;39:379.
- Public program of data analysis for research and innovation in health in Catalonia [Programa públic d'analítica de dades per a la recerca i la innovació en salut a Catalunya]. Health evaluation and quality agency of Catalonia. Health Department. Generalitat de Catalunya. http://aquas.gencat.cat/ca/detall/article/padris [Accessed April 2019].
- Monterde D, Vela E, Clèries M, et al. Adjusted morbidity groups: a new multiple morbidity measurement of use in Primary Care. Atención Primaria. 2016;48:674–682.
- Catalonia. SLT/353/2013, 13th of February, revision of public prices for sanitary services offered by the Catalan Institute of Health [revisió de preus públics corresponents als serveis sanitaris que presta l’Institut Català de la Salut]. (DOGC number 6326 - 1.3.2013).
- Catalonia. SLT/235/2013, 9th of September, that establishes the values of payment units for the services supplied by social health centres in 2013 [per la qual s'estableixen per a l'any 2013 els valors de les unitats de pagament per a la contraprestació dels serveis duts a terme pels centres sociosanitaris]. (DOGC number 6478 - 11.10.2013).
- Borkan J, Eaton CB, Novillo-Ortiz D, et al. Renewing primary care: lessons learned from the Spanish health care system. Health Aff (Millwood). 2010;29:1432–1441.
- Report of the Project of Stratification of the Population in GMA groups in the National Health System (2014–2016). [Informe del proyecto de Estratificación de la Población por Grupos de Morbilidad Ajustados (GMA) en el Sistema Nacional de Salud (2014–2016)]. Spanish Ministry of Health; 2018.
- Burton BK, Jones KB, Cederbaum S, et al. Prevalence of comorbid conditions among adult patients diagnosed with phenylketonuria. Mol Genet Metab. 2018;125:228–234.
- Alfheeaid H, Gerasimidis K, Năstase AM, et al. Impact of phenylketonuria type meal on appetite, thermic effect of feeding and postprandial fat oxidation. Clin Nutr. 2018;37:851–857.
- Bilder DA, Kobori JA, Cohen-Pfeffer JL, et al. Neuropsychiatric comorbidities in adults with phenylketonuria: a retrospective cohort study. Mol Genet Metab. 2017;121:1–8.
- Payne RA, Abel GA, Guthrie B, et al. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. CMAJ. 2013;185:E221–E228.
- Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307:2493–2494.
- MacDonald A, Smith TA, de Silva S, et al. The personal burden for caregivers of children with phenylketonuria: a cross-sectional study investigating time burden and costs in the UK. Mol Genet Metab Rep. 2016;9:1–5.
- Wang L, Zou H, Ye F, et al. Household financial burden of phenylketonuria and its impact on treatment in China: a cross-sectional study. J Inherit Metab Dis. 2017;40:369–376.
- Berry SA, Brown C, Grant M, et al. Newborn screening 50 years later: access issues faced by adults with PKU. Genet Med. 2013;15:591–599.