Abstract
Aims: To estimate the cost-effectiveness of isavuconazole compared with the standard of care, voriconazole, for the treatment of patients with invasive fungal infection disease when differential diagnosis of the causative pathogen has not yet been achieved at treatment initiation.
Materials and methods: The economic model was developed from the perspective of the UK National Health Service (NHS) and used a decision-tree approach to reflect real-world treatment of patients with invasive fungal infection (IFI) prior to differential pathogen diagnosis. It was assumed that 7.8% of patients with IFI prior to differential pathogen diagnosis at treatment initiation actually had mucormycosis, and confirmation of pathogen identification was achieved for 50% of all patients during treatment. To extrapolate to a lifetime horizon, the model considered expected survival based on the patients’ underlying condition. The model estimated the incremental costs (costs of drugs, laboratory analysis, hospitalization, and management of adverse events) and clinical outcomes (life-years (LYs) and quality-adjusted life-years (QALYs)) of first-line treatment with isavuconazole compared with voriconazole. The robustness of the results was assessed by conducting deterministic and probabilistic sensitivity analyses.
Results: Isavuconazole delivered 0.48 more LYs and 0.39 more QALYs per patient at an incremental cost of £3,228, compared with voriconazole in the treatment of patients with IFI prior to differential pathogen diagnosis. This equates to an incremental cost-effectiveness ratio (ICER) of £8,242 per additional QALY gained and £6,759 per LY gained. These results were driven by a lack of efficacy of voriconazole in mucormycosis. Results were most sensitive to the mortality of IA patients and treatment durations.
Conclusions: At a willingness to pay (WTP) threshold of £30,000 per additional QALY, the use of isavuconazole for the treatment of patients with IFI prior to differential pathogen diagnosis in the UK can be considered a cost-effective allocation of healthcare resources compared with voriconazole.
Introduction
IA and mucormycosis are rare fungal diseases caused by opportunistic fungi, Aspergillus and Mucorales, respectivelyCitation1–3. Typically affecting immunocompromised or critically ill patients, the incidence of IA and mucormycosis is rising as the size and survival of these patient groups increasesCitation4,Citation5.
The burden to patients and healthcare providers of IA and mucormycosis infection is often significant, meaning prompt and correct diagnosis is crucial to optimize patient outcomes and efficiently manage healthcare resources. For example, IA and mucormycosis disease progression is rapid and, particularly with disseminated infection, often results in deathCitation6,Citation7, with mortality rates ranging from 88–100%Citation6,Citation8. Furthermore, mortality rates for IA and mucormycosis can increase by at least 2-fold if antifungal coverage is delayed by 6–10 daysCitation9,Citation10. Management of IA and mucormycosis is also associated with lengthy hospital staysCitation11,Citation12, which further increases the clinical and economic burden on patients and healthcare institutions, respectively.
Unfortunately, establishing a prompt IA and/or mucormycosis pathogen confirmation is challenging, as patients typically present with non-specific symptomsCitation4, often similar to those of more common bacterial and viral infectionsCitation13. IA and mucormycosis may also occur as a co-infection, further complicating the likelihood of achieving a differential diagnosis, as confirmation of one does not rule out the otherCitation14. Consequently, treatment is often initiated before confirmation of a causative pathogen, or when co-infection cannot be ruled outCitation15. This can lead to patients receiving inappropriate treatment, including antifungals which may lack activity against the pathogen causing infectionCitation16, or broad-spectrum antibiotics, that, along with being ineffective against fungal diseases, may accelerate the problem of antimicrobial resistanceCitation17.
Broad-spectrum antifungal agents are typically used in the treatment of IA and mucormycosis, including isavuconazole, voriconazole, posaconazole, and lipid-based formulations of amphotericin BCitation18–32. Isavuconazole is indicated in the European Union (EU) in adults with IA and those with mucormycosis for whom amphotericin B is inappropriateCitation33, and in the United States (US) in patients aged 18 years and older with IA and mucormycosisCitation34. Two pivotal trials (SECURE and VITAL), have shown isavuconazole to have comparable efficacy and survival to voriconazole in patients with IA, as well as efficacy as primary or salvage therapy in the treatment of mucormycosisCitation35,Citation36. In a matched case-control analysis using controls from the FungiScope registry, isavuconazole demonstrated similar survival to amphotericin B in patients with mucormycosisCitation36.
Isavuconazole offers multiple advantages relative to voriconazole, which are likely to be of benefit in the treatment of patients with IA and also have positive economic consequences.
Unlike voriconazole that does not have Mucorales coverage, isavuconazole is active against Aspergillus, and Mucorales.Citation33,Citation37
Patients receiving isavuconazole experience significantly fewer moderate/severe treatment-emergent adverse events (AEs) than voriconazole.Citation38
Isavuconazole does not routinely require therapeutic drug monitoring—a necessity for the majority of patients with IA receiving voriconazole.Citation35,Citation39
Unlike voriconazole, isavuconazole does not require dose adjustment for use in patients with mild/moderate hepatic impairment, and its intravenous (IV) formulation that does not contain cyclodextrin can be used without restriction in patients with renal impairment.Citation23,Citation33
Economic analyses of isavuconazole have been performed relative to voriconazole in the treatment of IA and amphotericin B in mucormycosis, in several healthcare system settings. A US cost-effectiveness evaluation found isavuconazole to be a cost-effective treatment option relative to voriconazole in hospitalized patients with IACitation40. Another cost-effectiveness analysis took a Swedish healthcare payer perspective and found treatment of possible IA (at the point of treatment initiation, a differential diagnosis between IA and mucormycosis had not been achieved) with isavuconazole to be cost-effective compared with voriconazoleCitation41. Furthermore, cost-minimization models in ItalyCitation42, GermanyCitation43, and the UKCitation44 estimated that isavuconazole is cost-saving relative to liposomal amphotericin B (L-AMB) followed by posaconazole in the treatment of mucormycosis and of invasive mold disease in generalCitation45.
The current economic model is from the perspective of the UK NHS, and reflects real-world treatment approaches, by estimating the cost-effectiveness of isavuconazole for the treatment of IFI prior to differential pathogen diagnosis in adult patients, when, at the point of treatment initiation, a differential diagnosis between IA and mucormycosis has not been achieved.
Materials and methods
Model structure and key assumptions
The model is based on a decision-tree structure. A decision-tree approach was deemed appropriate as it reflects the short-term patient pathway from initial symptoms to eventual result after antifungal treatment (completion of antifungal treatment upon resolution of infection or death); .
Figure 1. Decision-tree model structure. First level decision nodes represent the treatment comparison; second level decision nodes represent the IA/mucormycosis pathogen split; third level decision nodes are associated with second-line treatment options; areas in grey represent the parts of the tree branch where pathogen information has/may have an effect on treatment decisions. Abbreviations. IA, Invasive aspergillosis; Mucor, Mucormycosis; L-AMB, Liposomal amphotericin-B.
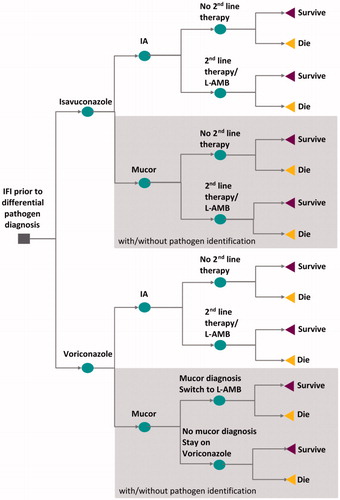
The model estimated that, based on NHS Hospital Episode Statistics data, 7.8% of patients with invasive mold disease, but without differential pathogen diagnosis, had mucormycosis (with the remaining 92.2% having IA)Citation46. The model assumed that, during treatment, differential pathogen diagnosis was achieved in 50% of patients with mucormycosis, necessitating switching to a treatment with mucormycosis coverage (i.e. L-AMB).
In line with previous cost-effectiveness analyses of antifungal treatments in the UKCitation47, a lifetime horizon was chosen to fully capture the long-term effects and costs of the two comparators. Results were extrapolated to a lifetime horizon using the average life expectancy (17 yearsCitation48) of acute myeloid leukemia survivors. A quality-of-life utility value of 0.82 was applied to all patients who successfully completed IFI treatment, irrespective of whether they had IA or mucormycosis, based on the reported EQ-5D scores of 88 acute myeloid leukemia survivorsCitation49. Acute myeloid leukemia was chosen as it was the most prevalent underlying health condition of patients included in the SECURECitation35 and VITALCitation36 trials.
The model used base case values for the UK. Default values were sourced from published literature, the SECURE and VITAL studies, isavuconazole’s and voriconazole’s Summary of Product Characteristics (SmPC) documents, and UK national price lists. Costs and health effects were discounted using a 3.5% discount rate as per The National Institute for Health and Care Excellence (NICE) requirementsCitation50.
Additional assumptions upon which the model is based include:
Patients with invasive mold disease initiate antifungal treatment prior to pathogen information being available to clinicians. This information is assumed to become available to clinicians before the end of a treatment course.
Drug administration costs are not included separately and are assumed to be part of the hospitalization costs applied in the model.
Patients that do not respond to first-line treatment switch treatment at an average of 21 days (a figure based on observed data from SECURE and VITAL shows that the majority of patients with no initial response switched treatment between days 1 to 42).
Treatment duration with L-AMB and voriconazole is assumed to be equivalent to the observed value of isavuconazole in the SECURE trial.
Patients with IA being treated with L-AMB step down to either posaconazole or voriconazole on a 50/50% split—clinical assumption.
Additional therapeutic drug monitoring for isavuconazole and voriconazole is factored into the model in accordance with the 2017 ESCMID-ECMM-ERS guideline (optional for base case).
Patients with IA, who receive voriconazole, are assumed to experience the same mortality as those receiving isavuconazole (since the difference observed in the SECURE trial was not statistically significant).
The percentage of patients requiring second-line treatment from the isavuconazole arm of the SECURE trial is applied to both isavuconazole and voriconazole cohorts (since the difference observed in the SECURE trial was not statistically significant).
It is assumed that the mortality figure from the isavuconazole arm of the SECURE trial can also be attributed to second-line treatment with L-AMB (without applying two mortality figures—for first- and second-line—in this sub-group).
Model parameters
Clinical inputs
The analysis incorporated a second-line treatment course with L-AMB for patients discontinuing first-line treatment. The percentage of patients receiving second-line treatment was calculated using data from the SECURE and VITAL trials by considering the number of patients that discontinued first-line treatment due to no or insufficient response to treatment, adverse events or intercurrent illness. The figure from the isavuconazole arm of the SECURE trial was applied to both isavuconazole and voriconazole cohorts as the difference observed in the SECURE trial was not statistically significant. It was also assumed that all patients with mucormycosis receiving voriconazole would switch to second-line treatment once pathogen information became available. The proportion of patients moving to second line treatment are summarized in .
Table 1. Proportion of patients moving to second line treatment.
All-cause mortality (ACM) data used in the model were sourced from the SECURECitation35 and VITAL studiesCitation36, and the literatureCitation51,Citation52. Patients with IA receiving voriconazole were assumed to experience the same ACM at day 84 as those receiving isavuconazole (29%)Citation35. This percentage was also assumed for second-line treatment of patients with L-AMB without sequentially applying two mortality figures. For patients treated with isavuconazole for mucormycosis, an ACM at day 84 of 43% was used, based on results from the VITAL studyCitation36. For patients treated with voriconazole who switched to L-AMB/posaconazole, a day 84 ACM rate of 83% was assumed, based on findings from a study analyzing the impact of delaying effective amphotericin B-based therapy on patients with hematologic malignancy and zygomycosis (mucormycosis)Citation51. For voriconazole-treated patients with mucormycosis for whom no pathogen confirmation became available, an ACM at day 84 of 96% was used, sourced from a meta-analysis of the mortality attributed to untreated mucormycosis patientsCitation52 ().
Table 2. All-cause mortality rates at day 84.
While there was no significant difference in the incidence of treatment-emergent adverse events, the SECURE trial manuscript by Maertens et al.Citation35 reported a statistically significant difference in the overall incidence of drug-related adverse events between patients treated with isavuconazole and voriconazole. A breakdown of individual drug-related treatment-emergent AEs experienced by a significantly different proportion of isavuconazole- and voriconazole-treated patients was reported in the European Medicines Agency (EMA) assessment report of isavuconazole. Significant differences were observed within various system organ classes, including hepatobiliary, psychiatric, eye, and respiratory, thoracic and mediastinal disorders, and laboratory investigationsCitation53 (). Risk of nephrotoxicity was also included for patients who received L-AMB, and was estimated to occur in 11.5%Citation54 of patients, associated with 9 days of additional hospital treatmentCitation47, at a cost of £472.55 per dayCitation55.
Table 3. Study drug-related treatment-emergent AEs experienced by isavuconazole and voriconazole patientsCitation53.
Treatment, hospitalization, and laboratory analysis inputs
For isavuconazole, voriconazole, and posaconazole, dosing regimens in the model were aligned to the recommended dosage in their corresponding SmPCCitation23,Citation33,Citation56,Citation57. For L-AMB, the dosing regimen was aligned to the dosage in the FungiScope registry matched case control analysisCitation58. For the IV formulations of voriconazole and L-AMB, weight-based dosing was calculated using the mean weight of 71.4 kg (standard deviation = 16.4 kg) as observed in the SECURE studyCitation35. In both treatment groups, the model assumed 75% of patients started treatment on IV therapy, subsequently switching to oral therapy, and 25% started on oral therapyCitation45.
For first-line treatment of IA, the treatment durations for isavuconazole and voriconazole were set according to the SECURE study (47.1 days in total; 8.1 days IV, 38.9 days oral)Citation59. For first-line treatment of mucormycosis, treatment duration of isavuconazole was set to the mean duration observed in the VITAL study (15.5 days IV, 133.5 days oral, or 149 days for those not receiving IV). Patients with mucormycosis were assumed to remain on voriconazole treatment unless pathogen information became available; at which point, they would switch to L-AMB on day 11 of treatment—the median time between clinical signs and mucormycosis diagnosis observed in the study by Xhaard et al.Citation60. Those who did not receive a diagnosis were assumed to be treated as per IA.
L-AMB was used in the model as a second-line treatment for both IA and mucormycosis after failure of both isavuconazole and voriconazole. It was dosed at 5 mg/kg for both IA and mucormycosis, with the assumption that the dosing for IA is the same as in the treatment of mucormycosisCitation33, and for mucormycosis, as in the matched case control analysis registry, FungiScopeCitation58. It was assumed L-AMB would be followed by oral step-down treatment with voriconazole/posaconazole for patients with IA, and by posaconazole for patients with mucormycosis.
In the treatment of IA, duration of second-line therapy was assumed to be equal to that of first-line therapy in patients who responded to treatment (70.7 days)—an assumption made in a previous economic modelCitation47. The duration of treatment with L-AMB was set to 14.5 days, as observed in the Leenders et al.Citation61 clinical study, and the duration of step-down treatment with posaconazole or voriconazole was calculated as the difference between 14.5 days and the total duration of second-line therapy. In the treatment of mucormycosis, the duration of treatment with L-AMB was set to 27.2 days, as observed in the matched cohort from the FungiScope registryCitation62, and following the same estimation approach as in IA, the duration of treatment with posaconazole was calculated as the difference between 27.2 days and the total duration of second-line therapy.
Outpatient laboratory analyses considered in the model were liver function tests and therapeutic drug monitoring. The number of tests per course of treatment was estimated based on requirements stated in the appropriate SmPCsCitation23,Citation33,Citation63 and the SECURE studyCitation35,Citation38.
Costs
Drug retail prices were taken from the British National Formulary (BNF)Citation64; . The Drug Tariff Price was only available for the tablet format of voriconazole, therefore for the other drugs the NHS Indicative Price was collected. For IV voriconazole, the lowest NHS Indicative Price was selected from the range of prices listed in the BNF.
Table 4. Drug prices.
To estimate the cost of hospitalization for patients with IA, the mean duration of initial hospitalization was based on the duration observed in the SECURE study, which was not statistically different between treatments (18.6 vs 19.4 daysCitation35, and 15.0 vs 16.0 daysCitation59 for isavuconazole vs voriconazole, respectively; p = non-significant for both). Patients on second-line treatment with L-AMB followed by posaconazole or voriconazole were assumed to have the same length of hospital stay as those undergoing first-line treatment in the SECURE study (18.6 days).
To estimate the cost of hospitalization for isavuconazole-treated patients with mucormycosis, the mean duration of initial hospitalization was based on data from the VITAL studyCitation36. For patients treated with L-AMB/posaconazole, the mean duration of hospitalization was set to equal the duration of IV therapy observed in the FungiScope registry case control analysisCitation58. Patients receiving voriconazole, prior to switching to L-AMB, were assumed to be hospitalized for the complete course of their short treatment prior to switching. Patients receiving voriconazole with no pathogen information available were assumed to be hospitalized as patients with mucormycosis in the VITAL studyCitation36.
The cost per hospital day used in the model was £645.79. This was calculated from NHS reference costsCitation55 as a weighted average cost of elective and non-elective inpatient days for patients with malignant disorders of lymphatic or hematological systems, who were considered to be representative of patients with the most prevalent underlying condition in the SECURECitation35 and VITALCitation36 studies.
To estimate laboratory analysis costs for liver function tests, a price of £9.54 was applied per test, which included both the test and an average cost of 20 minutes’ work of hospital nursing staffCitation65. For therapeutic drug monitoring for antifungal agents, a price per assay (£61.97) was sourced from a UK-based mycology reference centerCitation59.
The cost of managing AEs associated with each organ class included in the model (eye, hepatobiliary, psychiatric, respiratory, eye and mediastinal, plus laboratory investigations) was assumed to be equal to the cost of an outpatient visit to the relevant specialist according to NHS reference costsCitation55 ().
Uncertainty testing
To take account of uncertainty around the input point estimates for each parameter, the model was run 10,000 times, with a value for each of the different inputs randomly selected from its respective probability distribution; mean costs and mean QALYs were calculated using these values. The way in which distributions were defined reflected the nature of the data. Parameters bounded by 0 and 1, such as percentages and health utilities, were given a beta distribution, reflecting that they could be outside this range. Parameters with positive figures and bounded at 0 (i.e. costs) were given a gamma distribution (Supplementary Table S1).
A deterministic sensitivity analysis was also performed for a variety of variables to test the robustness of model assumptions. Parameters tested included, cost, mortality, quality-of-life, epidemiology and life expectancy, and treatment, clinical practice, laboratory analysis, and hospitalization. The impact on the base-case ICER for each variable was assessed at upper and lower bounds of +25% and –25%, respectively.
A scenario analysis was conducted to test the results’ sensitivity for specific groups of patients, including those with renal and/or hepatic impairment. These patient populations were built on inputs and assumptions identified in the literature and SmPC documents of drugs used for this analysis. Scenarios for patients with renal impairment included more urinalysis and serum creatinine tests, a change in length of hospital stay, and treatment with IV voriconazole. For patients with hepatic impairment, scenarios included more liver function tests and adjustment of the voriconazole dose. A combination of these scenarios was used for patients with renal and hepatic impairment.
Results
Base case analysis
Isavuconazole was found to be more effective and more expensive than voriconazole. Specifically, over a lifetime horizon, isavuconazole delivered 0.39 more QALYs and 0.48 more LYs per patient at an incremental cost of £3,228. This resulted in an ICER of £8,242 per additional QALY gained and £6,759 per LY gained.
In terms of cost drivers, isavuconazole was found to have higher drug acquisition costs compared with voriconazole (£11,657 vs £8,254). Hospitalization costs were found to be similar among the two comparators (£19,367 vs 19,409), while isavuconazole had lower AE (£160 vs £254) and laboratory analysis costs (£68 vs £107) ().
Table 5. Base case analysis results.
Isavuconazole was associated with a greater total number of QALYs (7.50 vs 7.11) and LYs (9.15 vs 8.67) compared with voriconazole. Since the model assumed equivalent clinical outcomes for patients with IA (with the exception of AEs), the difference in QALYs and LYs gained between the two comparators was due to patients with mucormycosis, where the results favored isavuconazole for both LYs (0.58 vs 0.11) and QALYs (0.48 vs 0.09). Isavuconazole was also estimated to result in fewer AEs (0.19 vs 0.57) and fewer deaths (0.30 vs 0.34) compared with voriconazole, resulting in an ICER of £8,507 per AE avoided and an ICER of £88,496 per death avoided, respectively.
Probabilistic sensitivity analysis
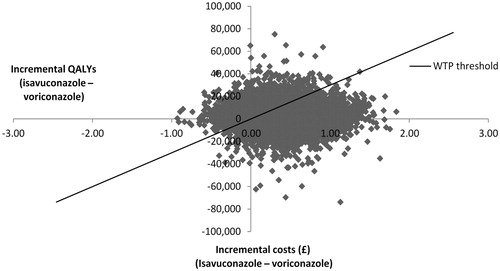
Analysis with the probabilistic values of the inputs was run 10,000 times, and the average ICER was compared against a WTP threshold of £30,000 per QALY, corresponding with the NICE WTP threshold of £20,000–£30,000, whereby products with an ICER below £30,000 per additional QALY gained are considered cost-effective and are likely to be recommended for reimbursement. Most of the scattered points, representing the differences in costs and effects among the two comparators, were in the top right quadrant of the graph, indicating that isavuconazole is more effective and more expensive in the majority of cases (). Furthermore, the majority of the scattered points fell under the threshold line where the results can be considered cost-effective.
Figure 2. Cost-effectiveness plane. Abbreviations. QALY, Quality-adjusted life years; WTP, Willingness-to-pay.
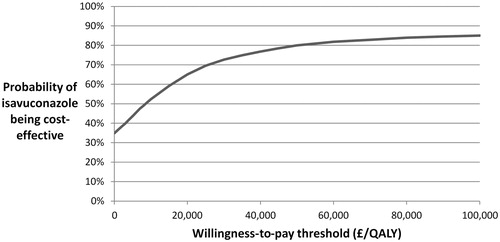
A cost-effectiveness acceptability curve (CEAC) was plotted to estimate the likelihood of isavuconazole being cost-effective at the NICE WTP threshold of £20,000–£30,000 (). The probability of isavuconazole being cost-effective at the lower bound, £20,000 per QALY gained, was 65%. At £30,000 per QALY gained, the upper bound of the threshold, this rose to 73%.
Deterministic sensitivity analysis
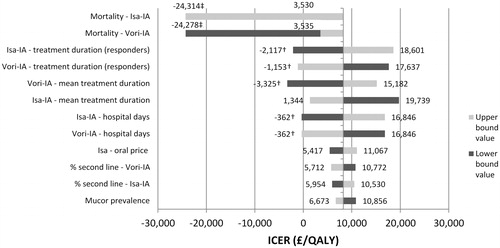
The values of 122 parameters were tested in the deterministic sensitivity analysis and the 12 parameters most influential to the model results were presented in a tornado plot (). Negative ICERs on the graph indicate isavuconazole being dominant over voriconazole (cheaper and more effective; marked with †) or dominated by voriconazole (more expensive and less effective; marked with ‡). Results were found to be sensitive primarily to changes in IA patients’ mortality, treatment duration, and hospital stay. Even so, changes in these parameters resulted in an ICER below the £20,000 threshold. Decreasing the mortality of IA patients treated with isavuconazole by 25% or increasing this input for voriconazole by the same amount decreased the ICER to ∼£3,500 per QALY. However, by increasing this variable for isavuconazole or decreasing it for voriconazole by 25%, isavuconazole became dominated by voriconazole. In addition, the length of treatment and the number of days spent in hospital also had a considerable effect on the results. Increasing the number of hospital days for isavuconazole by 25% or decreasing it for voriconazole by 25% approximately doubled the ICERs.
Scenario analysis
Three scenarios were tested in the scenario analysis: patients with renal impairment, patients with hepatic impairment, and patients with both renal and hepatic impairment. The parameters tested in the scenario analysis are outlined in Supplementary Table S2. As the inputs modified in the scenarios did not impact patient survival and quality-of-life, there was no change in the incremental LYs and QALYs. Compared to the base case results, ICERs varied only due to changes in costs.
In scenarios that included renal impairment it was estimated that isavuconazole dominated over voriconazole. For patients with hepatic impairment only, neither treatment dominated and the cost-effectiveness results were within the range of £10,000 to £13,000 per LY or per QALY gained ().
Table 6. Scenario analysis results.
Discussion
The isavuconazole cost-effectiveness model was developed to estimate the cost-effectiveness of isavuconazole compared with voriconazole from the perspective of the UK healthcare system in the treatment of adult patients with IFI prior to differential pathogen diagnosis.
The base case analysis suggested that isavuconazole is cost-effective compared with voriconazole at a WTP threshold range of £20,000–30,000 per QALY. Isavuconazole was 11.5% more costly than voriconazole, mainly due to higher drug acquisition costs (£11,657 per patient for isavuconazole compared with £8,254 for voriconazole), but this was partially offset by lower hospitalization, laboratory analysis, and AE costs. The increase in expenditure was accompanied by improved clinical outcomes with a gain of almost 0.4 QALYs and 0.5 LYs per patient compared with voriconazole-treated patients. The difference in QALYs and LYs between the two treatments was mainly attributed to the mucormycosis arm of the model and to the fact that voriconazole is not effective for the treatment of mucormycosis. It should be noted that the model results are based on 2017 costs and that, as of mid-2019, the price of oral voriconazole has decreased by 7%. However, this is unlikely to greatly impact the overall model results since reducing the cost of oral voriconazole by 25% in the deterministic sensitivity analysis only increased the ICER by ∼£500/QALY (Supplementary Table S3). Treatment with isavuconazole was also associated with a reduction in adverse events of 0.38 per patient and a more modest reduction in the number of deaths of 0.04 per patient compared with voriconazole.
Very few studies evaluating the cost-effectiveness of isavuconazole have been published. A US hospital perspective model was published in 2017 and evaluated the cost-effectiveness of isavuconazole and voriconazole in terms of incremental cost per death avoided and incremental cost per responder, based on data from the SECURE trialCitation40. The US model results favored the use of isavuconazole with a total cost per patient of $44,748 compared with $52,166 for voriconazole. The main driver of the difference in total costs was the cost of hospitalization. In terms of cost-effectiveness, the US model estimated that isavuconazole was dominant in terms of the incremental cost per death avoided and the incremental cost per responder. A Swedish model published in 2019 assessed the cost-effectiveness of isavuconazole vs voriconazole for the treatment of patients who, at the point of treatment initiation, did not have a confirmed differential diagnosis between IA and mucormycosis, in terms of incremental cost per QALY based on data from the SECURE trial, and case-control analysis between the VITAL study and FungiScope registryCitation41. The Swedish model results also favored the use of isavuconazole with 0.3 more QALYs per patient than voriconazole at an incremental cost of 52,191 Swedish krona (SEK), resulting in an ICER of 174,890 SEK per additional QALY gained. This was mainly due to the efficacy of isavuconazole against IA and mucormycosis, as opposed to voriconazole, which is only effective against IA. The results of the UK model described in this paper are, therefore, well-aligned with the previously-published US and Swedish models insomuch as all three are favorable to isavuconazole in the treatment of IFI prior to differential pathogen diagnosis. However, it is difficult to draw any more substantial conclusions from the studies due to variation in perspective and approach taken.
In order to obtain results for the base-case population, several assumptions were made concerning clinical practice, the patients’ underlying condition, and quality-of-life. These assumptions were necessary due to limited availability of published evidence in the area, driven by the low incidence of mucormycosis and scarce information about the long-term consequences of antifungal treatments. The model assumed that patients had underlying acute myeloid leukemia, which was the most prevalent underlying condition in the SECURE and VITAL studies, and applied utility values and survival figures associated with this condition. However, it is possible that the utility and survival rates associated with acute myeloid leukemia may differ for patients who survive IFI. Furthermore, in the real world patients with IFI may have different underlying conditions such as chronic obstructive pulmonary disease (COPD) or diabetes mellitus which may affect the underlying quality-of-life—patients with advanced COPD would be expected to have a lower quality-of-life, for example. However, the assumption that patients had acute myeloid leukemia is unlikely to have greatly impacted the overall results of the model, since the deterministic sensitivity analysis results for AML life expectancy and quality-of-life showed only a modest impact on the ICER, suggesting that there is a wide tolerance for uncertainty in this input (Supplementary Table S3). The model assumed that patients surviving a fungal infection do not carry any disutility as a result of their infection or treatment, whereas, in practice, some patients remain immunosuppressed after completing therapy and may require continued therapy and monitoring for lifeCitation66, leading to increased costs and reduced quality-of-life. However, any impact on the ICER should be minimal given that only a small sub-set of patients will require long-term monitoring. Patients switching to L-AMB as a result of pathogen confirmation were assumed to have an increased mortality rate due to the delay before appropriate treatment, while patients who continued to receive voriconazole were assumed to have a mortality rate applicable to untreated patients. These mortality assumptions are believed to reflect clinical experience and the natural progression of the diseaseCitation52.
Importantly, a number of clinical, pharmacokinetic, pharmacodynamic, safety and ease-of-use benefits have been documented with the use of isavuconazole, which fall outside of the scope of the model described here. For example, unlike voriconazole which may extend the QT interval, isavuconazole has a dose-related shortening effect, with no evidence of associated cardiac risk, thereby potentially reducing the cost of cardiac-associated AEsCitation67. Additionally, isavuconazole has fixed dose administration via either oral capsules or an intravenous infusionCitation33, whereas voriconazole must be administered twice daily, which will likely lead to increased costs associated with drug administration. Furthermore, isavuconazole has a lower potential for drug–drug interactions than voriconazoleCitation68,Citation69. Drug–drug interactions may lead to changes in the exposure dose of any concurrent treatments being administered and, therefore, demand increased clinical monitoring, leading to an increase in the overall treatment costs. Given that the model does not account for these additional benefits, it is possible that the reported ICER under-estimates the true cost-effectiveness of isavuconazole in the treatment of patients with IFI prior to differential pathogen diagnosis. Results from a 2018 UK budget impact analysis, whereby isavuconazole was estimated to reduce the total budget in patients with renal impairment, provide an example of how inclusion of additional clinical benefits with the use of isavuconazole instead of voriconazole may lead to economic advantagesCitation70.
Although the perspective of the model was the UK NHS, clinical inputs in the model were largely populated from clinical trial data (i.e. the SECURE and VITAL studies) which included patients from multiple countries across multiple continents. Clinical outcomes may have differed if a solely UK-based population had been included, which may have, in turn, impacted costs, resource use, and the overall ICER. It should also be noted that patients participating in clinical trials have to meet pre-specified inclusion criteria which may not reflect the diverse range of baseline characteristics observed in real-world practice. However, in the deterministic sensitivity analysis, the results were found to be robust under a number of alterations to key parameters, and all deterministic analysis results remained under NICE’s upper WTP threshold value of £30,000 per QALY gained. Probabilistic sensitivity analysis results scattered mostly in the top and bottom right quarters of the cost-effectiveness plane and the results showed some uncertainty around the parameters used in the model, expressed via the range of scatters on the horizontal QALY axis. This was mainly due to the small sample sizes in the studies used to source the clinical effectiveness figures. Nevertheless, the CEAC demonstrated that the probability of isavuconazole being cost-effective is over 73% at a threshold of £30,000 per QALY gained. The sensitivity analyses therefore suggest that, regardless of patient heterogeneity, isavuconazole will be cost-effective in real-world clinical use.
The scenario analysis focused only on alternate fragile patient populations due to the fact that IA and mucormycosis typically affect patients who are immunocompromised or critically ill. Since no other scenarios of methodological uncertainty, such as discount rates or time horizons were conducted, this is considered to be a limitation of the model. The scenario analysis for fragile patient populations demonstrated that all cost-effectiveness results were either more favorable to isavuconazole or below the NICE WTP threshold range. Additionally, all scenarios in which patients had renal impairment showed that isavuconazole was dominant over voriconazole, suggesting that isavuconazole may be of particular value in this sub-set of patients. Patients with IFI prior to differential pathogen diagnosis may also be receiving immunosuppressant medication, to prevent rejection following a transplant, for example. Voriconazole has a higher risk of drug–drug interactions with immunosuppressants compared with isavuconazoleCitation68,Citation69. These drug–drug interactions can lead to changes in immunosuppressant exposure and may, therefore, require additional clinical monitoringCitation71. Immunocompromised patients therefore represent a sub-group of particular interest. Unfortunately, it was not possible to perform a scenario analysis for immunocompromised patients in this study due to insufficient data, but future studies should aim to include this should the data become available.
Conclusions
IA and mucormycosis are fungal infections that typically affect immunocompromised and critically ill patients. Due to their severity, rapid and correct diagnosis is necessary to ensure effective treatment. While voriconazole is considered the standard of care for these fungal infections, isavuconazole offers several advantages in their treatment, including efficacy against both IA and mucormycosis. This study estimated the impact of using isavuconazole to treat patients with IFI prior to differential pathogen diagnosis from a UK NHS perspective. The results showed that isavuconazole is more expensive, but also more effective than voriconazole. Based on a willingness-to-pay threshold of £20,000–£30,000 isavuconazole can be considered cost-effective and, therefore, represents an attractive option in the treatment of patients with IFI prior to differential pathogen diagnosis.
Transparency
Declaration of funding
This study was sponsored by Pfizer. The sponsor was involved in study design, data collection, analysis, and interpretation of the data, in collaboration with the authors. The authors were responsible for the conception and design of the study.
Declaration of financial/other relationships
LF and DW were employees of Covance Market Access, who were paid consultants to Pfizer in connection with the development of this manuscript. AP was a paid consultant to Pfizer in connection with the development of this manuscript. AT is an employee of Pfizer Ltd. RC is an employee of Pfizer PFE. MG is an employee of Covance Market Access. AS is an employee of Pfizer Inc. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (34.3 KB)Acknowledgements
Editorial writing support was provided by Daniel Fielden and Michael Blackney at Covance Market Access and was funded by Pfizer.
References
- ema.europa.eu. [Internet] London: European Medicines Agency; July 2014 [cited Jan 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2014/07/WC500169890.pdf
- European Medicines Agency; July 2014 [cited Jan 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2014/07/WC500169714.pdf
- Richardson M, Lass FC. Changing epidemiology of systemic fungal infections. Clin Microbiol Infect. 2008;14:5–24.
- Badiee P, Hashemizadeh Z. Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 2014;139:195–204.
- Bitar D, Lortholary O, Le Strat Y, et al. Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis. 2014;20:1149–1155.
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–366.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin. Infect Dis. 2012;54:S23–S34.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653.
- Jeong SJ, Lee JU, Song YG, et al. Delaying diagnostic procedure significantly increases mortality in patients with invasive mucormycosis. Mycoses. 2015;58:746–752.
- von Eiff M, Roos N, Schulten R, et al. Pulmonary aspergillosis: early diagnosis improves survival. Respiration. 1995;62:341–347.
- Ceesay MM, Sadique Z, Harris R, et al. Prospective evaluation of the cost of diagnosis and treatment of invasive fungal disease in a cohort of adult haematology patients in the UK. J Antimicrob Chemother. 2015;70:1175–1181.
- Dodds AE, Drew R, Johnson M, et al. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy. 2012;32:890–901.
- Huijskens EGW, Koopmans M, Palmen FMH, et al. The value of signs and symptoms in differentiating between bacterial, viral and mixed aetiology in patients with community-acquired pneumonia. J Med Microbiol. 2014;63:441–452.
- Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98:492–504.
- Pfaller MA, Pappas PG, Wingard JR. Invasive fungal pathogens: current epidemiological trends. Clin Infect Dis. 2006;43:S3–S14.
- Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118:1216–1224.
- Denning DW, Perlin DS, Muldoon EG, et al. Delivering on antimicrobial resistance agenda not possible without improving fungal diagnostic capabilities. Emerging Infect Dis. 2017;23:177–183.
- medicines.org.uk. [Internet] Middlesex: emc; Nov 2017 [cited Feb 2018]. Available from: https://www.medicines.org.uk/emc/medicine/559/SPC/Fungizone±50mg±Powder±for±Sterile±Concentrate
- medicines.org.uk. [Internet] Middlesex: emc; Jan 2017 [cited Feb 2018]. Available from: https://www.medicines.org.uk/emc/product/5407
- medicines.org.uk. [Internet] Middlesex: emc; Jul 2016 [cited Feb 2018]. Available from: https://www.medicines.org.uk/emc/product/2226
- medicines.org.uk. [Internet] Middlesex: emc; Aug 2017 [cited Feb 2018]. Available from: https://www.medicines.org.uk/emc/product/5914
- medicines.org.uk. [Internet] Middlesex: emc; Apr 2017 [cited Dec 2017]. Available from: https://www.medicines.org.uk/emc/medicine/1236
- ec.europa.eu. [Internet] London: European Commission; 2005 [cited Feb 2018]. Available from: http://ec.europa.eu/health/documents/community-register/2005/200503299473/anx_9473_en.pdf
- ema.europa.eu. [Internet] London: European Medicines Agency; 2015 [cited Mar 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002734/WC500196128.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Mar 2015 [cited Feb 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Oct 2008 [cited Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050740s016lbl.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Feb 2005 [cited Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/21227s015lbl.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Nov 2015 [cited Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022003s018s020,0205053s002s004,0205596s001s003lbl.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Feb 2015 [cited Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021266s038,021267s047,021630s028lbl.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Apr 2012 [cited Mar 2018]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020083s048s049s050lbl.pdf
- aidsinfo.nih.gov. [Internet] US: US Department of Health and Human Services; Dec 2017 [cited Mar 2018]. Available from: https://aidsinfo.nih.gov/drugs/6/amphotericin-b/115/professional
- aidsinfo.nih.gov. [Internet] US: US Department of Health and Human Services; Dec 2017 [cited Mar 2018]. Available from: https://aidsinfo.nih.gov/drugs/6/amphotericin-b/117/professional
- ema.europa.eu. [Internet] London: European Medicines Agency; Oct 2015 [cited Mar 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002734/WC500196128.pdf
- accessdata.fda.gov. [Internet] US: Food and Drug Administration; Mar 2015 [cited Jan 2019]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–769.
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16:828–837.
- Sabatelli F, Patel R, Mann PA, et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemoth. 2006;50:2009–2015.
- Ullmann AJ, Selleslag D, Heinz W, et al. A comparison of the safety profiles of isavuconazole vs voriconazole in the Phase 3 SECURE study in patients with invasive mould infections. Poster EP018 presented at 25th European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Congress, Copenhagen, Denmark, April 25–28, 2015.
- Ashbee HR, Barnes RA, Johnson EM, et al. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69:1162–1176.
- Harrington R, Lee E, Yang H, et al. Cost-effectiveness analysis of isavuconazole vs. voriconazole as first-line treatment for invasive aspergillosis. Adv Ther. 2017;34:207–220.
- Floros L, Kuessner D, Posthumus J, et al. Cost-effectiveness analysis of isavuconazole versus voriconazole for the treatment of patients with possible invasive aspergillosis in Sweden. BMC Infect Dis. 2019;19:134.
- Bagshaw E, Carotti A, Blackney M, et al. The cost of treating mucormycosis with isavuconazole compared with liposomal amphotericin B followed by posaconazole in Italy: Economic evaluation of the phase III vital study and FungiScope matched case-control analysis, in Poster presented at: 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation, Marseille, France, March 26–29, 2017.
- Bagshaw E, Blackney M, Heimann SM, et al. The cost of treating mucormycosis with isavuconazole compared with liposomal amphotericin B followed by posaconazole in Germany: economic evaluation of the phase III VITAL study and FungiScope matched case-control analysis, in Poster presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, April 22–25, 2017.
- Bagshaw E, Kuessner D, Posthumus J, et al. The cost of treating mucormycosis with isavuconazole compared with standard therapy in the UK. Future Microbiol. 2017;12:515–525.
- Bagshaw E, Enoch DA, Blackney M, et al. Economic impact of treating invasive mold disease with isavuconazole compared with liposomal amphotericin B in the UK. Future Microbiol. 2018;13:1283–1293.
- digital.nhs.uk. [Internet] UK: NHS Digital; 2016/2017 [cited Feb 2019]. Available from: https://files.digital.nhs.uk/publication/7/d/hosp-epis-stat-admi-diag-2016-17-tab.xlsx
- Bruynesteyn K, Gant V, McKenzie C, et al. A cost-effectiveness analysis of caspofungin vs. liposomal amphotericin B for treatment of suspected fungal infections in the UK. Eur J Haematol. 2007;78:532–539.
- Bower H, Bjorkholm M, Andersson T-L, et al. Continued improvement in survival of acute myeloid leukemia patients: an application of the loss in expectation of life. Blood Cancer J. 2016;6:e390.
- Leunis A, Redekop WK, Uyl-de Groot CA, et al. Impaired health-related quality of life in acute myeloid leukemia survivors: a single-center study. Eur J Haematol. 2014;93:198–206.
- nice.org.uk. [Internet] UK: National Institute for Health and Care Excellence; Apr 2013 [cited Jan 2019]. Available from: https://www.nice.org.uk/process/pmg9/chapter/the-reference-case#discounting
- Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–509.
- accessdata.fda.gov. [Internet] US: Center For Drug Evaluation And Research; July 2014 [cited Jan 2019]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207500Orig1207501Orig1s000MedR.pdf
- ema.europa.eu. [Internet] London: European Medicines Agency; July 2015 [cited Feb 2018]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002734/WC500196130.pdf
- Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402.
- assets.publishing.service.gov.uk. [Internet] UK: Department of Health; 2016 [cited Jan 2019]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/577083/Reference_Costs_2015-16.pdf
- medicines.org.uk. [Internet] Middlesex: emc; July 2018 [cited Jan 2019]. Available from: https://www.medicines.org.uk/emc/product/5388/smpc
- medicines.org.uk. [Internet] Middlesex: emc; Jul 2018 [cited Jan 2019]. Available from: https://www.medicines.org.uk/emc/product/3435/smpc
- Fluckiger U, Marchetti O, Bille J, et al. Treatment options of invasive fungal infections in adults. Swiss Med Weekly. 2006;136:447–463.
- Horn D, Goff D, Khandelwal N, et al. Hospital resource use of patients receiving isavuconazole versus voriconazole for invasive mold infections in the phase III SECURE trial. J Med Econ. 2016;19:728–734.
- Xhaard A, Lanternier F, Porcher R, et al. Mucormycosis after allogeneic haematopoietic stem cell transplantation: a French Multicentre Cohort Study (2003–2008). Clin Microbiol Infect. 2012;18:E396–E400.
- Leenders A, Daenen S, Jansen RLH, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103:205–212.
- Matched-case analysis of primary mucormycosis patients from isavuconazole study 9766-CL-0103/WSA-CS-003 to patients from FungiScope registry database. 2014.
- medicines.org.uk. [Internet] Middlesex: emc; 2018 [cited Jan 2019]. Available from: https://www.medicines.org.uk/emc/product/1022/smpc
- medicinescomplete.com. [Internet] UK: Medicines Complete; 2019 [cited Jan 2019]. Available from: https://about.medicinescomplete.com/#/browse/bnf
- Curtis L, Burns A, [Internet] UK: PSSRU; 2017 [cited Jan 2019]. Available from: https://kar.kent.ac.uk/65559/40/65559_rep_UCR-2017-v13finalKAR.pdf
- Patterson TF, Thompson GR, 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–e60.
- Keirns J, Desai A, Kowalski D, et al. QT interval shortening with isavuconazole: in vitro and in vivo effects on cardiac repolarization. Clin Pharmacol Ther. 2017;101:782–790.
- Natesan SK, Chandrasekar PH. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. IDR. 2016;9:291–300.
- Townsend R, Desai A, Azie N, et al. Drug interaction profiles of isavuconazole, voriconazole and posaconazole with immunosuppressants metabolized by CYP4503A4 (CYP3A4). Mycoses. 2015;58:218–219.
- Weidlich D, Hicks M, Floros L, et al. Estimating the budget and clinical impact of introducing isavuconazole for the treatment of patients with possible invasive aspergillosis in the United Kingdom. 2018, Poster presented at ISPOR Europe, 10–14 Nov, 2018.
- Groll AH, Desai A, Han D, et al. Pharmacokinetic assessment of drug-drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin Pharmacol Drug Dev. 2017;6:76–85.